Published online Mar 7, 2014. doi: 10.3748/wjg.v20.i9.2168
Revised: December 21, 2013
Accepted: January 14, 2014
Published online: March 7, 2014
Processing time: 106 Days and 19.6 Hours
Chronic alcohol exposure can lead to alcoholic liver disease, including hepatitis, cirrhosis and hepatocellular carcinoma, and chronic inflammation can simultaneously cause systemic medical illness. Recent evidence suggests that alcoholic liver disease is a predictor for liver-related diseases, cardiovascular disease, immunologic disease, and bone disease. Chronic inflammation in alcoholic liver disease is mediated by a direct inflammatory cascade from the alcohol detoxification process and an indirect inflammatory cascade in response to gut microflora-derived lipopolysaccharides (LPS). The pathophysiology of alcoholic liver disease and its related systemic illness is characterized by oxidative stress, activation of the immune cascade, and gut-liver interactions. Integrative therapeutic strategies for alcoholic liver disease include abstaining from alcohol consumption; general anti-inflammatories such as glucocorticoid, pentoxifylline, and tumour necrosis factor-α antagonist; antioxidants such as N- acetylcysteine; gut microflora and LPS modulators such as rifaximin and/or probiotics. This review focuses on the impact of chronic liver inflammation on systemic health problems and several potential therapeutic targets.
Core tip: Beyond the natural course in the liver, alcoholic liver disease can be implicated in many health problems that affect the quality of life and disease progression. Evidence suggests that alcoholic liver disease is a predictor for liver-related diseases, cardiovascular disease, immunologic disease, and bone disease. Chronic inflammation in alcoholic liver disease and related systemic illness is mediated by a direct response to alcohol and an indirect inflammatory response. Alcoholic liver disease should be considered from the perspective of chronic inflammation. Accordingly, integrative therapeutic strategies including anti-inflammatory targeting are needed for alcohol-induced liver inflammation management and prevention of systemic medical problems.
- Citation: Park BJ, Lee YJ, Lee HR. Chronic liver inflammation: Clinical implications beyond alcoholic liver disease. World J Gastroenterol 2014; 20(9): 2168-2175
- URL: https://www.wjgnet.com/1007-9327/full/v20/i9/2168.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i9.2168
Chronic alcohol consumption is a major risk factor for chronic liver disease worldwide. Cardinal features of alcoholic liver disease include simple fatty liver, alcoholic hepatitis, fibrosis or, more seriously, cirrhosis and hepatocellular carcinoma. Alcohol has been recognized as a true hepatotoxin, an agent able to cause liver damage, for many years[1]. Although abstaining from alcohol is the primary recommendation for managing alcoholic liver disease, the chronic features of alcoholic liver disease and its progression can affect a patient’s attitude toward consumption. Alcohol is an important cause of hepatocellular carcinoma in Korea in addition to the hepatitis B virus and the hepatitis C virus[2]. Additionally, up to 48% of cirrhosis-related deaths have been associated with alcohol in the United States[3].
Recent evidence has determined that inflammation is closely linked with development of alcoholic liver disease[3-7]. Acute inflammation as a defense against noxious stimuli is very important for homeostasis in the body, whereas chronic exposure to an agent that induces inflammation may cause a dysregulated or unresolved inflammatory response, which causes chronic inflammation. Finally, various inflammatory components can influence systemic medical conditions. The major sources of chronic low-grade inflammation in alcoholic liver disease are categorized as follows: a direct inflammatory cascade from the alcohol detoxification process and an indirect inflammatory cascade in response to gut microflora-derived lipopolysaccharides (LPS).
The liver plays a key role in detoxifying alcohol and its related toxic products and is also responsible for immunologic effects. However, chronic alcohol consumption can lead to alcoholic liver disease and simultaneous systemic medical illness because of chronic inflammation. Beyond the natural course in the liver, alcoholic liver disease can be implicated in many health problems that affect the quality of life and disease progression. Therefore, alcoholic liver disease should be considered from the perspective of chronic inflammation. This review focuses on the impact of chronic liver inflammation on systemic health problems and several potential therapeutic targets.
