Published online Mar 7, 2014. doi: 10.3748/wjg.v20.i9.2237
Revised: December 14, 2013
Accepted: January 19, 2014
Published online: March 7, 2014
Processing time: 135 Days and 6.5 Hours
Pancreatic ductal adenocarcinoma (PDA) is among the deadliest cancers in the United States and in the world. Late diagnosis, early metastasis and lack of effective therapy are among the reasons why only 6% of patients diagnosed with PDA survive past 5 years. Despite development of targeted therapy against other cancers, little progression has been made in the treatment of PDA. Therefore, there is an urgent need for the development of new treatments. However, in order to proceed with treatments, the complicated biology of PDA needs to be understood first. Interestingly, majority of the tumor volume is not made of malignant epithelial cells but of stroma. In recent years, it has become evident that there is an important interaction between the stromal compartment and the less prevalent malignant cells, leading to cancer progression. The stroma not only serves as a growth promoting source of signals but it is also a physical barrier to drug delivery. Understanding the tumor-stroma signaling leading to development of desmoplastic reaction and tumor progression can lead to the development of therapies to decrease stromal activity and improve drug delivery. In this review, we focus on how the current understanding of biology of the pancreatic tumor microenvironment can be translated into the development of targeted therapy.
Core tip: This is a comprehensive review and an update on recent progresses in understanding the role of tumor microenvironment in the growth, invasion and metastasis of pancreatic cancer. The role of tumor microenvironment in anti-tumor immune response and treatment of pancreatic cancer is also reviewed. How our knowledge in tumor microenvironment is translated into the development of pancreatic cancer therapy is discussed.
- Citation: Rucki AA, Zheng L. Pancreatic cancer stroma: Understanding biology leads to new therapeutic strategies. World J Gastroenterol 2014; 20(9): 2237-2246
- URL: https://www.wjgnet.com/1007-9327/full/v20/i9/2237.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i9.2237
Pancreatic ductal adenocarcinoma (PDA) is a devastating disease. It is the 4th leading cause of cancer related deaths in the United States and according to latest statistics the incidence rate is on a rise. The high mortality rates are founded on the fact that PDA is very resistant to chemotherapy and radiation. Most patients are diagnosed at late/metastatic stages of the disease. Less than 20% of patients diagnosed with PDA are eligible for surgical resection, and out of those most present with high incidence of metastasis after resection[1]. This aggressiveness contradicts the finding that majority of the tumor volume is not composed of neoplastic cells, but consists of the stroma/desmoplastic reaction to the cancer[2,3]. In recent years, it has become evident that the desmoplastic reaction is not only a bystander, but it is a source of cellular and molecular components that promote tumor progression and metastasis[4,5]. Importantly, increased levels of stroma correlate with poor prognosis[6] and depletion of the stromal compartment has been associated with improved prognosis in both preclinical and clinical trials[7-9] making pancreatic tumor stroma a valid therapeutic target. Despite the broader understanding of PDA biology very little progress has been made in terms of treatment development. Gemcitabine was approved for PDA treatment over a decade ago, however it still remains the standard of care[10]. The recent breakthrough phase III clinical trial evaluating combination therapy FOLFIRINOX (oxaliplatin/irinotecan/5-FU/leucovorin) showed increase in overall survival by 4.3 mo when compared to gemcitabine but it also resulted in increased toxicity. Results of the study led to approval of this drug combination for patients with metastatic PDA and good performance status[11]. The development of new therapeutics for PDA has been progressing very slowly, nevertheless the devastating PDA statistics call for an urgent advance in effective treatment strategy. In this review, we will discuss the current understanding of PDA biology and how this knowledge is being translated into development of novel, targeted therapies for PDA patients.
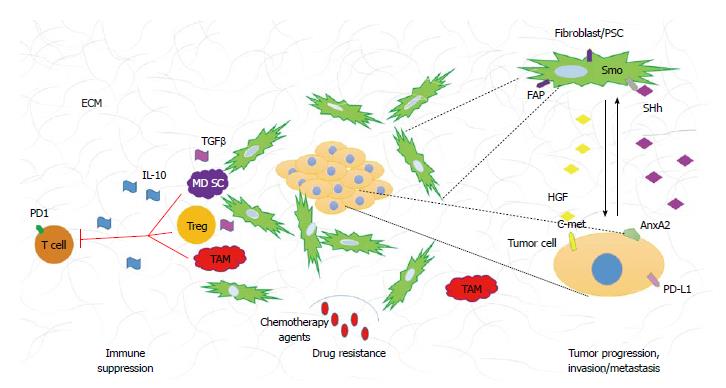
The histological hallmark of PDA is the dense stroma surrounding malignant epithelial cells. The stroma, also referred to as desmoplastic reaction consist of numerous cellular as well as acellular constituents. The cellular components include fibroblasts, stellate cells, immune cells, endothelial cells, and nerve cells. The acellular compartment is comprised of extracellular matrix (ECM) (i.e., collagen, fibrinogen, hyaluronan, and fibrin) as well as variety of other proteins, enzymes, and growth factors (Figure 1).
Activated fibroblasts, also referred to as pancreatic stellate cells (PSCs), have been given much attention in the past years. PSCs in their quiescent form are found in minimal numbers in normal, healthy pancreas[12]. Their homeostatic role is still poorly understood, however they have been shown to contain fat droplets in their cytoplasm; indicating potential role in lipid metabolism; have low mitotic index and low capability of ECM synthesis[13]. In PDA, on the other hand, PSCs become activated, as determined by their myofibroblastic phenotype and expression of alpha smooth muscle actin[14]. Activated PSCs have been shown to be a source of ECM, growth factors and immune modulatory signals[15-17]. Molecular signals originating from PSCs are conveyed to neoplastic cell promoting tumor proliferation and invasion, cancer stem cell maintenance and generation of immunosuppressive environment[13,16-21]. Similarly, neoplastic cells send stimulatory signals to PSCs providing a positive feedback loop that promotes cancer progression[22]. The population of stromal fibroblasts is very heterogeneous and numerous markers have been utilized to characterize stromal cells[23]. PSCs, which are regarded as alpha smooth muscle actin expressing cells are phenotypically similar to a broader population of fibroblasts marked by the surface glycoprotein expression of fibroblast activation protein (FAP)[14]. This similarity is based on the ability of both cell types to promote tumor proliferation and invasion, secretion of collagen types I, III, and IV, fibronectin, laminin, hyaluronan, and various growth factors[14,24]. Pro-tumorigenic properties of FAP expressing fibroblasts have made them an attractive target for PDA therapy (discussed later).
