Published online Feb 28, 2014. doi: 10.3748/wjg.v20.i8.2014
Revised: December 9, 2013
Accepted: January 14, 2014
Published online: February 28, 2014
Processing time: 155 Days and 13.4 Hours
This article addresses the use of computed tomographic colonography (CTC) for the diagnosis and management of colorectal cancer, focusing on presurgical evaluation of the colon proximal to an occlusive cancer and surveillance after cancer resection surgery. The key evidences accumulated in the literature and future work needed are summarized. CTC is a technically robust and the most practical method to evaluate the colon proximal to an occlusive cancer, which prevents colonoscopic examination past the occlusion, either before or after metallic stent placement. The high sensitivity of CTC for detecting cancers and advanced adenomas in the proximal colon can help prevent additional surgical procedures in patients showing negative results. However, the accuracy of CTC for distinguishing intramural cancers from adenomas is low, and the technique is limited in guiding management when a medium-sized lesion that do not show invasive features such as pericolic extension or nodal metastasis is found in the proximal colon. A maximal diameter ≥ 15 mm has been proposed as a criterion for surgical removal of proximal lesions. However, this needs to be verified in a larger cohort. In addition, the influence of presurgical CTC results on the current post-cancer resection colonic surveillance timeline remains to be determined. CTC can be readily added to the routine abdominopelvic CT in the form of contrast-enhanced CTC, which can serve as an effective stand-alone tool for post-cancer resection surveillance of both the colorectum and extracolonic organs. Although the accuracy of CTC has been demonstrated, its role in the current colonoscopy-based postoperative colonic surveillance protocols remains to be determined. Readers of CTC also need to be knowledgeable on the colonic lesions that are unique to the postoperative colon.
Core tip: Computed tomographic colonography (CTC) is technically robust and the most practical method to evaluate the colon proximal to an occlusive cancer either before or after metallic stent placement. Contrast-enhanced CTC may serve as an effective stand-alone tool for post-cancer resection surveillance of both the colorectum and extracolonic organs. However, several issues discussed in this article should be addressed further and clarified.
- Citation: Hong N, Park SH. CT colonography in the diagnosis and management of colorectal cancer: Emphasis on pre- and post-surgical evaluation. World J Gastroenterol 2014; 20(8): 2014-2022
- URL: https://www.wjgnet.com/1007-9327/full/v20/i8/2014.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i8.2014
Computed tomographic (CT) colonography (CTC) (also known as virtual colonoscopy) is a recently developed radiological imaging technology for the evaluation of the colorectum, enabled by advances in CT scan and three-dimensional image processing technologies[1,2]. CTC is less invasive and generally safer than optical colonoscopy[1,3]. CTC can visualize the lumen of the colorectum in various three-dimensional views in addition to the conventional colonoscopy-like endoluminal navigation as well as in two-dimensional multiplanar cross-sectional views[2,4,5]. This variety in visualization modes allows for accurate and efficient evaluation of the colorectum. Unlike optical colonoscopy, which is limited to the endoluminal examination of the colorectum, CTC enables the evaluation of extracolonic organs, particularly when performed with intravenous contrast enhancement. The clinical usefulness of CTC has been studied extensively, largely focusing on screening/surveillance of the general population for colorectal cancer, and CTC has repeatedly shown acceptably high accuracy comparable to colonoscopy for detecting clinically-relevant colorectal neoplasms[6-12]. Accordingly, CTC has now been included in the guidelines for colorectal cancer screening in several countries, for instance, the Joint Guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology[13] and Korean guidelines[14]. On the other hand, CTC has yet to be completely accepted as a tool for population screening in terms of reimbursement as CTC is only incompletely reimbursed in some countries[15,16]: the decision by the Centers for Medicare and Medicaid Services in the United States to deny coverage for CTC in the recent past was such an example[17,18]. Nevertheless, new clinical evidences and data have been accumulated and are likely to resolve the prior concerns regarding widespread adoption of CTC in population screening for colorectal cancer[19,20]. Likewise, CTC is steadily gaining clinical acceptance and increasingly utilized as a screening examination [13-15].
