INTRODUCTION
Novel therapies for human pancreatic cancer are needed to treat the more than 45000 people in the United States who will be diagnosed with this cancer in 2013[1]. Death due to this cancer approaches the same number, with estimates of more than 38000 persons dying of pancreatic cancer in 2013. Males have increased incidence and death rates relative to females; black ethnic groups have the highest incidence[1,2]. Five-year survival rates range between 5% and 6%, and have not changed in more than a decade of research[2]. Therapeutics based on underlying mechanisms of disease are needed.
The standard of care for pancreatic cancer is gemcitabine. This anti-metabolite is a nucleoside analog that blocks DNA replication, or inhibits ribonucleotide reductase, an enzyme needed to produce the deoxyribonucleotides necessary for DNA replication; both pathways induce apoptosis and slow tumor growth[3]. However, gemcitabine cannot be used if the patient has allergies (e.g., dye, additives, food), other diseases (e.g., kidney, liver, hepatitis, heart, lung, diabetes, gout), or infections. Radiation therapy cannot be combined with gemcitabine, and women of child-bearing age are encouraged not to take gemcitabine as it may cause birth defects[3].
New endogenous peptide pathways have been identified that provide novel targets for non-toxic therapeutic alternatives for pancreatic cancer. Our knowledge about the biology of pancreatic cancer supports the need for treatments that target the biology of this cancer.
ENDOGENOUS OPIOIDS
An endogenous opioid peptide and its receptor were first identified more than 3 decades ago as being an important inhibitor of human cancer cell proliferation[4,5]. Ongoing studies on the opioid growth factor (OGF)-OGF receptor (OGFr) axis have characterized this pathway and defined mechanistic approaches for the treatment of neoplasia[6]. The peptide is chemically termed [Met5]-enkephalin, and is a 5-amino acid neuropeptide secreted by the brain and originally identified as an endogenous opioid by scientists in the mid-1970s[7-9]. This peptide was renamed OGF after discoveries of its growth modulating characteristics in mouse neuroblastoma cells and developing rat brain[4,5,10], and to distinguish its pharmacological function from neurotransmission. OGF is derived from both preproenkephalin and pro-opiomelanocortin genes[11], and is rapidly translated and degraded in human blood. Studies have shown that OGF is autocrine and paracrine produced in tissues originating from dermal derivatives, with brain and gut tissues having the greatest levels of the peptide[12,13].
The inhibitory effects of OGF on cell replication were first recorded in developing rat brain[14,15] and in tissue culture studies on mouse and human neuroblastoma[16-19]. OGF inhibits DNA synthesis and cell replication of normal cells and tissues[20,21], human neoplasia[22], and bacteria[23]. The main action of OGF is to upregulate inhibitory kinases in the cell cycle process. OGF’s activity is receptor-mediated, dose-related, time-dependent, and reversible. The peptide is present in developing and renewing tissues, and has been localized in embryonic tissues and many human cancers[24-27].
OPIOID RECEPTORS
Classical opioid receptors were discovered in 1973 by three independent laboratories[28-30] and were identified in brain and gastrointestinal tissues. Based on their binding activities to radioisotopes, three classes of receptors - mu (MOR), delta (DOR), and kappa (KOR) were characterized in membrane homogenates as 7-member transmembrane cytoplasmic receptors. The gene and protein structures for the classical receptors are homologous, and many of the opioid ligands cross-react with more than one receptor. OGF binds to DOR and MOR receptors. However, a series of biochemical and pharmacological studies demonstrated that OGF also binds to a new opioid receptor, OGFr, that is located on the nuclear membrane[31-33]. Sequencing of OGFr reveals little or no homology with classical opioid receptors. However, OGFr does display pharmacological characteristics of opioid receptors such as stereospecificity of ligands and opioid antagonist blockade[34]. Subcellular fractionation studies of OGFr in developing rat brain and neuroblastoma cells reveal that OGFr is associated with the nuclear membrane, and immunoelectron microscopy studies have shown that OGF co-localizes with OGFr on the outer nuclear membrane and within the nucleus[35].