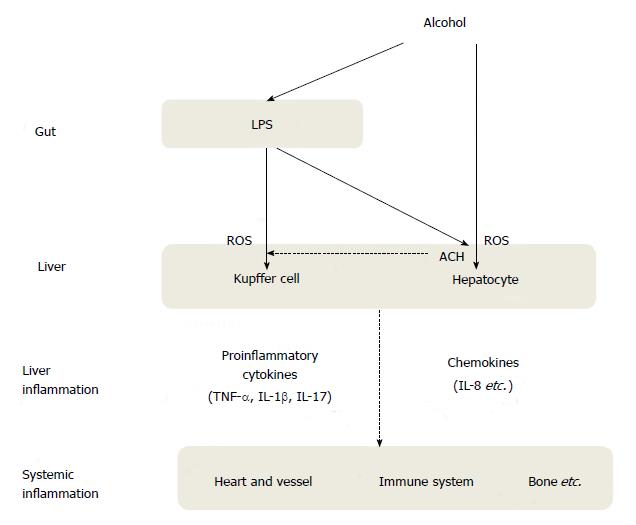
Emerging evidence suggests that alcoholic liver disease predicts not only liver-related diseases, but also atherosclerotic cardiovascular disease (CVD). Recent data suggest that some pathogenic mechanisms are involved in atherosclerotic CVD. In patients with alcoholic liver disease, alcohol, acetaldehyde, and excessive free fatty acids (FFA) in hepatocytes generate an excess of reactive oxygen species (ROS). This formation leads to lipid peroxidation, cytokine production, and hepatic inflammation[8], which contribute to a higher oxidative-inflammatory response. Thus, alcoholic liver disease may actively involve chronic low-grade inflammation in the arterial wall[9]. Moreover, pro-inflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-8 (IL-8), and interleukin-17 (IL-17) are produced by Kupffer cells in the liver in response to LPS, which, in turn, play a key role in inducing acute phase reactants in the liver, such as C-reactive protein (CRP), ferritin, and amyloid A[10,11]. These inflammatory cascades can also synergistically or interactively contribute to arterial inflammation. Indeed, several epidemiologic studies have shown that various inflammatory markers, such as TNF-α and CRP, are elevated in patients with alcoholic liver disease[12-14]. Furthermore, chronic low-grade inflammation plays a crucial role in regulating arterial wall tone by affecting the release of nitric oxide (NO) and endothelin-1[15,16]. These cascades may cause endothelial dysfunction and alter arterial elastic properties, leading to arterial stiffness. In addition, when a hepatic cell is damaged, hepatic stellate cells also secrete angiotensin II[17], a major pro-atherogenic and vasoconstrictive peptide that acts on the arterial wall. The overproduction of hematostatic factors such as plasminogen activator inhibitor-1 (PAI-1) may have a direct atherogenic effect on blood vessels[18] in patients with alcoholic liver disease. Lastly, chronic alcohol consumption has a tendency for increased plasma homocysteine levels, albeit the results are inconsistent according to amount and types of alcoholic beverage consumed, or underlying diseases[19]. However, hyperhomocysteinemia induced by chronic alcohol consumption may be one of the important risk factors for CVD[20,21].
Recent research has shown that alcoholic liver disease may alter immune regulation, which can lead to immunodeficiency and autoimmunity[22]. Additionally, individuals with chronic alcohol consumption are more susceptible to bacterial pneumonia and septicemia[23-25]. There is also an increased incidence of pulmonary tuberculosis or human immunodeficiency virus (HIV) in patients with alcoholic liver disease[26-28]. In addition, less common infectious diseases such as meningitis, diphtheria, lung abscess, or cellulitis are more prevalent in alcoholic liver disease[27].
Alcoholic hepatitis and alcoholic liver cirrhosis have been associated with autoimmune properties[22]. Liver function in patients with alcoholic hepatitis can decrease for several weeks (at least two weeks) after abstinence from alcohol, and resuming drinking after recovering from alcoholic hepatitis can lead to more severe alcoholic hepatitis. In this regard, autoantibodies against the liver may be an important cause of the liver damage and scarring in alcoholic liver disease.
Other autoimmune diseases or allergic reactions are also seen in alcoholic liver disease. Immunoglobulin A (IgA) has been found in skin and kidney deposits as well as the liver in many patients with alcoholic liver disease[29]. Also, alcohol consumption contributes to immunoglobulin E (IgE)-mediated reactions in susceptible humans, such as individuals with food allergies or asthma[30]. Chronic alcohol use has been found to increase total IgE level[30-32]. Therefore, understanding altered immunity and cytokines in alcoholic liver disease can be important for assessing potential immunologic risk and could provide insight into therapeutic targets.
Hepatic osteodystrophy is abnormal bone metabolism that has been identified in association with chronic liver disease, including such conditions as osteopenia, osteoporosis, and osteomalacia. The prevalence of osteopenia in patients with alcoholic liver disease is between 34% and 48%, and the prevalence of osteoporosis is between 11% and 36%[33-35]. However, osteomalacia has rarely been confirmed in patients with chronic liver disease and low vitamin D level[36,37].