In addition to the role in general screening/surveillance for colorectal cancer, the dual function of CTC in colorectal and extracolonic evaluation suggests that this technique could be applicable to other clinical scenarios. One particular area of interest is the role of CTC in the management of patients who have already been diagnosed with colorectal cancer[21], and multiple studies have addressed this use of CTC, albeit not as extensively as the research on the general screening/surveillance role of CTC. The present review summarizes and discusses the results of such studies, placing emphasis on (1) the use of CTC for presurgical evaluation of the colonic segments proximal to an occlusive cancer preventing colonoscopic examination beyond the level of occlusion, and (2) the use of CTC for post-cancer resection surveillance. The review highlights key evidence accumulated in the literature and further work that needs to be done. This article does not address the general technical issues or principles of CTC, as these are already well explained in the literature elsewhere[1,2,21]. A few technical issues unique to the practice of CTC for such non-screening indications will be briefly addressed.
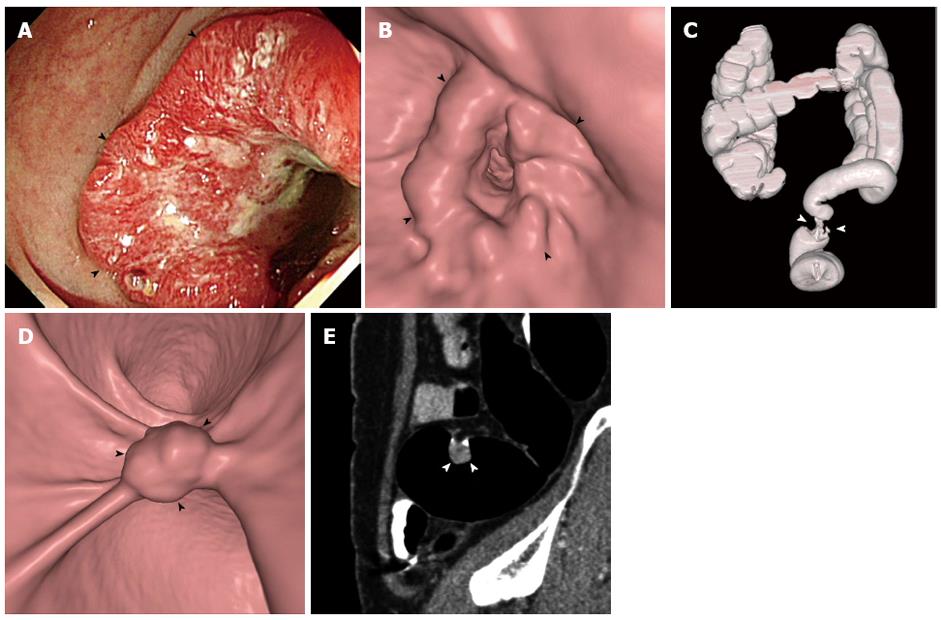
Patients with colorectal cancer may present with an occlusive mass that prevents colonoscopic examination beyond the level of the occlusion. Complete presurgical evaluation of the entire colon is important in patients diagnosed with colorectal cancer because identification of synchronous cancers, which are present in 1%-7% of these patients[22,23], may determine the extent of surgical resection. The presurgical diagnosis of these synchronous cancers is important to prevent a second surgery or even failure of curative treatment. Various options are available for proximal colonic evaluation, including double-contrast barium enema, CTC, intraoperative colonoscopy, and surgical palpation. Of these, CTC is currently regarded as the standard procedure (Figure 1). Double-contrast barium enema, despite its historical use for proximal colonic evaluation in occlusive colorectal cancers[24], has low sensitivity even in the absence of an occlusive cancer[25], in which case bowel preparation is relatively easier compared with in patients with occlusive cancer. In addition, barium is associated with a risk of barium desiccation in the colon proximal to an obstructing cancer. Intraoperative colonoscopy is possible but is not a practical option[26]. By contrast, CTC is a technically robust method that can be performed successfully if the insufflated gas can be delivered across the tumor-induced occlusion to adequately distend the colonic segments proximal to the occlusion. This is in contrast to colonoscopy, which requires the passage of the scope across the narrowing. Therefore, almost all cases of failed colonoscopy due to occlusive cancer can be examined successfully with CTC using the low-pressure carbon dioxide colon insufflation system widely adopted for screening CTC[27-29]. CTC is known to be a safe procedure, particularly when it is performed using the low-pressure carbon dioxide insufflation, as the reported rates of overall procedure-related colonic perforations ranged from 0.009% to 0.06% and nearly all the perforated cases were associated with manual insufflation[3,30-32]. However, the data were largely from screening CTC practices or from patients who did not have colonic