OGF-OGFR AXIS: PRECLINICAL IN VITRO STUDIES
OGF and OGFr are present in human pancreatic cells (PANC-1, MIA PaCa-2, BxPC-3 and Capan-1) grown in culture, xenografted to nude mice, or surgical specimens obtained during tumor resection[36-39]. In vitro studies using PANC-1 cells reveal that the receptor has specific and saturable binding affinity to radiolabeled [Met5]-enkephalin, with enriched binding in the nuclear fraction of cells[38]. Competition experiments using ligands for classical opioid receptors [e.g., [D-Ala2, NMe-Phe4, Gly-ol5]-enkephalin] (DAMGO), [D-Pen2,5]-enkephalin (DPDPE), dynorphin A1-8, morphine] do not displace the affinity of [Met5]-enkephalin for OGFr[38] supporting the selectivity of peptide and receptor.
The efficacy of OGF has been characterized in a series of tissue culture studies[37]. OGF inhibits DNA synthesis and the growth of PANC-1 cells in a dose-dependent (42% reductions) and temporal manner (up to 48% reductions at 120 h) relative to control cultures, with its action being receptor-mediated and reversible. Absorption of endogenous ligand using OGF antibodies negated growth inhibition associated with exogenous peptide administration. OGF has a ubiquitous effect on pancreatic cancer cells derived from tumors at different stages of differentiation. Administration of OGF for 72 h inhibits growth (up to 37%) of Capan-1 and MIA PaCa-2 cells, well-differentiated cancer cell lines[40,41], BxPC-3, a moderately well to poorly differentiated cell line[42], as well as PANC-1, an undifferentiated pancreatic cancer cell line[43].
Combinatorial therapeutic regimens are often more effective than single agent therapy. Gemcitabine is the standard of care for advanced pancreatic neoplasia, and also acts through inhibition of DNA synthesis[3]. Utilizing MIA PaCa-2 cells grown in log phase conditions, the combination of OGF (10-6 mol/L) and gemcitabine (10-8 mol/L) reduces cell number from control levels by more than 45% over 48 h, whereas each compound alone inhibits growth by less than 22% in the same time period. The action of OGF, but not gemcitabine, is mediated by a naloxone-sensitive receptor and is reversible. Combining OGF with 5-fluorouracil (5-FU; 10-6 mol/L) also increases inhibitory action. Over a 96 h period, OGF and 5-FU reduce cell number up to 30%, whereas each compound alone reduces cell number by up to 18%[39].
The specificity of OGF and OGFr has been documented in a variety of experiments using different human pancreatic cancer cell lines. The specificity of OGF has been confirmed by addition of multiple ligands that are specific for classical opioid receptors yet have no effect on cell proliferation and/or growth of cells or tumors[37-39]. Absorption of OGF by antibodies to the endogenous peptide depresses growth, demonstrating the specificity of this peptide. Competition binding assays using classical ligands such as DAMGO, DPDPE, morphine, ethylketocyclazocine and others results in no loss of binding of OGF to OGFr, suggesting little or no affinity of other ligands for OGFr[38].