Bone is a dynamic tissue that is remodeled through constant bone resorption and formation. Bone turnover accounts for up to 15% of the annual renovation of total bone mass[38]. Decreased bone density, commonly seen in hepatic osteodystrophy, results from decreased bone formation or increased bone resorption. Bone mineral density, measured by dual energy X-ray absorptiometry, is reported with a Z score and T score; the former is used to compare the patient’s bone mineral density with an age-matched mean value, and the latter is used to compare the bone mineral density with that of healthy young individuals. Osteopenia is identified when a T score ranges from -1.0 to -2.5, and osteoporosis is defined by a T score < 2.5. Osteomalacia is characterized by abnormal bone matrix mineralization, which can be confirmed by bone biopsy. These metabolic bone diseases are very common and can be important complications in patients with alcoholic liver disease. Although the mechanism of metabolic bone diseases remains complex and multifactorial, osteoclastogenic inflammatory cytokines, such as interleukin 1 (IL-1) and TNF-α, have been known to have a key role in the pathogenesis of bone metabolic disease that is related to alcoholic liver disease[39]. Early assessment and therapeutic intervention in patients with hepatic osteodystrophy can be important for minimizing future fracture risk and maintaining the quality of life.
It is known that alcohol increases ROS, which are chemically reactive molecules that can damage various cellular components such as proteins, lipids, or deoxyribonucleic acid (DNA)[40]. Moreover, acetaldehyde, an intermediate alcohol metabolite, is a highly reactive compound and is highly toxic to hepatocytes, promotes glutathione depletion, lipid peroxidation, and mitochondrial damage[41-42]. Evidence suggests that oxidative stress can contribute to the development of alcoholic liver disease and has been associated with various major diseases including cardiovascular diseases, type 2 diabetes, neurodegenerative disease, and carcinogenesis[43-46].
Although multiple mechanisms are involved in alcohol-related ROS production, cytochrome P450 E21 and the mitochondrial electron transport chain are important targets[40,47,48]. Moreover, alcohol-derived ROS may directly trigger the systemic inflammatory response[49]. ROS could activate nuclear factor kappa B (NF-κB), which leads to production of inflammatory cytokines such as TNF-α. Alcohol-derived ROS may play a role in initiating a vicious cycle via the liver cell damage mechanism with additional inflammatory cytokines and ROS production[50]. Therefore, alcohol-derived ROS may be important for understanding systemic inflammation accompanied with alcoholic liver disease.
As described above, individuals with chronic alcohol consumption are more susceptible to immunodeficiency and autoimmunity. Understanding altered innate and acquired immunity in alcoholic liver disease may be important for assessing the potential risk of opportunistic infections and allergic diseases such as food allergies and bronchial asthma.
Alcohol consumption causes gut microflora dysbiosis and bacterial over-growth and ultimately increases gut permeability and the translocation of LPS from the gut to the liver. In Kupffer cells, gut microflora derived-LPS interacts with toll-like receptor 4 (TLR4), and pro-inflammatory cytokines and chemokines such as TNF-α, IL-8, IL-17 are produced, leading to the production of ROS and alcohol-induced liver damage[51,52]. Interestingly, activation of TLR4 also induces Kupffer cells to produce hepatoprotective cytokines such as IL-6 which reduces hepatocyte necrosis-associated inflammation, albeit having proinflammatory roles, and anti-inflammatory cytokines such as interleukin-10 (IL-10)[53]. However, long-term alcohol consumption may generate lipid peroxidation products such as malondialdehyde (MDA) as a result of ROS cascades, which can modify many proteins linked to the adaptive immune response[54,55]. Patients with alcoholic liver disease have increased levels of circulating antibodies against lipid peroxidation products and increased numbers of T and B cells in the liver, which contribute to adaptive immunity activation in alcoholic liver disease[54,55].
Optimal functioning of the gut-liver axis depends on healthy gut integrity and mucosal microflora and a healthy liver; however, chronic alcohol exposure impairs both gut and liver health. These changes affect each other and ultimately contribute to the increased blood levels of LPS, or endotoxemia, in patients with alcoholic liver disease. Major inducers of chronic low-grade inflammation in alcoholic liver disease are broadly summarized as a direct inflammatory injury from alcohol and its metabolites or an indirect inflammatory injury in response to LPS. The microflora-derived LPS enters systemic circulation in two different ways, either via a portal vein or through gastrointestinal lymphatic vessels[56,57]. Most LPS in the lymphatic system move through mesenteric lymph nodes and eventually enter the systemic circulation at the thoracic duct opening, whereas most LPS in the portal vein can be detoxified and excreted.
Alcohol can alter gut integrity and permeability in both direct and indirect manners. Alcohol and its metabolites such as acetaldehyde can directly alter both gut permeability and microflora content and composition. Alcohol and acetaldehyde can weaken the intestinal epithelial barrier, such as tight junctions between intestinal enterocytes. Moreover, increased gut permeability in alcoholic liver disease may be aggravated by increased expression of inducible nitric oxide synthase (iNOS) and NF-κB, which, in turn, enhance the translocation of LPS between tight junctions of adjacent enterocytes[58,59]. This increased gut permeability is also called leaky gut syndrome (LGS). Patients with alcoholic hepatitis commonly show elevated LPS levels in plasma, implicating a crucial role of LPS-induced inflammation in the pathogenesis of alcoholic liver disease[60]. Thus, alcohol facilitates the translocation of endotoxin from the intestinal lumen to the portal vein, thereby aggravating the risk of liver injury.