Another component of the tumor microenvironment is the ECM. This acellular part of pancreatic tumor stroma is composed of variety of fibrous proteins (i.e., collagen), polysaccharides (i.e., hyaluronan) and glycoproteins (i.e., fibronectin). Additionally, diversity of growth factors and other proteins are found in the ECM of PDA. This mesh of fibrous molecules not only provides support to the surrounding tissues but it also plays a role in differentiation, remodeling and homeostasis, in healthy organs[25]. Not surprisingly, different components of pancreatic stroma ECM have been shown to have tumorigenic properties. In particular, collagen I has been associated with higher expression of transgelin (gene used in this study to determine PSCs activation) when compared to other non-activating matrices[26]. In other studies, collagen I was linked to resistance to gemcitabine, a standard cytotoxic drug used for pancreatic cancer treatment[27,28]. Hyaluronan (HA), a non-typical glycosaminoglycan with high capacity of water retention, has recently become an attractive target for pancreatic cancer therapy. HA is expressed in high levels in PDA and its abundance has been connected to increased intratumoral fluid pressure and consequent vascular collapse[29,30]. PDA, unlike many solid tumors is hypovascular, moreover, the blood vessels that are present in the intratumoral space have been reported to be mostly nonfunctional[30]. The limited numbers of functional blood vessels in PDA and the dense stroma are believed to be among the reasons why intravenous chemotherapeutic agents as well as the recently tested antiangiogenic drugs[31] do not elicit great effect on the tumor cells.
Broad repertoire of immune cells including both adaptive and innate cell types are also present in the PDA tumor microenvironment. Tumor infiltrating immune cells have been implicated in tumor progression, chemotherapy resistance and metastasis[32-34]. Additionally, the immune infiltration is evident in early premalignant lesions and increases with PDA progression[32]. Immune suppression and immune tolerance to tumor associated antigens is one of the characteristics of PDA and it is associated with poor prognosis[33,35]. The abundance of suppressive cells leads to low numbers of effector CD8+ T cells in the PDA stroma and consequently limited anti-tumor cytotoxicity[32]. Among the most plentiful tumor infiltrating immune cells characterized by their suppressive phenotype are myeloid derived suppressive cells (MDSCs), T regulatory cells (Tregs), and tumor associated macrophages (TAMs). The suppressive cell population is characterized by its ability to prevent activation and functionality of effector cells leading to diminished tumor cytotoxicity[36]. The immune modulatory cell population regulates effector cells anti-tumor responses by variety of mechanisms. MDSC inhibit CD8+ T cell function via arginase, and reactive oxygen species secretion, which requires direct cell contact[37,38]. Tregs ability to decrease effectors function is partially due to their ability to secrete suppressive cytokines such as interleukin 10 (IL-10) and tumor growth factor β (TGFβ) but they can also be cell contact dependent where proteins like CTLA-4 and PD-1 are involved[39]. TAMs can be divided into two functional subtypes: M1 (pro-inflammatory) and M2 (immunosuppressive). The M2 subtype cells are a source of anti-inflammatory cytokines such as IL-10 and have been shown to induce Th2 responses (also found to be immunosuppressive in PDA)[40]. In addition to the presence of immunosuppressive cell populations in the PDA microenvironment, the numbers of effector cells such as CD4+, CD8+ T cells and NK cells are minimal. More importantly, infiltrating CD8+ and CD4+ T cells have either naïve phenotype or are nonfunctional, antigen experienced effectors[32]. There are numerous mechanisms that have been implicated in the non-functionality of antigen experienced T cells. Checkpoints and inhibitory receptors like PD-1 are examples of proteins that transduce inhibitory signals during lymphocyte activation[41]. Tumor cells can also express ligands such as the PD-L1 protein that have been shown to dampen immune anti-tumor responses. Upregulation of those inhibitory molecules, PD-L1 in particular, has been associated with poor prognosis[42,43]. Lastly, TGFβ has been shown to play a role in Th17 subtype differentiation[44]. Interestingly, TGFβ dependent differentiation of Th17 cells has been implicated with increased immunosuppressive abilities[45]. Both tumor cells and cancer associated fibroblast secrete TGFβ and increased levels of IL-17 secreting CD4+ cells (Th17) have been found in PDA tumor microenvironment[46]. To date, the role of Th17 subtype of immune cells remains controversial in cancer biology, as it has been shown to have both pro- and anti-tumorigenic properties[47-49]. It is important to mention, that the role of Th17 cells is well documented in promoting fibrosis[50,51]. Hepatic stellate cells (HSCs) have been shown to become activated in response to Th17 secreted factors[50,52], and because PSCs resemble HSCs, it would be interesting to investigate the role of Th17 immune subtype on PSCs activation and desmoplastic reaction.
Subsequently, modulation of the pro-tumorigenic immune infiltration by either depletion of suppressive cells, polarization of the cell population to more anti-tumor phenotype, checkpoint blockade or increase of activity of the effector cells can be exploited in cancer therapy.
Tumor-stroma interactions create a very complicated signaling network that drives tumor progression. Many signaling pathways have been associated with PDA tumorigenesis, in this review we will focus on paracrine pathways that originate in the neoplasm and contribute to the development of desmoplastic reaction (Figure 1).
Sonic hedgehog (SHh) is a developmental signaling pathway that is crucial for organ development during embryogenesis. Briefly, in the absence of ligand (SHh) the signaling pathway is inactive and the cell surface receptor Patched (Ptch) inhibits translocation of smoothened (Smo) to the cell surface. Upon ligand binding, Ptch relives the repression on Smo allowing it to translocate to cell surface. The translocation of Smo is a key activating step in downstream signaling. Gli1/2/3, which belong to the zinc-finger transcription factor family are the downstream effectors of Smo activation. Ligand binding to Ptch, results in the translocation of Gli1 (activator) to the nucleus allowing expression of SHh associated genes. During activation of the pathway, Gli2/3 (repressors) are nonfunctional. In the absence of ligand binding, Gli2/3, undergo proteolytic cleavage, move to nucleus and repress transcription of SHh dependent genes. In the inactive state, Gli1 is rendered nonfunctional[53]. SHh is overexpressed by PDA tumor cells, however its function is restricted to the stromal compartment forming a paracrine signaling network that promotes and maintains desmoplasia[54-56]. It has been also noted that only cancer associated fibroblasts and not the neoplastic cells show SHh pathway activation and Smo receptor overexpression[57]. Importantly, Olive et al[7] demonstrated that the use of a SHh inhibitor in preclinical mouse model of pancreatic cancer, resulted in better delivery of gemcitabine through reduction of stroma and increase of vascular density. It is important to mention that even though cancer associated fibroblasts (CAFs) are an established target of SHh pathway activation, recent study of pancreatic cancer stem cells (cancer initiating cells) showed that Smo is overexpressed in this population of tumor cells. Pancreatic cancer stem cells alike CAFs have been shown to be susceptible to SHh inhibition and should be considered a target[58].