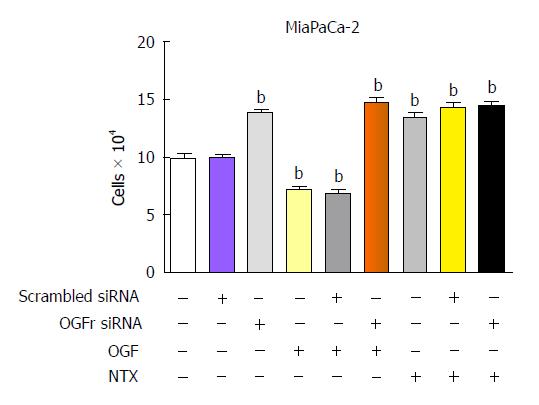
OGFr selectivity and specificity for the ligand OGF have been shown in several experiments. In tissue culture, siRNA knockdown of RNA and protein expression of OGFr results in cultures that grow faster than controls because there is no receptor available for interaction with endogenous inhibitory OGF. Addition of exogenous OGF to cultures lacking OGFr has no inhibitory action (Figure 1). Finally, transient transfection experiments that knockdown classical opioid receptors using siRNAs to MOR, DOR, or KOR results in no alteration in cell proliferation in homeostatic conditions or following the addition of OGF[44].
Figure 1 Opioid growth factor receptor is required for the inhibitory action of opioid growth factor and the stimulatory action of NTX on growth of MIA PaCa-2 human pancreatic cell cultures.
Cells were transfected with opioid growth factor receptor (OGFr) siRNA or scrambled siRNA for 24 h and then treated with 10-6 mol/L. Opioid growth factor (OGF) or NTX, or 100 μL of sterile water for 72 h; compounds and media were replaced daily. Values are expressed as mean ± SE for cell counts from 2 aliquots/well and at least 2 wells/treatment. bP < 0.001 vs untransfected cultures.
IN VIVO STUDIES ON OGF INHIBITION OF PANCREATIC TUMOR GROWTH
Transplantation of BxPC-3 human pancreatic cancer cell lines into nude mice has established a model to study how OGF inhibits tumor incidence and growth[36]. Athymic mice were inoculated subcutaneously with BxPC-3 cells and injected intraperitoneally 3 times daily with 5 mg/kg OGF or sterile saline. OGF-treated mice exhibited a delay of 43% in the time of initial tumor appearance relative to controls (10.6 d). Importantly, 62% of the OGF-treated mice did not have tumors on the day when 100% of all saline-injected mice had visible tumors, suggesting that OGF inhibits proliferation of tumor cells in such a way as to prevent tumor appearance. For those mice receiving OGF that did develop tumors, growth was markedly slower relative to mice injected with saline.
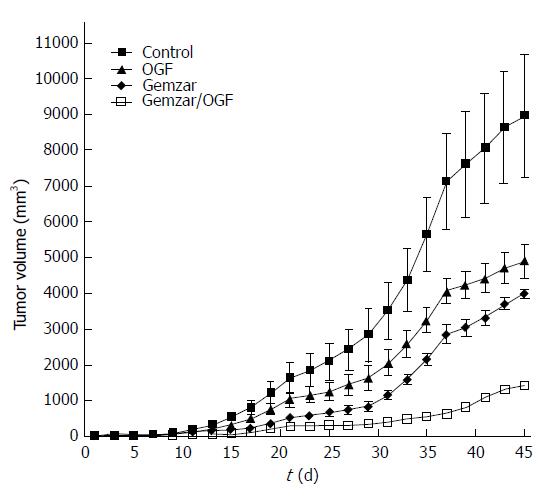
Studies utilizing MIA PaCa-2 cells were conducted in nude mice receiving combination therapy of OGF and/or gemcitabine[39]. Measurement of human pancreatic tumor growth (MIA PaCa-2) in nude mice revealed marked reductions in tumor progression under all treatment modalities, but combination therapy resulted in tumors with marked reductions in size relative to control mice, as well as mice receiving either OGF alone (10 mg/kg daily) or gemcitabine alone (120 mg/kg every 3rd day) (Figure 2). Tumor volumes after 45 d of treatment were reduced approximately 83% in the combination therapy group relative to controls (8900 mm3), whereas reductions in tumor volume were 45% and 56% for mice receiving OGF alone or gemcitabine alone, respectively, relative to controls.
Figure 2 Tumor growth of MIA PaCa-2 tumors xenografted into nude mice.