Individuals with chronic alcohol use are more susceptible to small intestinal bacterial overgrowth and dysbiosis compared to counterpart non-alcoholics or abstainers[61,62]. Excessive alcohol ingestion facilitates the overgrowth of Gram-negative bacteria, contributing to increased endotoxin levels[60]. In addition, micronutrient deficiency, such as zinc, is common in alcoholic liver disease, which adversely affects the integrity of the intestinal epithelium[63]. More recently, evidence suggests that increased gut permeability may be an important factor in the pathogenesis of alcoholic liver disease.
As intestinal permeability increases, endotoxin and other bacterial toxins increase the sensitivity of Kupffer cells to LPS stimulation in the liver, where increased pro-inflammatory cytokines lead to neutrophil activation, increased sinusoidal permeability, generation of ROS, and mitochondrial damage in the liver[64]. These cascades may cause systemic low-grade inflammation in addition to liver inflammation (Figure 1).
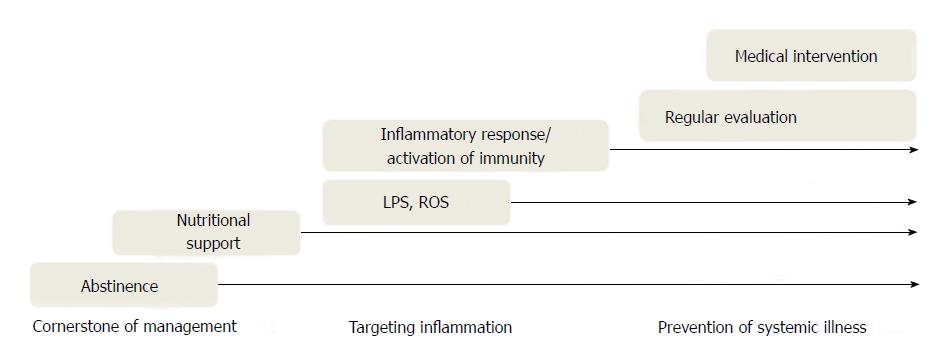
The first-line therapy for alcoholic liver disease is alcohol abstinence with nutritional support[3,7,65]. However, therapeutic lifestyle changes are not easy in clinical practice and may not be sufficient for some patients. Corticosteroids, pentoxifylline, and TNF-α antagonist have been identified as therapeutic agents for severe alcoholic hepatitis. Among them, corticosteroids and pentoxifylline are currently recommended[7]. Glucocorticoids could reduce immune activation by blocking cytotoxic and inflammatory signal pathways, and pentoxifyllin plays an anti-inflammatory role as a non-selective phosphodiesterase inhibitor and TNF-α suppressor[66]. Although TNF-α has been regarded as a predictor for the severity of alcoholic hepatitis and TNF-α antagonist reduces liver damage in alcohol-fed animals, clinical trials with TNF-α antibody have not shown consistent results[67-69]. TNF-α antibody or corticosteroids may induce a condition that causes patients to be susceptible to infections because of immune suppression[7,69]. Pentoxifylline may be considered in patients with severe alcoholic hepatitis who cannot use corticosteroids[3].
Antioxidants such as N-acetylcysteine have been reported to reduce inflammatory markers and liver fat accumulation in alcohol-fed animals[49]. S-adenosylmethionine could increase cellular antioxidant glutathione in patients with alcoholic liver disease[70]. Betaine, precursor to S-adenosylmethionine, has also been reported to attenuate alcoholic liver disease[71]. In clinical trials, S-adenosylmethionine has shown improved survival in patients with less advanced liver cirrhosis[72] but has not been consistently effective in treating alcoholic liver disease[73]. Antioxidants including phytochemicals such as resveratrol and carotenoids are successful for treating alcohol-fed animals, but lack convincing benefits in human patients[74-76]. Oxidative stress may be more pronounced in early stages of alcoholic liver disease, which is found in most animal models, but plays a minor role in later stages of alcoholic liver disease. Actually, administration of antioxidants cocktail has shown inferior survival rates compared to corticosteroid administration in patients with severe alcoholic hepatitis[77].