The notion that depletion of stromal compartment allows for better drug delivery in PDA brought upon reexamination of another signaling pathway that has been linked to regulation of desmoplastic reaction, the TGFβ signaling pathway. This signaling cascade involves three TGFβ ligands and three receptors. In short, binding of ligand to its receptor (type II) results in recruitment and phosphorylation of type I receptor and downstream propagation of molecular signals. The effector molecules in this cascade are the proteins of SMAD family, which upon phosphorylation, dimerize, translocate to the nucleus and regulate expression of TGFβ associated genes[59]. TGFβ is overexpressed in PDA and its overexpression correlates with poor survival[60]. TGFβ’s involvement in pancreatic cancer is complicated as it has been shown to affect both the stromal and the neoplastic compartments. Elevated levels of TGFβ have been shown to impact cell proliferation, immunosuppression and activation of PSCs[61-64]. In mouse models, overexpression of SMAD 7 (TGFβ inactivator) showed decreased ECM production, less fibrosis and more importantly diminished PSCs activation[65]. Additionally, TGFβ has been demonstrated to drive epithelial to mesenchymal transition (EMT) process, believed to be the initial step of metastasis[66]. EMT was first characterized in development, in which the process is vital for embryogenesis and organogenesis. The cellular characteristics of EMT include the loss of epithelial cells polarity, cell adhesion, gain of mobility and invasive properties resulting in phenotypical changes that resemble mesenchymal cells[67]. On a molecular level, EMT is described by the changes in gene/protein expression that occur in this process. Specifically, upregulation of mesenchymal markers (vimentin, fibronectin, N-cadherin, Snail and Slug) and downregulation of epithelial markers (E-cadherin, zonula-occludens and nuclear translocation of β-catenin) are routinely used to determine the presence of EMT[67,68]. In recent years, many different pathways have been implicated in the initiation of EMT and consequent cancer invasion and metastasis, of which TGFβ is an example[66,69-74]. EMT has also been linked to induction and maintenance of cancer stem cell population in PDA[75]. Importantly, the presence of EMT markers (as discussed above) has been shown to correlate with higher lymph node metastasis and decreased survival in PDA patients[76]. Taken together, the pleotropic functionality of TGFβ in cancer makes it a valid target for patients with PDA.
There are many other signaling pathways that have been associated with PDA development, progression and metastasis. We will discuss them briefly. Expression of Delta-like ligand 4, a protein involved in the developmental Notch pathway has been linked to worst prognosis in patients who underwent surgical resection of pancreatic tumor[77]. Moreover, inhibition of γ-secretase, a protein that allows Notch signaling propagation to take place and that is often constitutively active in PDA, showed regression of primary tumors, reduced metastasis and decrease of pancreatic stem cell population when combined with gemcitabine[78]. Another pathway that recently gained attention is the c-met pathway. It is well documented that c-met receptor and its ligand HGF are upregulated in PDA. C-met and HGF are detected early in PDA development but are not sufficient to promote tumorigenesis without other oncogenic changes[79]. Recently the expression of c-met has been linked to the stem cell population and because HGF is exclusively secreted by stromal fibroblasts, paracrine relationship between the stroma and neoplasm to promote cancer progression has been suggested. Importantly, studies with c-met inhibitors showed increase of apoptosis and sensitivity to gemcitabine in malignant cells[80]. Moreover, stromal expression of HGF was correlated with decreased disease free survival[81] proposing that HGF/c-met targeting can be beneficial to patients with PDA. Another attractive target is the annexinA2 pathway shown to play an important role in pancreatic tumor metastasis and EMT. Inhibition of tyrosine 23 phosphorylation of annexinA2 was shown to reduce invasion in vitro, and metastasis in vivo. Although, the kinases responsible for annexinA2 phosphorylation in PDA remain to be confirmed, IGF-1R and Src have been proposed to be involved[74].
Nuclear factor kappa-B (NFκ-B), a transcription factor, regulates genes involved in inflammation, cell proliferation and survival[82]. NF-κB signaling pathway activity has been documented to be upregulated in PDA but not in normal pancreatic tissue[83,84]. Activation of this signaling cascade has also been linked to early processes in PDA development. Liou et al[85], recently reported that macrophage secreted cytokines initiate acinar to ductal metaplasia via activation of NF-κB and consequent upregulation of matrix metalloprotinases (MMPs). To date, direct targeting of NF-κB has been shown to be challenging[86,87]. As an alternative to the direct NF-κB inhibition, upstream activators and downstream effectors of the signaling pathway should be evaluated.
Numerous signaling pathways have been explored as potential targets for pancreatic cancer therapy, however review of all of them goes beyond the scope of this article.
Understanding complex stromal constituents and involvement of numerous signaling pathways in PDA progression and desmoplastic reaction is crucial to the development of novel therapies. It has become evident that targeting the stromal components has undeniable benefit for preclinical mouse models of PDA. However, translating those findings to the patients care can be challenging. There are numerous ongoing clinical trials utilizing the above described targets that show encouraging results. In this part of the review we will briefly discuss the most promising ones (Table 1).