obstruction; and, in fact, there is no large data regarding the risk of colonic perforation of CTC performed for patients with an occlusive cancer. The majority of the reported cases of colonic perforations associated with CTC had underlying colonic lesions including inflammatory and/or obstructive lesions[31,33,34]. Also, according to a recent systematic review, large bowel obstruction is among the risk factors for colonic perforation following CTC[33]. Therefore, more careful attention while performing the procedure would be prudent.
Several studies have investigated the accuracy of CTC for detecting synchronous colonic lesions proximal to an occlusive cancer, and have demonstrated a high sensitivity of CTC for the detection of proximal synchronous cancers[28,35-39](Table 1). Most of these studies were preliminary studies that included a small number of patients[35-39]; however, one recent large study (the largest report thus far)[28] included 427 consecutive patients with stenosing colorectal cancer, of which 284 were ultimately analyzed to determine the accuracy of CTC. The results showed 100% and 88.6% sensitivities of CTC for detecting patients harboring synchronous colorectal cancer and advanced neoplasia (i.e., advanced adenoma[40] or cancer), respectively, in the proximal colon. As a result, the corresponding negative predictive values of CTC (i.e., the probability of the proximal colon being devoid of the lesions when CTC is negative) were 100% for proximal synchronous cancer and 97.4% for advanced neoplasia. Therefore, negative CTC findings in the proximal colon exclude the need for additional surgical procedures in the proximal colon with high confidence. These results are highly promising. Nevertheless, given the low prevalence of proximal synchronous cancers[22,23], future multi-institutional efforts aimed at accumulating additional data and evidence would be indicated.
| Patients with occlusive cancer | Sensitivity | Specificity | |||
| Target lesions | Per-patient | Per-lesion | |||
| Park et al[28] | 284 | Adenocarcinoma | 100% (6/6) | 100% (8/8) | 87.9 (181/206) |
| Advanced neoplasia1 | 88.6% (39/44) | 80% (52/65) | |||
| Fenlon et al[35] | 29 | Adenocarcinoma | 100% (2/2) | 100% (2/2) | NA |
| Neri et al[36] | 17 | Adenocarcinoma | 100% (3/3) | 100% (3/3) | NA |
| Coccetta et al[37] | 43 | Adenocarcinoma | 100% (1/1) | 100% (1/1) | NA |
| Galia et al[38] | 19 | Adenocarcinoma | 100% (2/2) | 100% (2/2) | NA |
| Kim et al[39] | 67 | Adenocarcinoma | 100% (3/3) | 100% (3/3) | 95 (NA) |
Another advantage of CTC to this particular group of patients is that it can serve as a one-stop examination for the proximal colonic evaluation as well as for overall pretreatment cancer staging of the abdomen and pelvis when performed with intravenous contrast enhancement. Contrast-enhanced CTC is essentially the same imaging method as the routine contrast-enhanced abdominopelvic CT used for abdominopelvic staging of colorectal cancer[41,42], except for the use of gaseous colonic distention in the former. Therefore, the two methods are expected to be similarly effective and accurate for tumor staging, although published data on the accuracy of contrast-enhanced CTC for general TNM staging of colorectal cancers are limited. According to several published studies, the accuracy of contrast-enhanced CTC for tumor staging is 83%-95% for T-staging, 80%-85% for N-staging, and 100% for M-staging[43-46].