The gut-liver interaction has been identified as an important interaction for liver health and prevention of systemic inflammation. In this regard, the modulation of gut microflora and LPS pathway could be used to treat alcoholic liver disease[3,78,79] (Figure 2). For the former, probiotics and bioactive extracts may provide therapeutic benefit in patients with alcoholic liver disease[79,80]. In addition, non-absorbable antibiotics such as rifaximin and/or probiotics can modify the gut microflora and help reduce the risk of hepatic encephalopathy[81]. For the latter, TLR4 antagonists that modify the LPS pathway have been proposed as therapeutic materials for chronic liver disease[82].
Several surrogate agents for treating alcoholic liver disease are being investigated. Global suppression of inflammatory responses could lead to undesirable side effects such as immune suppression. Therefore, specific anti-inflammatory targeting may be more promising. Recent studies have shown that IL-22 has hepatoprotective properties including antioxidant, anti-steatotic, and anti-microbial effects[83]. Moreover, the IL-22 receptor exists only on epithelial cells such as hepatocytes, and side effects that target this receptor may be minimal. Also, IL-8 and IL-17 have been related to neutrophil infiltration, TNF-α augmentation, and autoimmunity[7,84]. Therefore, based on the cytokine and immune cell profiles, specific intervention may merit serious consideration to reduce the inflammatory response with minimal side effects.
Chronic inflammation in alcoholic liver disease and related systemic illness is mediated by a direct response to alcohol and an indirect inflammatory response to gut microflora-derived LPS, leading to a stronger oxidative-inflammatory response. In addition to alcohol abstinence, integrative therapeutic strategies to reduce inflammatory cascades may be needed to treat and prevent alcoholic liver disease.
P- Reviewers: Buechler C, Saito T, Yang SC S- Editor: Qi Y L- Editor: A E- Editor: Liu XM
| 1. | Lieber CS, Jones DP, Decarli LM. Effects of prolonged ethanol intake: production of fatty liver despite adequate diets. J Clin Invest. 1965;44:1009-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 433] [Cited by in RCA: 392] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 2. | Song IH, Kim KS. Current status of liver diseases in Korea: hepatocellular carcinoma. Korean J Hepatol. 2009;15 Suppl 6:S50-S59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1495] [Article Influence: 106.8] [Reference Citation Analysis (0)] |
| 4. | Enomoto N, Ikejima K, Bradford BU, Rivera CA, Kono H, Goto M, Yamashina S, Schemmer P, Kitamura T, Oide H. Role of Kupffer cells and gut-derived endotoxins in alcoholic liver injury. J Gastroenterol Hepatol. 2000;15 Suppl:D20-D25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 94] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Wang HJ, Zakhari S, Jung MK. Alcohol, inflammation, and gut-liver-brain interactions in tissue damage and disease development. World J Gastroenterol. 2010;16:1304-1313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 162] [Cited by in RCA: 200] [Article Influence: 13.3] [Reference Citation Analysis (1)] |
| 6. | Hernandez-Gea V, Friedman SL. Pathogenesis of liver fibrosis. Annu Rev Pathol. 2011;6:425-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1096] [Cited by in RCA: 1380] [Article Influence: 98.6] [Reference Citation Analysis (0)] |
| 7. | Wang HJ, Gao B, Zakhari S, Nagy LE. Inflammation in alcoholic liver disease. Annu Rev Nutr. 2012;32:343-368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 236] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 8. | Park SH, Kim BI, Yun JW, Kim JW, Park DI, Cho YK, Sung IK, Park CY, Sohn CI, Jeon WK. Insulin resistance and C-reactive protein as independent risk factors for non-alcoholic fatty liver disease in non-obese Asian men. J Gastroenterol Hepatol. 2004;19:694-698. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 155] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 9. | Wang CC, Lin SK, Tseng YF, Hsu CS, Tseng TC, Lin HH, Wang LY, Kao JH. Elevation of serum aminotransferase activity increases risk of carotid atherosclerosis in patients with non-alcoholic fatty liver disease. J Gastroenterol Hepatol. 2009;24:1411-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428-435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3548] [Cited by in RCA: 4381] [Article Influence: 257.7] [Reference Citation Analysis (0)] |
| 11. | Baumann H, Gauldie J. The acute phase response. Immunol Today. 1994;15:74-80. [PubMed] |
| 12. | Kogiso T, Moriyoshi Y, Shimizu S, Nagahara H, Shiratori K. High-sensitivity C-reactive protein as a serum predictor of nonalcoholic fatty liver disease based on the Akaike Information Criterion scoring system in the general Japanese population. J Gastroenterol. 2009;44:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Wieckowska A, Papouchado BG, Li Z, Lopez R, Zein NN, Feldstein AE. Increased hepatic and circulating interleukin-6 levels in human nonalcoholic steatohepatitis. Am J Gastroenterol. 2008;103:1372-1379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 443] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 14. | Bahcecioglu IH, Yalniz M, Ataseven H, Ilhan N, Ozercan IH, Seckin D, Sahin K. Levels of serum hyaluronic acid, TNF-alpha and IL-8 in patients with nonalcoholic steatohepatitis. Hepatogastroenterology. 2005;52:1549-1553. [PubMed] |