| Stromal component | Therapeutic target | Treatments in preclinical and clinical trials | Up to date preclinical/clinical trial results |
| PSCs/fibroblasts | FAP | Sibrotuzumab (colorectal cancer) | Hofheinz et al[99], 2003 |
| ECM | Hyaluronan | PEGPH20 | Strimpakos et al[91], 2013 |
| MMPs | BAY 12-9566 | Moore et al[100], 2003 | |
| Marimastat | Bramhall et al[101], 2002 | ||
| Immune cells | PD-L1 | BMS-936559 | Brahmer et al[102], 2012 |
| CTLA-4 | Ipilimumab | Le et al[95], 2013 | |
| CD8+ T cells | GVAX | Lutz et al[93], 2011 | |
| Laheru et al[94], 2008 | |||
| CD40 | CP-870,893 | Beatty et al[103], 2013 | |
| Signaling pathways mediating tumor-stroma interactions | Smo/SHh | Vismodegib (GDC-0449) | Stephenson et al[90], 2011 |
| IPI-926 | |||
| Type II TGFβ receptor | Trabedersen | Oettle et al[92], 2009 | |
| γ-secretase (Notch pathway) | PF-03084014 (preclinical) | Yabuuchi et al[78], 2013 | |
| (preclinical) | |||
| HGF/c-met | Many different compounds (solid cancers) | Venepalli et al[104], 2013 | |
| (solid cancers) | |||
| Different molecules in NF-κB cascade | Many different compounds (i.e., curcumin, proteasome inhibitor) | Arlt et al[105], 2012 |
SHh pathway inhibition shows beneficial effect in patients with other cancers such as basal cell carcinoma for which Vismodegib (GDC-0449) has been FDA approved in 2012[88]. Inhibition of SHh in preclinical mouse models showed better gemcitabine delivery, stromal depletion and increased vascularization of PDA tumors[7]. Thus, different SHh inhibitors have recently been tested in clinical trials in combination with gemcitabine or FOLFIRINOX for metastatic PDAs[89]. Additionally, GDC-0449 is now being tested in combination with nab-paclitaxel (human-albumin-bound paclitaxel, Abraxane) and gemcitabine in phase II clinical trial in patients with previously untreated metastatic PDA (clinical trial # NCT01088815) to evaluate disease free survival and toxicity. Although IPI-926 (Smo inhibitor) given in combination with gemcitabine showed partial responses in 3 out of 9 patients, the combination of IPI-926 and gemcitabine did not yield any survival benefit comparing to gemcitabine alone[90]. Therefore, targeting the stroma of PDA through SHh inhibition and simultaneous modulation of other stromal signaling should be explored.
Another stromal target showing encouraging results in phase Ib clinical trials is hyaluronan. As shown in mouse models of PDA, enzymatic degradation of hyaluronan resulted in increased gemcitabine tumor cytotoxicity due to relief of vascular collapse[30]. Those prove of principle experiments lead to the development of PEGPH20 (pegylated recombinant human hyaluronidase- an enzyme that degrades hyaluronan). Administration of PEGPH20 to PDA patients with advanced disease (stage IV) in combination with gemcitabine revealed partial response in 43% of patients and stable disease in additional 30% patients in phase Ib clinical trials. More impressively, the partial response rate was 64% in those patients whose PDAs expressed high level of hyaluronan[91]. This high response rate has led to further testing of PEGPH20 in combination with gemcitabine and nab-paclitaxel in a randomized phase II clinical trial.
Trabedersen, a type II TGFβ antisense inhibitor is also being tested in clinical trials in patients with advanced pancreatic adenocarcinoma and malignant melanoma. Although phase II clinical trial results have not been released yet, phase I reports revealed that trabedersen was well tolerated and showed median overall survival to be 13.2 mo. In addition, one patient presented with a stable disease after 14.8 mo of last treatment[92].
Immunotherapy approaches are being tested in clinical trials for PDA with a goal to induce tumor infiltration and activation of effector cells (i.e., CD8+ T cells) and consequent CD8+ T cell dependent tumor lysis.
Multiple clinical trials of a lethally irradiated allogeneic GM-CSF secreting whole cell vaccine (GVAX) administered to patients with resected PDA or metastatic PDA demonstrated that enhanced response of interferon-γ secreting mesothelin-specific CD8+ T cells in peripheral lymphocytes correlates with better survival[93,94]. A pilot study testing the combination of GVAX and ipilimumab (an anti-CTLA-4 therapeutic antibody) comparing to ipilimumab alone showed a trend of increase in overall survival in metastatic PDA patients that have been previously treated with multiple lines of chemotherapy and thus supported the role of CTLA-4 blockade in enhancing anti-tumor response of GVAX[95]. However, it remains to be explored how vaccine-based immunotherapy activates anti-tumor effector cells within tumor microenvironment. Identification of new targets in tumor microenvironment may enhance the development of immune modulatory therapies.
A potential immune modulatory target in tumor microenvironment is CD40. CD40 is a costimulatory molecule found on antigen presenting cells (APCs) that is required for their activation by CD4+ helper cells. Only activated APCs can in turn activate naïve CD8+ T cells into cytotoxic effector cells. Key studies showed that using CD40 activating antibody can effectively stimulate APCs in the absence of CD4+ helper cells, which then can successfully prime and activate CD8+ T cells[96]. Those preclinical studies led to development of activating CD40 antibodies, which have been tested in clinical trials. One study showed that combination of CD40 agonist with gemcitabine resulted in tumor regression in patients not eligible for tumor resection. Interestingly, it was noted that the tumoricidal cells were CD40 activated macrophages and not CD8+ T cells as originally expected. The treatment with CD40 agonist resulted in stroma depletion and increased numbers of tumor infiltrating activated macrophages[8].
How stromal fibroblast cells can modulate anti-tumor immune response has been investigated in preclinical studies. One study demonstrated that depletion of fibroblast activation protein-α (FAP)-expressing stromal cells in PDA resulted in an immune-mediated hypoxic necrosis of both tumor and stroma cells[97]. Additionally, targeting of cancer stroma fibroblasts with FAP-activated promelittin protoxin, showed increased tumor lysis and growth inhibition in xenograft mouse models of breast and prostate cancer[98]. However, targeting FAP positive stromal cells with humanized anti-FAP antibodies tested in phase II clinical trials in patients with metastatic colorectal cancer did not report encouraging results[99]. Taking into consideration the outcomes from both preclinical and clinical studies, it is reasonable to propose that FAP-targeted stromal depletion shows immune activating effect, but requires additional immune modulation to be effective. It is plausible that simultaneous FAP-targeted stromal depletion and immune activation, by either vaccination or immune checkpoint blockade would result in increased benefits for PDA patients.
Despite the broad number of clinical trials, there is still a lack of groundbreaking therapies for patients affected by pancreatic cancer. Thus, targeting only the neoplastic cells has not resulted in a substantially improved PDA treatment. It is now well established that the desmoplastic reaction present in PDA is not just a bystander but it is a source of different cellular and acellular factors that promote tumor progression, immunosuppression and metastasis. Targeted therapies to deplete stromal compartments have shown improved chemotherapy delivery and reduction of immunosuppression in preclinical models. There is still much work to be done in order to decipher the complicated interactions between stroma and neoplastic cells in PDA. It is clear, however, that future studies should not be limited to one component of PDA. Application of targeted therapy to deplete the tumorigenic stromal compartment along with inhibition of cancer promoting signaling pathways should be evaluated. Moreover, future studies ought to test the combination of agents that target the stroma and those that activate anti-tumor immune responses. Treatments that can reduce desmoplastic reaction, overcome immune suppression and inhibit tumorigenic signaling pathways may lead to more successful patient care.