Despite the high accuracy of CTC for detecting synchronous lesions in the colon proximal to an occlusive cancer, the clinical impact of CTC in the management of occlusive cancer patients remains a bit unclear. First, even if CTC accurately detects proximal colonic lesions, unless it can clearly tell which of the detected lesions should be removed by surgery rather than endoscopy after resection of the occlusive cancer, the patient management remains ambiguous. The distinction would be straightforward for small polyps (i.e., endoscopic removal) or large invasive advanced cancers (i.e., surgical excision). However, it is difficult for CTC to distinguish adenomas from relatively small medium-sized cancers confined within the colonic wall without pericolic extension or nodal metastasis[28]. Therefore, a certain degree of over-interpretation (i.e., overcalling noncancerous polyps as cancers) or under-interpretation (i.e., undercalling small cancers as noncancerous polyps) at CTC, which may result in unnecessarily extensive surgery or repeat colonic surgery, respectively, seems inevitable. Robust criteria for the selection of surgical removal versus postsurgical endoscopic resection for a proximal colonic lesion detected by CTC remain to be developed. One study[28] suggested a maximum lesion diameter of 15 mm or greater as the criterion for surgical removal, which yielded 87.5% sensitivity and 70% positive predictive value for proximal synchronous cancers. The need for specific characterization of the colonic lesions detected by CTC is a unique aspect of CTC performed in occlusive cancer patients. By contrast, the general screening/surveillance CTC is only concerned with detecting colonic lesions, as its key clinical role is to determine who should be sent for colonoscopy. Secondly, it is unclear if and how the adoption of CTC in the presurgical evaluation of occlusive colorectal cancer patients should affect the current postsurgical colonoscopic surveillance timeline. The current guidelines for the management of colorectal cancer (as proposed by The National Comprehensive Cancer Network, the American Cancer Society, and the US Multi-Society Task Force on Colorectal Cancer) stipulate that early postoperative follow-up colonoscopy to evaluate the proximal colon should be performed 3-6 mo after surgical removal of an occlusive cancer in addition to the routine colonoscopic surveillance approximately 1 year after surgery or perioperative clearance of the colon[41,42,47]. These “current” guidelines are largely based on the data and experience from the pre-CTC era. Given the higher accuracy of CTC compared with other methods, particularly the high sensitivity of CTC for detecting cancer that is approaching 100%[48], negative preoperative CTC findings in the proximal colon could potentially provide a confident clearance for the proximal colon and could potentially eliminate the need for early postoperative colonoscopy. If this notion is proven, it would help reduce redundancy and the costs of postsurgical colonic surveillance, and would also mean a substantial convenience factor for patients who are recuperating from major surgery. Further investigations in this area would therefore be worthwhile.