| 15. | Bhagat K, Vallance P. Effects of cytokines on nitric oxide pathways in human vasculature. Curr Opin Nephrol Hypertens. 1999;8:89-96. [PubMed] |
| 16. | Kahaleh MB, Fan PS. Effect of cytokines on the production of endothelin by endothelial cells. Clin Exp Rheumatol. 1997;15:163-167. [PubMed] |
| 17. | Bataller R, Sancho-Bru P, Ginès P, Lora JM, Al-Garawi A, Solé M, Colmenero J, Nicolás JM, Jiménez W, Weich N. Activated human hepatic stellate cells express the renin-angiotensin system and synthesize angiotensin II. Gastroenterology. 2003;125:117-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 251] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 18. | Sookoian S, Castaño GO, Burgueño AL, Rosselli MS, Gianotti TF, Mallardi P, Martino JS, Pirola CJ. Circulating levels and hepatic expression of molecular mediators of atherosclerosis in nonalcoholic fatty liver disease. Atherosclerosis. 2010;209:585-591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 97] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 19. | Sakuta H, Suzuki T. Alcohol consumption and plasma homocysteine. Alcohol. 2005;37:73-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Welch GN, Loscalzo J. Homocysteine and atherothrombosis. N Engl J Med. 1998;338:1042-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1440] [Cited by in RCA: 1381] [Article Influence: 51.1] [Reference Citation Analysis (0)] |
| 21. | Hankey GJ, Eikelboom JW. Homocysteine and vascular disease. Lancet. 1999;354:407-413. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 706] [Cited by in RCA: 634] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 22. | Cook RT. Alcohol abuse, alcoholism, and damage to the immune system--a review. Alcohol Clin Exp Res. 1998;22:1927-1942. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Chen CW, Jong GM, Shiau JJ, Hsiue TR, Chang HY, Chuang YC, Chen CR. Adult bacteremic pneumonia: bacteriology and prognostic factors. J Formos Med Assoc. 1992;91:754-759. [PubMed] |
| 24. | Cortese MM, Wolff M, Almeido-Hill J, Reid R, Ketcham J, Santosham M. High incidence rates of invasive pneumococcal disease in the White Mountain Apache population. Arch Intern Med. 1992;152:2277-2282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 55] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Kuikka A, Syrjänen J, Renkonen OV, Valtonen VV. Pneumococcal bacteraemia during a recent decade. J Infect. 1992;24:157-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Friedman LN, Sullivan GM, Bevilaqua RP, Loscos R. Tuberculosis screening in alcoholics and drug addicts. Am Rev Respir Dis. 1987;136:1188-1192. [PubMed] |
| 27. | MacGregor RR, Louria DB. Alcohol and infection. Curr Clin Top Infect Dis. 1997;17:291-315. [PubMed] |
| 28. | Bagasra O, Bachman SE, Jew L, Tawadros R, Cater J, Boden G, Ryan I, Pomerantz RJ. Increased human immunodeficiency virus type 1 replication in human peripheral blood mononuclear cells induced by ethanol: potential immunopathogenic mechanisms. J Infect Dis. 1996;173:550-558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 62] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 29. | Paronetto F. Immunologic reactions in alcoholic liver disease. Semin Liver Dis. 1993;13:183-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Gonzalez-Quintela A, Vidal C, Gude F. Alcohol, IgE and allergy. Addict Biol. 2004;9:195-204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | González-Quintela A, Vidal C, Gude F. Alcohol-induced alterations in serum immunoglobulin e (IgE) levels in human subjects. Front Biosci. 2002;7:e234-e244. [PubMed] |
| 32. | Gonzalez-Quintela A, Dominguez-Santalla MJ, Perez LF, Lojo S, Vidal C. Serum levels of soluble CD30 and total IgE in alcoholics. Allergol Intern. 2002;51:33-37. |
| 33. | Sokhi RP, Anantharaju A, Kondaveeti R, Creech SD, Islam KK, Van Thiel DH. Bone mineral density among cirrhotic patients awaiting liver transplantation. Liver Transpl. 2004;10:648-653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 34. | Ninkovic M, Love SA, Tom B, Alexander GJ, Compston JE. High prevalence of osteoporosis in patients with chronic liver disease prior to liver transplantation. Calcif Tissue Int. 2001;69:321-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 74] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 35. | Hay JE, Guichelaar MM. Evaluation and management of osteoporosis in liver disease. Clin Liver Dis. 2005;9:747-766, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 36. | Leslie WD, Bernstein CN, Leboff MS. AGA technical review on osteoporosis in hepatic disorders. Gastroenterology. 2003;125:941-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Collier JD, Ninkovic M, Compston JE. Guidelines on the management of osteoporosis associated with chronic liver disease. Gut. 2002;50 Suppl 1:i1-i9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 134] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 38. | Sanchez AJ, Aranda-Michel J. Liver disease and osteoporosis. Nutr Clin Pract. 2006;21:273-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Collier J. Bone disorders in chronic liver disease. Hepatology. 