Animals were injected with 10 mg/kg opioid growth factor (OGF) daily, 120 mg/kg gemcitabine every 3 d (gemzar), both OGF and Gemzar, or 0.1 mL of sterile saline daily (control). Tumor volumes were monitored with calipers over a 45-d period of time. Values represent mean ± SE for all mice in the group. See original manuscript[39] for statistical comparisons.
The relationship between OGFr levels and the progression of human pancreatic tumors in nude mice was investigated by assaying OGFr binding activity and OGFr gene expression in tumors of small, medium, or large volume[45]. Binding capacity of OGFr, and transcriptional activity of OGFr, were not dependent on the size of tumor and were unaltered between small and large tumors. Interestingly, OGF plasma levels were decreased up to 7.9-fold in untreated mice with tumors relative to normal, non-tumorigenic mice suggesting that production of the inhibitory peptide, but not the receptor, may be deficient as cancer progresses[45]. These data support that exogenous administration of OGF is important as a therapy because the receptor is present and functioning in late-stage mouse tumors.
Overexpression of OGFr in tumor cells transplanted into nude mice confirmed that the OGF-OGFr axis provides tonic, homeostatic regulatory control of pancreatic neoplasia[46,47]. MIA PaCa-2 cells were stably transfected to overexpress OGFr, selectively cloned and expanded, and inoculated into nude mice; phenotypic changes in tumorigenicity were monitored. Analysis of receptor number showed that transfected tumor tissue had more than 4 times the binding capacity compared to wildtype tumors. Tumor incidence in mice receiving the molecularly manipulated cells was reduced up to 50% from animals inoculated with wildtype or empty vector transfected cell lines. Latency for the appearance of a measurable tumor was increased 30%, whereas tumor volumes were decreased 70% in comparison to measurements in mice receiving cells transfected with empty vector cDNA constructs. Treatment of mice with an overexpression of OGFr reduced tumor volumes even more with reductions up to 55% recorded[47]. Therefore, OGFr is a regulator of neoplastic cell proliferation that impacts human pancreatic tumorigenic expression. Modification of receptor number alone may prevent or delay human pancreatic cancer.
OGF-OGFR AXIS: MECHANISM OF ACTION
The mechanism of action of OGF is targeted to DNA synthesis and is directed to the p21 cyclin-dependent inhibitory kinase pathway in human pancreatic cancer[37,48,49]. OGF action is mediated by the receptor OGFr. Unlike the mechanistic pathways of many of the standard chemotherapies, investigations have shown that OGF is non-toxic and does not induce apoptosis[50]. Using a variety of human cancer cell lines, studies have demonstrated that OGF does not reduce cell number by changing other biological pathways associated with migration, differentiation, or cell death[50-52]. Flow cytometric analyses of BxPC-3 cell lines treated with OGF reveal a notable increase in cell number in the G0 /G1 phase and compensatory reduction in the proportion of cells in the S and G2/M phases. The percentage of labeled mitotic cells was increased in the G0 /G1 phase[48]. Further studies utilizing synchronized cultures of BxPC-3 pancreatic cells were directed at deciphering the specific pathway in the cell cycle that is targeted by OGF and focused on the retinoblastoma pathway[49]. It was found that OGF decreased phosphorylation of retinoblastoma protein, but did not change the overall level of retinoblastoma protein. The change was correlated with a reduction in cyclin dependent kinase activity (cdk-2), and increased p21 expression[49]. In general, human pancreatic cancer cells express p21 cyclin-dependent inhibitory kinase pathways, whereas many other cancers (e.g., squamous cell carcinoma of the head and neck) utilize p16 cyclin-dependent inhibitory kinase pathways because of deletions or mutations in the p21 pathway. The presence of one intact pathway is important to maintain a homeostatic balance of cellular replication, allowing for one pathway to be mutated as is often the case in neoplasia. The requirement of an intact OGF-OGFr axis for regulation of pancreatic neoplasia was corroborated in a study[44] whereby more than 30 human cancer cell lines were transfected to repress OGFr cDNA and OGFr expression. The lack of OGFr rendered OGF ineffective in inhibiting proliferation.