Patients with advanced colorectal cancer causing acute severe colonic obstruction require urgent decompression to avoid colonic perforation. Self-expandable metallic colonic stents are currently widely used in patients with acute severe colonic obstruction caused by colorectal cancer, as a bridging treatment to one-stage elective surgery[49,50]. In these cases, proximal colonic evaluation requiring passage through the metallic stent to find synchronous colonic lesions becomes an issue[51-53]. Colonoscopy involving passage through the stent can be performed safely without any major complications and a success rate of 88.9%-93.4% has been reported[51,52]. However, the extent of clinical application of this procedure is unknown. Among the concerns raised, long-term instrumental damage to a colonoscope caused by passing it through a metallic stent appears to be one important reason for the reluctance in performing colonoscopy under these conditions[54]. CTC could provide an alternative tool for this diagnostic task. According to one study[53], which included 50 consecutive patients who underwent CTC after metallic stent placement for acute severe cancer obstruction, CTC was performed adequately in 94% of the patients using the standard techniques used for screening or other indications and no procedure-related adverse events were reported. Although the diagnostic performance of CTC in this setting was not evaluated thoroughly because of the small number of patients analyzed, the preliminary results were promising. The per-patient and per-lesion sensitivities for lesions 6 mm or larger in diameter in the colon proximal to the stent were 90% and 85.7%, respectively, and CTC correctly identified two proximal synchronous cancers present in the study cohort[53]. One potential diagnostic pitfall noted in the study was some degree of lesion obscuration by colonic obstruction-related mural edema[53], which may need further clarification. Furthermore, a technical consideration is that an additional scout CT scan of the abdomen and pelvis using low-dose radiation prior to gaseous colonic distention is recommended in this group of patients to detect any clinically silent colonic perforation, given the relatively high risk of colonic perforation associated with the metallic stent placement procedure (3.8% according to one systematic review[50]). The scout scan would be a prudent step to avoid the risk of exacerbating a clinically silent perforation by inadvertently performing CTC.
Colorectal cancer is unique in that, unlike other gastrointestinal malignancies, timely second curative-intent treatment of the recurred/metastatic cancer that developed after the initial curative-intent treatment can improve the ultimate patient survival[55-57]. Therefore, preemptive (i.e., performed for all postsurgical patients regardless of their symptoms) surveillance for recurrent disease after curative-intent treatment of colorectal cancer is crucial in the management of this disease. Both colonic and extracolonic surveillance are important, as the recurrent disease may occur in any location. Most recurrences occur as distant extracolonic metastatic disease and, in the case of local or (peri-)anastomotic recurrence, more often than not without an intraluminal colonic component[58-60]. As a result, current postsurgical surveillance guidelines generally include a combination of clinical assessment, serum carcinoembryonic antigen measurement, colonoscopy, and contrast-enhanced CT[41,42,61]. Considering that contrast-enhanced abdominopelvic CT is already a standard postoperative surveillance examination[41,42], and that CTC can be readily added to the routine abdominopelvic CT in the form of contrast-enhanced CTC, which would effectively cover both the colorectum and extracolonic organs simultaneously, contrast-enhanced CTC may potentially represent an attractive stand-alone examination for combined colonic and extracolonic postoperative surveillance of colorectal cancer patients[62,63]. Adding the essential colonographic techniques (i.e., bowel preparation and colonic distention) to contrast-enhanced abdominopelvic CT would not incur much extra cost, another hospital visit, or other complexity in patient management.
At present, a relatively small amount of data regarding the use of CTC as a tool for post-cancer resection surveillance exists (Table 2), and most such research reports were feasibility studies in nature that only included a small number of patients[64-67]. On the other hand, one recent study[68] analyzed a large retrospective cohort of 742 consecutive patients who had no apparent clinical or laboratory evidence of recurrent disease after curative-intent colorectal cancer surgery and underwent contrast-enhanced CTC for postsurgical surveillance[68]. In the study, the per-patient sensitivity of CTC was 100% for metachronous or anastomotic recurrent cancers and 81.8% for advanced neoplasia. The corresponding negative predictive value of CTC was 100% for metachronous or anastomotic recurrent cancers and 99.1% for advanced neoplasia. The maximum referral rate for colonoscopy after CTC in this asymptomatic postsurgical population was 19%. These results imply that performing CTC as an adjunct to the routine postsurgical contrast-enhanced abdominopelvic CT could theoretically prevent surveillance colonoscopy in as much as approximately 80% of the patients (on an assumption that colonoscopy is to be performed at a similar time to CT) by confidently excluding those patients who would not need colonoscopy because they do not harbor advanced neoplasia or cancer. As the frequency and timing of surveillance colonoscopy and surveillance abdominopelvic CT do not always coincide in the real-world clinical setting, the actual benefit of contrast-enhanced CTC would be smaller. However, the study at least demonstrated that CTC could be a viable alternative to colonoscopy for postsurgical surveillance and may therefore help decrease the burden or redundancy of the colonoscopic surveillance.