2007;46:1271-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 40. | Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 446] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 41. | Setshedi M, Wands JR, Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev. 2010;3:178-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 238] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 42. | Farfán Labonne BE, Gutiérrez M, Gómez-Quiroz LE, Konigsberg Fainstein M, Bucio L, Souza V, Flores O, Ortíz V, Hernández E, Kershenobich D. Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biol Toxicol. 2009;25:599-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 66] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 43. | Kehrer JP. Free radicals as mediators of tissue injury and disease. Crit Rev Toxicol. 1993;23:21-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 985] [Cited by in RCA: 901] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 44. | Knight JA. Free radicals: their history and current status in aging and disease. Ann Clin Lab Sci. 1998;28:331-346. [PubMed] |
| 45. | Cederbaum AI. Iron and CYP2E1-dependent oxidative stress and toxicity. Alcohol. 2003;30:115-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 46. | Singh M, Gupta S, Singhal U, Pandey R, Aggarwal SK. Evaluation of the oxidative stress in chronic alcoholics. J Clin Diagn Res. 2013;7:1568-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 47. | Zakhari S. Overview: how is alcohol metabolized by the body. Alcohol Res Health. 2006;29:245-254. [PubMed] |
| 48. | Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 246] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 49. | Zhou Z, Wang L, Song Z, Lambert JC, McClain CJ, Kang YJ. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am J Pathol. 2003;163:1137-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 161] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 50. | McVicker BL, Tuma PL, Kharbanda KK, Lee SM, Tuma DJ. Relationship between oxidative stress and hepatic glutathione levels in ethanol-mediated apoptosis of polarized hepatic cells. World J Gastroenterol. 2009;15:2609-2616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 51. | Yin M, Wheeler MD, Kono H, Bradford BU, Gallucci RM, Luster MI, Thurman RG. Essential role of tumor necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. 1999;117:942-952. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 52. | Hritz I, Mandrekar P, Velayudham A, Catalano D, Dolganiuc A, Kodys K, Kurt-Jones E, Szabo G. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. 2008;48:1224-1231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 332] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 53. | Wang H, Lafdil F, Kong X, Gao B. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci. 2011;7:536-550. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 174] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 54. | Mottaran E, Stewart SF, Rolla R, Vay D, Cipriani V, Moretti M, Vidali M, Sartori M, Rigamonti C, Day CP. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:38-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 55. | Thiele GM, Freeman TL, Klassen LW. Immunologic mechanisms of alcoholic liver injury. Semin Liver Dis. 2004;24:273-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Olofsson P, Nylander G, Olsson P. Endotoxin: routes of transport in experimental peritonitis. Am J Surg. 1986;151:443-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 57. | Olofsson P, Nylander G, Olsson P. Endotoxin-transport routes and kinetics in intestinal ischemia. Acta Chir Scand. 1985;151:635-639. [PubMed] |
| 58. | Ferrier L, Bérard F, Debrauwer L, Chabo C, Langella P, Buéno L, Fioramonti J. Impairment of the intestinal barrier by ethanol involves enteric microflora and mast cell activation in rodents. Am J Pathol. 2006;168:1148-1154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 197] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 59. | Rao R. Endotoxemia and gut barrier dysfunction in alcoholic liver disease. Hepatology. 2009;50:638-644. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 375] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 60. | Fukui H, Brauner B, Bode JC, Bode C. Plasma endotoxin concentrations in patients with alcoholic and non-alcoholic liver disease: reevaluation with an improved chromogenic assay. J Hepatol. 1991;12:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 354] [Cited by in RCA: 351] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 61. | Bode JC, Bode C, Heidelbach R, Dürr HK, Martini GA. Jejunal microflora in patients with chronic alcohol abuse. Hepatogastroenterology. 1984;31:30-34. [PubMed] |
| 62. | Hauge T, Persson J, Danielsson D. Mucosal bacterial growth in the upper gastrointestinal tract in alcoholics (heavy drinkers). Digestion. 1997;58:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 48] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 63. | Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Preservation of intestinal structural integrity by zinc is independent of metallothionein in alcohol-intoxicated mice. Am J Pathol. 2004;164:1959-1966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 64. | Thurman RG. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605-G611. [PubMed] |