CLINICAL STUDIES ON THE SAFETY AND EFFICACY OF OGF FOR TREATMENT OF HUMAN PANCREATIC CANCER
Preclinical studies on OGF have shown no toxicity and significant efficacy toward repressing pancreatic cancer progression. Clinical trials to assess OGF treatment of advanced pancreatic cancer were conducted by Zagon et al[52] and Smith et al[53] at The Pennsylvania State University College of Medicine. The maximum tolerated dose (MTD) was established at 250 μg/kg infused over a period of 30 min[52]. Patients with unresectable advanced pancreatic adenocarcinoma were treated with the MTD to establish safety and toxicity. No adverse effects related to cardiac rhythm, blood values, neurological status or other laboratory tests were reported; hypotension was the dose-limiting toxicity. Of interest were the signs of efficacy shown by the small number of patients in this phase I trial. Mean survival time for the patients in the study, including those receiving only one dosage of OGF, was over 8.5 mo, and two patients had resolution of liver metastases. These observations support further clinical trials on OGF as a treatment of advanced pancreatic cancer.
A prospective phase II open-labeled clinical trial with 24 patients who failed standard chemotherapy for advanced pancreatic cancer was conducted whereby patients were treated weekly with 250 μg/kg OGF by intravenous infusion[54]. Outcomes were tumor size measured by computer tomography, survival time, and quality of life. Blood samples were evaluated for levels of OGF after 4 and 8 wk of infusion. Data on the OGF treatment were compared to results obtained from a control group (n-166) of patients of equivalent age who failed therapy and were discharged to hospice care. OGF-treated patients had a three-fold increase in median survival time in comparison to untreated patients. Tumor size was stabilized or reduced in 62% of the cancer patients receiving OGF and surviving more than 8 wk in order to conduct the tomography. Plasma enkephalin levels were significantly increased at 4 and 8 wk with blood levels reaching approximate 55 pg/mL in comparison to baseline values of 8 pg/mL. Finally, no adverse effects on blood chemistry were noted, confirming the safety and lack of toxicity of OGF. Feedback from patients receiving OGF and their caregivers on quality of life indicated that OGF infusion did not indicate any stress or pain.
OGF BIOTHERAPY
Preclinical studies using a variety of human pancreatic adenocarcinoma cell lines that represent undifferentiated to well differentiated pancreatic neoplasms have demonstrated that OGF inhibits DNA synthesis and cell proliferation in vitro. The action of OGF is mediated by OGFr, is reversible, and does not involve apoptotic pathways. OGF is an endogenous peptide that is readily degraded, without alteration of cell migration, differentiation, or survival and thus can be considered a biotherapy. The specificity and selectivity of the OGF-OGFr axis substantiate that this axis is a determinant of cell proliferation in a variety of human cancers.
Investigations of the OGF-OGFr axis in mouse models of cancer with human cell lines transplanted into nude mice confirmed and extended tissue culture studies. Exogenous OGF repressed tumor progression under all situations, and tumors grown from cells overexpressing OGFr were inhibited in their growth. Combination OGF and chemotherapy provided enhanced efficacy at reducing tumor size.
Clinically, OGF is a safe, non-toxic biotherapy that extends survival and reduces tumor burden in patients with unresectable pancreatic cancer. In summary, the OGF-OGFr axis should be explored both as a primary therapy for pancreatic cancer, and as an adjuvant pathway with other chemotherapies.
ACKNOWLEDGMENTS
The authors acknowledge the technicians and faculty collaborators who have assisted in this research.
P- Reviewers: Cheng JT, Shi CJ S- Editor: Ma YJ L- Editor: A E- Editor: Wu HL