| Patients | Sensitivity | Specificity | ||||
| n | Characteristics | Target lesions1 | Per-patient | Per-lesion | ||
| Amitai et al[64] | 29 | Routine surveillance | (Peri) anastomotic recurrence | 100% (2/2) | 100% (2/2) | NA |
| Metachronous polyps | 100% (NA) | 93% (28/30) | 71% (NA) | |||
| Fletcher et al[65] | 50 | Routine surveillance | (Peri) anastomotic recurrence | 100% (2/2) | NA | 94% (45/48) |
| Metachronous polyps ≥ 5 mm | 60% (3/5) | NA | 84% (38/45) | |||
| You et al[67] | 80 | Suspicion of recurrence | (Peri) anastomotic recurrence | 100% (51/51) | 100% (51/51) | 83% (24/29) |
| Kim et al[68] | 548 | Routine surveillance | Metachronous cancer and (peri) anastomotic recurrence | 100% (6/6) | 100% (7/7) | 93.1% (421/452) |
| Advanced neoplasia2 | 81.8% (18/22) | 80.8% (21/26) | ||||
| All adenomatous lesions3≥ 6 mm | 80% (52/65) | 78.5% (62/79) | ||||
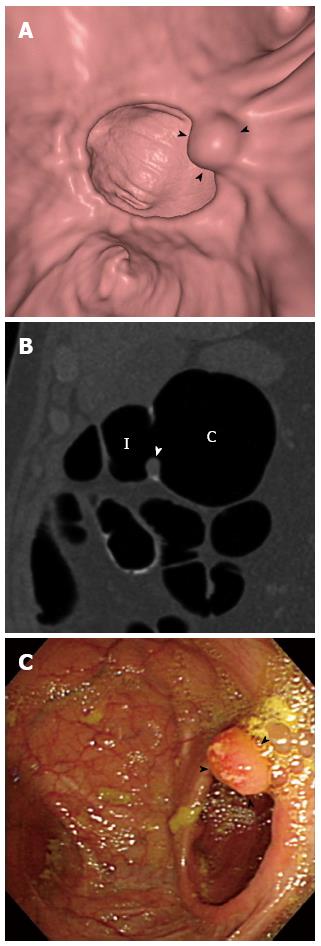
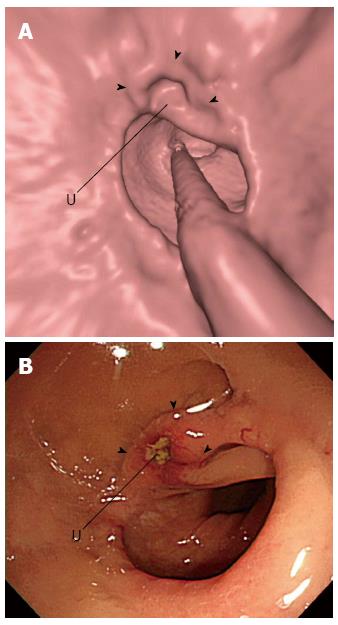
Although CTC may have diagnostically acceptable accuracy for postsurgical colonic surveillance, how it may fit into the current colonoscopy-based colonic surveillance practice remains to be determined. The current guidelines for colonic surveillance recommend colonoscopy at 1 year after the curative-intent surgery or after perioperative colonoscopic clearance of synchronous lesions, then in 3 years if negative at 1 year, and every 5 years if negative at the prior colonoscopy[41,42,47]. However, as revealed in a recent study[69], postsurgical colonoscopies are being performed more frequently than recommended by the guidelines at many institutions. Considering the relatively higher rates of metachronous cancers in the early postsurgical period[47], the use of colonoscopy for surveillance during the early postsurgical period, such as at 1 year, and CTC at later times may be appropriate. In addition, because CTC is less sensitive for small and subtle lesions than colonoscopy, while colonoscopy has a greater amount of blind areas compared with CTC[70], the alternating use of colonoscopy and CTC for postsurgical surveillance may be worth investigating, as it could capitalize on their complementary strengths and may contribute to improved patient survival. Another issue that may need to be addressed for the successful implementation of CTC in post-cancer resection surveillance is the reader familiarity with colonic lesions that are unique to the postoperative colon and are unencountered in general screening practice, including anastomotic inflammatory polyps (Figure 2) and anastomotic recurrences (Figure 3). Inflammatory polyps are by far the most common type of polypoid lesion occurring in the anastomosis that do not require treatment and typically manifest as well-circumscribed discrete 5- to 15-mm polyps located in the anastomotic line[62,71]. Anastomotic recurrent tumors may present as friable mucosa, irregular mucosa with shallow ulceration, sessile-to-flat infiltrative lesions, or luminal stenosis instead of showing mass-like or polypoid appearance, as they do not develop through the polypoid growth of the adenoma-carcinoma sequence[62,72].