P- Reviewers: De Ridder M, Yamaue H S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8406] [Cited by in RCA: 8970] [Article Influence: 690.0] [Reference Citation Analysis (0)] |
| 2. | Farrow B, Albo D, Berger DH. The role of the tumor microenvironment in the progression of pancreatic cancer. J Surg Res. 2008;149:319-328. [PubMed] |
| 3. | Lewin CS, Allen RA, Amyes SG. Mechanisms of zidovudine resistance in bacteria. J Med Microbiol. 1990;33:235-238. [PubMed] |
| 4. | Waghray M, Yalamanchili M, di Magliano MP, Simeone DM. Deciphering the role of stroma in pancreatic cancer. Curr Opin Gastroenterol. 2013;29:537-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 5. | Feig C, Gopinathan A, Neesse A, Chan DS, Cook N, Tuveson DA. The pancreas cancer microenvironment. Clin Cancer Res. 2012;18:4266-4276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 841] [Cited by in RCA: 1027] [Article Influence: 85.6] [Reference Citation Analysis (0)] |
| 6. | Erkan M, Michalski CW, Rieder S, Reiser-Erkan C, Abiatari I, Kolb A, Giese NA, Esposito I, Friess H, Kleeff J. The activated stroma index is a novel and independent prognostic marker in pancreatic ductal adenocarcinoma. Clin Gastroenterol Hepatol. 2008;6:1155-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 286] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 7. | Olive KP, Jacobetz MA, Davidson CJ, Gopinathan A, McIntyre D, Honess D, Madhu B, Goldgraben MA, Caldwell ME, Allard D. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457-1461. [PubMed] |
| 8. | Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, Huhn RD, Song W, Li D, Sharp LL. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1152] [Cited by in RCA: 1301] [Article Influence: 92.9] [Reference Citation Analysis (0)] |
| 9. | Heinemann V, Reni M, Ychou M, Richel DJ, Macarulla T, Ducreux M. Tumour-stroma interactions in pancreatic ductal adenocarcinoma: rationale and current evidence for new therapeutic strategies. Cancer Treat Rev. 2014;40:118-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403-2413. [PubMed] |
| 11. | Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4838] [Cited by in RCA: 5634] [Article Influence: 402.4] [Reference Citation Analysis (1)] |
| 12. | Bachem MG, Schneider E, Gross H, Weidenbach H, Schmid RM, Menke A, Siech M, Beger H, Grünert A, Adler G. Identification, culture, and characterization of pancreatic stellate cells in rats and humans. Gastroenterology. 1998;115:421-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 744] [Cited by in RCA: 798] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 13. | Bachem MG, Zhou S, Buck K, Schneiderhan W, Siech M. Pancreatic stellate cells--role in pancreas cancer. Langenbecks Arch Surg. 2008;393:891-900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 14. | Franco OE, Shaw AK, Strand DW, Hayward SW. Cancer associated fibroblasts in cancer pathogenesis. Semin Cell Dev Biol. 2010;21:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 15. | Spector I, Zilberstein Y, Lavy A, Nagler A, Genin O, Pines M. Involvement of host stroma cells and tissue fibrosis in pancreatic tumor development in transgenic mice. PLoS One. 2012;7:e41833. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Apte MV, Wilson JS, Lugea A, Pandol SJ. A starring role for stellate cells in the pancreatic cancer microenvironment. Gastroenterology. 2013;144:1210-1219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 366] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 17. | Mace TA, Ameen Z, Collins A, Wojcik S, Mair M, Young GS, Fuchs JR, Eubank TD, Frankel WL, Bekaii-Saab T. Pancreatic cancer-associated stellate cells promote differentiation of myeloid-derived suppressor cells in a STAT3-dependent manner. Cancer Res. 2013;73:3007-3018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 371] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 18. | Hamada S, Masamune A, Takikawa T, Suzuki N, Kikuta K, Hirota M, Hamada H, Kobune M, Satoh K, Shimosegawa T. Pancreatic stellate cells enhance stem cell-like phenotypes in pancreatic cancer cells. Biochem Biophys Res Commun. 2012;421:349-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 162] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 19. | Mace TA, Bloomston M, Lesinski GB. Pancreatic cancer-associated stellate cells: A viable target for reducing immunosuppression in the tumor microenvironment. Oncoimmunology. 2013;2:e24891. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Ene-Obong A, Clear AJ, Watt J, Wang J, Fatah R, Riches JC, Marshall JF, Chin-Aleong J, Chelala C, Gribben JG. Activated pancreatic stellate cells sequester CD8+ T cells to reduce their infiltration of the juxtatumoral compartment of pancreatic ductal adenocarcinoma. Gastroenterology. 2013;145:1121-1132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 446] [Article Influence: 37.2] [Reference Citation Analysis (0)] |
| 21. | Kadaba R, Birke H, Wang J, Hooper S, Andl CD, Di Maggio F, Soylu E, Ghallab M, Bor D, Froeling FE. Imbalance of desmoplastic stromal cell numbers drives aggressive cancer processes. J Pathol. 2013;230:107-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 22. | Apte MV, Park S, Phillips PA, Santucci N, Goldstein D, Kumar RK, Ramm GA, Buchler M, Friess H, McCarroll JA. Desmoplastic reaction in pancreatic cancer: role of pancreatic stellate cells. Pancreas. 2004;29:179-187. [PubMed] |
| 23. | Sugimoto H, Mundel TM, Kieran MW, Kalluri R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol Ther. 2006;5:1640-1646. [PubMed] |
| 24. | Lee HO, Mullins SR, Franco-Barraza J, Valianou M, Cukierman E, Cheng JD. FAP-overexpressing fibroblasts produce an extracellular matrix that enhances invasive velocity and directionality of pancreatic cancer cells. BMC Cancer. 2011;11:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 241] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 25. | Frantz C, Stewart KM, Weaver VM. The extracellular matrix at a glance. J Cell Sci. 2010;123:4195-4200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2308] [Cited by in RCA: 2787] [Article Influence: 199.1] [Reference Citation Analysis (0)] |