| 65. | Day CP. Treatment of alcoholic liver disease. Liver Transpl. 2007;13:S69-S75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 66. | Tan HH, Virmani S, Martin P. Controversies in the management of alcoholic liver disease. Mt Sinai J Med. 2009;76:484-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 67. | Spahr L, Rubbia-Brandt L, Frossard JL, Giostra E, Rougemont AL, Pugin J, Fischer M, Egger H, Hadengue A. Combination of steroids with infliximab or placebo in severe alcoholic hepatitis: a randomized controlled pilot study. J Hepatol. 2002;37:448-455. [PubMed] |
| 68. | Naveau S, Chollet-Martin S, Dharancy S, Mathurin P, Jouet P, Piquet MA, Davion T, Oberti F, Broët P, Emilie D. A double-blind randomized controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. 2004;39:1390-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 297] [Cited by in RCA: 322] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 69. | Boetticher NC, Peine CJ, Kwo P, Abrams GA, Patel T, Aqel B, Boardman L, Gores GJ, Harmsen WS, McClain CJ. A randomized, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. 2008;135:1953-1960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 265] [Cited by in RCA: 255] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 70. | Vendemiale G, Altomare E, Trizio T, Le Grazie C, Di Padova C, Salerno MT, Carrieri V, Albano O. Effects of oral S-adenosyl-L-methionine on hepatic glutathione in patients with liver disease. Scand J Gastroenterol. 1989;24:407-415. [PubMed] |
| 71. | Purohit V, Abdelmalek MF, Barve S, Benevenga NJ, Halsted CH, Kaplowitz N, Kharbanda KK, Liu QY, Lu SC, McClain CJ. Role of S-adenosylmethionine, folate, and betaine in the treatment of alcoholic liver disease: summary of a symposium. Am J Clin Nutr. 2007;86:14-24. [PubMed] |
| 72. | Mato JM, Cámara J, Fernández de Paz J, Caballería L, Coll S, Caballero A, García-Buey L, Beltrán J, Benita V, Caballería J. S-adenosylmethionine in alcoholic liver cirrhosis: a randomized, placebo-controlled, double-blind, multicenter clinical trial. J Hepatol. 1999;30:1081-1089. [PubMed] |
| 73. | Medici V, Virata MC, Peerson JM, Stabler SP, French SW, Gregory JF, Albanese A, Bowlus CL, Devaraj S, Panacek EA. S-adenosyl-L-methionine treatment for alcoholic liver disease: a double-blinded, randomized, placebo-controlled trial. Alcohol Clin Exp Res. 2011;35:1960-1965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 74. | Bishayee A, Darvesh AS, Politis T, McGory R. Resveratrol and liver disease: from bench to bedside and community. Liver Int. 2010;30:1103-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 75. | Sindhu ER, Firdous AP, Preethi KC, Kuttan R. Carotenoid lutein protects rats from paracetamol-, carbon tetrachloride- and ethanol-induced hepatic damage. J Pharm Pharmacol. 2010;62:1054-1060. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Stewart S, Prince M, Bassendine M, Hudson M, James O, Jones D, Record C, Day CP. A randomized trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol. 2007;47:277-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (1)] |
| 77. | Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O’Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis--a randomised clinical trial. J Hepatol. 2006;44:784-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 144] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 78. | Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med. 1994;205:243-247. [PubMed] |
| 79. | Kirpich IA, Solovieva NV, Leikhter SN, Shidakova NA, Lebedeva OV, Sidorov PI, Bazhukova TA, Soloviev AG, Barve SS, McClain CJ. Probiotics restore bowel flora and improve liver enzymes in human alcohol-induced liver injury: a pilot study. Alcohol. 2008;42:675-682. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 399] [Cited by in RCA: 388] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 80. | Ewaschuk JB, Diaz H, Meddings L, Diederichs B, Dmytrash A, Backer J, Looijer-van Langen M, Madsen KL. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1025-G1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 413] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 81. | Bass NM, Mullen KD, Sanyal A, Poordad F, Neff G, Leevy CB, Sigal S, Sheikh MY, Beavers K, Frederick T. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362:1071-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 868] [Cited by in RCA: 868] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 82. | Mencin A, Kluwe J, Schwabe RF. Toll-like receptors as targets in chronic liver diseases. Gut. 2009;58:704-720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 273] [Cited by in RCA: 268] [Article Influence: 16.8] [Reference Citation Analysis (1)] |
| 83. | Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, Gao B. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 364] [Cited by in RCA: 367] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 84. | Lemmers A, Moreno C, Gustot T, Maréchal R, Degré D, Demetter P, de Nadai P, Geerts A, Quertinmont E, Vercruysse V. The interleukin-17 pathway is involved in human alcoholic liver disease. Hepatology. 2009;49:646-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 306] [Article Influence: 19.1] [Reference Citation Analysis (0)] |