| 26. | Apte MV, Yang L, Phillips PA, Xu Z, Kaplan W, Cowley M, Pirola RC, Wilson JS. Extracellular matrix composition significantly influences pancreatic stellate cell gene expression pattern: role of transgelin in PSC function. Am J Physiol Gastrointest Liver Physiol. 2013;305:G408-G417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Dangi-Garimella S, Sahai V, Ebine K, Kumar K, Munshi HG. Three-dimensional collagen I promotes gemcitabine resistance in vitro in pancreatic cancer cells through HMGA2-dependent histone acetyltransferase expression. PLoS One. 2013;8:e64566. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 28. | Miyamoto H, Murakami T, Tsuchida K, Sugino H, Miyake H, Tashiro S. Tumor-stroma interaction of human pancreatic cancer: acquired resistance to anticancer drugs and proliferation regulation is dependent on extracellular matrix proteins. Pancreas. 2004;28:38-44. [PubMed] |
| 29. | Michl P, Gress TM. Improving drug delivery to pancreatic cancer: breaching the stromal fortress by targeting hyaluronic acid. Gut. 2012;61:1377-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 30. | Provenzano PP, Cuevas C, Chang AE, Goel VK, Von Hoff DD, Hingorani SR. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1408] [Cited by in RCA: 1639] [Article Influence: 126.1] [Reference Citation Analysis (0)] |
| 31. | Assifi MM, Hines OJ. Anti-angiogenic agents in pancreatic cancer: a review. Anticancer Agents Med Chem. 2011;11:464-469. [PubMed] |
| 32. | Clark CE, Hingorani SR, Mick R, Combs C, Tuveson DA, Vonderheide RH. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 2007;67:9518-9527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 653] [Cited by in RCA: 763] [Article Influence: 42.4] [Reference Citation Analysis (0)] |
| 33. | Protti MP, De Monte L. Immune infiltrates as predictive markers of survival in pancreatic cancer patients. Front Physiol. 2013;4:210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 34. | Mielgo A, Schmid MC. Impact of tumour associated macrophages in pancreatic cancer. BMB Rep. 2013;46:131-138. [PubMed] |
| 35. | Vonderheide RH, Bayne LJ. Inflammatory networks and immune surveillance of pancreatic carcinoma. Curr Opin Immunol. 2013;25:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 36. | Zheng L, Xue J, Jaffee EM, Habtezion A. Role of immune cells and immune-based therapies in pancreatitis and pancreatic ductal adenocarcinoma. Gastroenterology. 2013;144:1230-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 253] [Article Influence: 21.1] [Reference Citation Analysis (1)] |
| 37. | Bronte V, Serafini P, De Santo C, Marigo I, Tosello V, Mazzoni A, Segal DM, Staib C, Lowel M, Sutter G. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J Immunol. 2003;170:270-278. [PubMed] |
| 38. | Kusmartsev S, Nefedova Y, Yoder D, Gabrilovich DI. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J Immunol. 2004;172:989-999. [PubMed] |
| 39. | Beyer M, Schultze JL. Regulatory T cells in cancer. Blood. 2006;108:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 516] [Cited by in RCA: 548] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 40. | Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 812] [Cited by in RCA: 919] [Article Influence: 54.1] [Reference Citation Analysis (0)] |
| 41. | Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543-9553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1248] [Cited by in RCA: 1490] [Article Influence: 74.5] [Reference Citation Analysis (0)] |
| 42. | Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13:2151-2157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 645] [Cited by in RCA: 701] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 43. | Loos M, Giese NA, Kleeff J, Giese T, Gaida MM, Bergmann F, Laschinger M, W Büchler M, Friess H. Clinical significance and regulation of the costimulatory molecule B7-H1 in pancreatic cancer. Cancer Lett. 2008;268:98-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 44. | Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9:641-649. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1075] [Cited by in RCA: 1061] [Article Influence: 62.4] [Reference Citation Analysis (0)] |
| 45. | Regateiro FS, Howie D, Nolan KF, Agorogiannis EI, Greaves DR, Cobbold SP, Waldmann H. Generation of anti-inflammatory adenosine by leukocytes is regulated by TGF-β. Eur J Immunol. 2011;41:2955-2965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 146] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 46. | He S, Fei M, Wu Y, Zheng D, Wan D, Wang L, Li D. Distribution and clinical significance of th17 cells in the tumor microenvironment and peripheral blood of pancreatic cancer patients. Int J Mol Sci. 2011;12:7424-7437. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 47. | Qi W, Huang X, Wang J. Correlation between Th17 cells and tumor microenvironment. Cell Immunol. 2013;285:18-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 48. | Greten TF, Zhao F, Gamrekelashvili J, Korangy F. Human Th17 cells in patients with cancer: Friends or foe. Oncoimmunology. 2012;1:1438-1439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Ye J, Livergood RS, Peng G. The role and regulation of human Th17 cells in tumor immunity. Am J Pathol. 2013;182:10-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 133] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 50. | Tan Z, Qian X, Jiang R, Liu Q, Wang Y, Chen C, Wang X, Ryffel B, Sun B. IL-17A plays a critical role in the pathogenesis of liver fibrosis through hepatic stellate cell activation. J Immunol. 2013;191:1835-1844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 256] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 51. | Al-Muhsen S, Letuve S, Vazquez-Tello A, Pureza MA, Al-Jahdali H, Bahammam AS, Hamid Q, Halwani R. Th17 cytokines induce pro-fibrotic cytokines release from human eosinophils. Respir Res. 2013;14:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 52. | Gao B, Waisman A. Th17 cells regulate liver fibrosis by targeting multiple cell types: many birds with one stone. Gastroenterology. 2012;143:536-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 53. | Murone M, Rosenthal A, de Sauvage FJ. Sonic hedgehog signaling by the patched-smoothened receptor complex. Curr Biol. 1999;9:76-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 246] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 54. | Tian H, Callahan CA, DuPree KJ, Darbonne WC, Ahn CP, Scales SJ, de Sauvage FJ. Hedgehog signaling is restricted to the stromal compartment during pancreatic carcinogenesis. Proc Natl Acad Sci USA. 2009;106:4254-4259. [PubMed] |
| 55. | Li X, Ma Q, Duan W, Liu H, Xu H, Wu E. Paracrine sonic hedgehog signaling derived from tumor epithelial cells: a key regulator in the pancreatic tumor microenvironment. Crit Rev Eukaryot Gene Expr. 2012;22:97-108. [PubMed] |
| 56. | Bailey JM, Swanson BJ, Hamada T, Eggers JP, Singh PK, Caffery T, Ouellette MM, Hollingsworth MA. Sonic hedgehog promotes desmoplasia in pancreatic cancer. Clin Cancer Res. 2008;14:5995-6004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 425] [Cited by in RCA: 410] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 57. | Walter K, Omura N, Hong SM, Griffith M, Vincent A, Borges M, Goggins M. Overexpression of smoothened activates the sonic hedgehog signaling pathway in pancreatic cancer-associated fibroblasts. Clin Cancer Res. 2010;16:1781-1789. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 58. | Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030-1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2377] [Cited by in RCA: 2428] [Article Influence: 134.9] [Reference Citation Analysis (0)] |
| 59. | Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2875] [Cited by in RCA: 2919] [Article Influence: 104.3] [Reference Citation Analysis (0)] |
| 60. | Friess H, Yamanaka Y, Büchler M, Ebert M, Beger HG, Gold LI, Korc M. Enhanced expression of transforming growth factor beta isoforms in pancreatic cancer correlates with decreased survival. Gastroenterology. 1993;105:1846-1856. [PubMed] |
| 61. | Jaschinski F, Rothhammer T, Jachimczak P, Seitz C, Schneider A, Schlingensiepen KH. The antisense oligonucleotide trabedersen (AP 12009) for the targeted inhibition of TGF-β2. Curr Pharm Biotechnol. 2011;12:2203-2213. [PubMed] |
| 62. | Schnurr M, Duewell P. Breaking tumor-induced immunosuppression with 5’-triphosphate siRNA silencing TGFβ and activating RIG-I. Oncoimmunology. 2013;2:e24170. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 63. | Tahara H, Sato K, Yamazaki Y, Ohyama T, Horiguchi N, Hashizume H, Kakizaki S, Takagi H, Ozaki I, Arai H. Transforming growth factor-α activates pancreatic stellate cells and may be involved in matrix metalloproteinase-1 upregulation. Lab Invest. 2013;93:720-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 64. | Schlingensiepen KH, Jaschinski F, Lang SA, Moser C, Geissler EK, Schlitt HJ, Kielmanowicz M, Schneider A. Transforming growth factor-beta 2 gene silencing with trabedersen (AP 12009) in pancreatic cancer. Cancer Sci. 2011;102:1193-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 98] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 65. | He J, Sun X, Qian KQ, Liu X, Wang Z, Chen Y. Protection of cerulein-induced pancreatic fibrosis by pancreas-specific expression of Smad7. Biochim Biophys Acta. 2009;1792:56-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 66. | Creighton CJ, Gibbons DL, Kurie JM. The role of epithelial-mesenchymal transition programming in invasion and metastasis: a clinical perspective. Cancer Manag Res. 2013;5:187-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 82] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 67. | Lim J, Thiery JP. Epithelial-mesenchymal transitions: insights from development. Development. 2012;139:3471-3486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 466] [Cited by in RCA: 485] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 68. | Kong D, Li Y, Wang Z, Sarkar FH. Cancer Stem Cells and Epithelial-to-Mesenchymal Transition (EMT)-Phenotypic Cells: Are They Cousins or Twins. Cancers (Basel). 2011;3:716-729. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 262] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 69. | de Graauw M, Tijdens I, Smeets MB, Hensbergen PJ, Deelder AM, van de Water B. Annexin A2 phosphorylation mediates cell scattering and branching morphogenesis via cofilin Activation. Mol Cell Biol. 2008;28:1029-1040. [PubMed] |
| 70. | Lee MY, Chou CY, Tang MJ, Shen MR. Epithelial-mesenchymal transition in cervical cancer: correlation with tumor progression, epidermal growth factor receptor overexpression, and snail up-regulation. Clin Cancer Res. 2008;14:4743-4750. [PubMed] |
| 71. | Yang J, Weinberg RA. Epithelial-mesenchymal transition: at the crossroads of development and tumor metastasis. Dev Cell. 2008;14:818-829. [PubMed] |
| 72. | Ouyang G, Wang Z, Fang X, Liu J, Yang CJ. Molecular signaling of the epithelial to mesenchymal transition in generating and maintaining cancer stem cells. Cell Mol Life Sci. 2010;67:2605-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 73. | Hiraga R, Kato M, Miyagawa S, Kamata T. Nox4-derived ROS signaling contributes to TGF-β-induced epithelial-mesenchymal transition in pancreatic cancer cells. Anticancer Res. 2013;33:4431-4438. [PubMed] |
| 74. | Zheng L, Foley K, Huang L, Leubner A, Mo G, Olino K, Edil BH, Mizuma M, Sharma R, Le DT. Tyrosine 23 phosphorylation-dependent cell-surface localization of annexin A2 is required for invasion and metastases of pancreatic cancer. PLoS One. 2011;6:e19390. [PubMed] |
| 75. | Castellanos JA, Merchant NB, Nagathihalli NS. Emerging targets in pancreatic cancer: epithelial-mesenchymal transition and cancer stem cells. Onco Targets Ther. 2013;6:1261-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 76. | Yamada S, Fuchs BC, Fujii T, Shimoyama Y, Sugimoto H, Nomoto S, Takeda S, Tanabe KK, Kodera Y, Nakao A. Epithelial-to-mesenchymal transition predicts prognosis of pancreatic cancer. Surgery. 2013;154:946-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 77. | Chen HT, Cai QC, Zheng JM, Man XH, Jiang H, Song B, Jin G, Zhu W, Li ZS. High expression of delta-like ligand 4 predicts poor prognosis after curative resection for pancreatic cancer. Ann Surg Oncol. 2012;19 Suppl 3:S464-S474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 78. | Yabuuchi S, Pai SG, Campbell NR, de Wilde RF, De Oliveira E, Korangath P, Streppel MM, Rasheed ZA, Hidalgo M, Maitra A. Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett. 2013;335:41-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 79. | Yu J, Ohuchida K, Mizumoto K, Ishikawa N, Ogura Y, Yamada D, Egami T, Fujita H, Ohashi S, Nagai E. Overexpression of c-met in the early stage of pancreatic carcinogenesis; altered expression is not sufficient for progression from chronic pancreatitis to pancreatic cancer. World J Gastroenterol. 2006;12:3878-3882. [PubMed] |
| 80. | Hage C, Rausch V, Giese N, Giese T, Schönsiegel F, Labsch S, Nwaeburu C, Mattern J, Gladkich J, Herr I. The novel c-Met inhibitor cabozantinib overcomes gemcitabine resistance and stem cell signaling in pancreatic cancer. Cell Death Dis. 2013;4:e627. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 100] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 81. | Ide T, Kitajima Y, Miyoshi A, Ohtsuka T, Mitsuno M, Ohtaka K, Miyazaki K. The hypoxic environment in tumor-stromal cells accelerates pancreatic cancer progression via the activation of paracrine hepatocyte growth factor/c-Met signaling. Ann Surg Oncol. 2007;14:2600-2607. [PubMed] |
| 82. | Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12:86. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2313] [Cited by in RCA: 2531] [Article Influence: 210.9] [Reference Citation Analysis (0)] |
| 83. | Chandler NM, Canete JJ, Callery MP. Increased expression of NF-kappa B subunits in human pancreatic cancer cells. J Surg Res. 2004;118:9-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 84. | Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119-127. [PubMed] |
| 85. | Liou GY, Döppler H, Necela B, Krishna M, Crawford HC, Raimondo M, Storz P. Macrophage-secreted cytokines drive pancreatic acinar-to-ductal metaplasia through NF-κB and MMPs. J Cell Biol. 2013;202:563-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 177] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 86. | Carbone C, Melisi D. NF-κB as a target for pancreatic cancer therapy. Expert Opin Ther Targets. 2012;16 Suppl 2:S1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 87. | Wong HH, Lemoine NR. Pancreatic cancer: molecular pathogenesis and new therapeutic targets. Nat Rev Gastroenterol Hepatol. 2009;6:412-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 166] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 88. | Sobanko JF, Okman J, Miller C. Vismodegib: a hedgehog pathway inhibitor for locally advanced and metastatic basal cell carcinomas. J Drugs Dermatol. 2013;12:s154-s155. [PubMed] |
| 89. | Liss AS, Thayer SP. Therapeutic targeting of pancreatic stroma. Pancreatic Cancer and Tumor Microenvironment. Trivandrum (India): Transworld Research Network Transworld Research Network 2012; . |
| 90. | Stephenson J, Richards DA, Wolpin BM, Becerra C, Hamm JT, Messersmith WA, Devens S, Cushing J, Goddard J, Schmalbach T. The safety of IPI-926, a novel hedgehog pathway inhibitor, in combination with gemcitabine in patients (pts) with metastatic pancreatic cancer. J Clin Oncol. 2011;29 suppl:Abstr 4114. |
| 91. | Strimpakos AS, Saif MW. Update on phase I studies in advanced pancreatic adenocarcinoma. Hunting in darkness. JOP. 2013;14:354-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 92. | Oettle H, Hilbig A, Seufferlein T, Schmid RM, Luger T, von Wichert G, Schmaus S, Heinrichs H, Schlingensiepen K. Interim results of the phase I/II study of trabedersen (AP 12009) in patients with pancreatic carcinoma, malignant melanoma, or colorectal carcinoma. J Clin Oncol. 2009;27 suppl:Abstr 4619. |
| 93. | Lutz E, Yeo CJ, Lillemoe KD, Biedrzycki B, Kobrin B, Herman J, Sugar E, Piantadosi S, Cameron JL, Solt S. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann Surg. 2011;253:328-335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 318] [Cited by in RCA: 297] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 94. | Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14:1455-1463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 285] [Cited by in RCA: 268] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 95. | Le DT, Lutz E, Uram JN, Sugar EA, Onners B, Solt S, Zheng L, Diaz LA, Donehower RC, Jaffee EM. Evaluation of ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J Immunother. 2013;36:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 418] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 96. | Diehl L, den Boer AT, Schoenberger SP, van der Voort EI, Schumacher TN, Melief CJ, Offringa R, Toes RE. CD40 activation in vivo overcomes peptide-induced peripheral cytotoxic T-lymphocyte tolerance and augments anti-tumor vaccine efficacy. Nat Med. 1999;5:774-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 365] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 97. | Kraman M, Bambrough PJ, Arnold JN, Roberts EW, Magiera L, Jones JO, Gopinathan A, Tuveson DA, Fearon DT. Suppression of antitumor immunity by stromal cells expressing fibroblast activation protein-alpha. Science. 2010;330:827-830. [PubMed] |
| 98. | LeBeau AM, Brennen WN, Aggarwal S, Denmeade SR. Targeting the cancer stroma with a fibroblast activation protein-activated promelittin protoxin. Mol Cancer Ther. 2009;8:1378-1386. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 133] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 99. | Hofheinz RD, al-Batran SE, Hartmann F, Hartung G, Jäger D, Renner C, Tanswell P, Kunz U, Amelsberg A, Kuthan H. Stromal antigen targeting by a humanised monoclonal antibody: an early phase II trial of sibrotuzumab in patients with metastatic colorectal cancer. Onkologie. 2003;26:44-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 238] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 100. | Moore MJ, Hamm J, Dancey J, Eisenberg PD, Dagenais M, Fields A, Hagan K, Greenberg B, Colwell B, Zee B. Comparison of gemcitabine versus the matrix metalloproteinase inhibitor BAY 12-9566 in patients with advanced or metastatic adenocarcinoma of the pancreas: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 2003;21:3296-3302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 277] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 101. | Bramhall SR, Schulz J, Nemunaitis J, Brown PD, Baillet M, Buckels JA. A double-blind placebo-controlled, randomised study comparing gemcitabine and marimastat with gemcitabine and placebo as first line therapy in patients with advanced pancreatic cancer. Br J Cancer. 2002;87:161-167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 402] [Cited by in RCA: 397] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 102. | Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455-2465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5599] [Cited by in RCA: 6283] [Article Influence: 483.3] [Reference Citation Analysis (0)] |
| 103. | Beatty GL, Torigian DA, Chiorean EG, Saboury B, Brothers A, Alavi A, Troxel AB, Sun W, Teitelbaum UR, Vonderheide RH. A phase I study of an agonist CD40 monoclonal antibody (CP-870,893) in combination with gemcitabine in patients with advanced pancreatic ductal adenocarcinoma. Clin Cancer Res. 2013;19:6286-6295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 359] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 104. | Venepalli NK, Goff L. Targeting the HGF-cMET Axis in Hepatocellular Carcinoma. Int J Hepatol. 2013;2013:341636. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 105. | Arlt A, Schäfer H, Kalthoff H. The ‘N-factors’ in pancreatic cancer: functional relevance of NF-κB, NFAT and Nrf2 in pancreatic cancer. Oncogenesis. 2012;1:e35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 37] [Article Influence: 2.8] [Reference Citation Analysis (0)] |