In summary, CT colonography has important current and potential roles in the management of patients who have been diagnosed with colorectal cancer. It is technically robust and the most practical method for the evaluation of the colon proximal to an occlusive cancer, either before or after metallic stent placement. CT colonography may also serve as an effective stand-alone tool for post-cancer resection surveillance of both the colorectum and extracolonic organs.
P- Reviewers: Kita H, Triantafyllou K S- Editor: Gou SX L- Editor: A E- Editor: Wang CH
| 1. | Lin OS. Computed tomographic colonography: hope or hype? World J Gastroenterol. 2010;16:915-920. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 2. | Park SH, Yee J, Kim SH, Kim YH. Fundamental elements for successful performance of CT colonography (virtual colonoscopy). Korean J Radiol. 2007;8:264-275. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 26] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 3. | Pickhardt PJ. Incidence of colonic perforation at CT colonography: review of existing data and implications for screening of asymptomatic adults. Radiology. 2006;239:313-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 141] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Chang KJ, Soto JA. Computed tomographic colonography: image display methods. Atlas of virtual colonoscopy. 2nd ed. New York: Springer 2011; 111-132. |
| 5. | Dachman AH, Lefere P, Gryspeerdt S, Morin M. CT colonography: visualization methods, interpretation, and pitfalls. Radiol Clin North Am. 2007;45:347-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 6. | Graser A, Stieber P, Nagel D, Schäfer C, Horst D, Becker CR, Nikolaou K, Lottes A, Geisbüsch S, Kramer H. Comparison of CT colonography, colonoscopy, sigmoidoscopy and faecal occult blood tests for the detection of advanced adenoma in an average risk population. Gut. 2009;58:241-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 270] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 7. | Regge D, Laudi C, Galatola G, Della Monica P, Bonelli L, Angelelli G, Asnaghi R, Barbaro B, Bartolozzi C, Bielen D. Diagnostic accuracy of computed tomographic colonography for the detection of advanced neoplasia in individuals at increased risk of colorectal cancer. JAMA. 2009;301:2453-2461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 148] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 8. | Atkin W, Dadswell E, Wooldrage K, Kralj-Hans I, von Wagner C, Edwards R, Yao G, Kay C, Burling D, Faiz O. Computed tomographic colonography versus colonoscopy for investigation of patients with symptoms suggestive of colorectal cancer (SIGGAR): a multicentre randomised trial. Lancet. 2013;381:1194-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 178] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 9. | Halligan S, Wooldrage K, Dadswell E, Kralj-Hans I, von Wagner C, Edwards R, Yao G, Kay C, Burling D, Faiz O. Computed tomographic colonography versus barium enema for diagnosis of colorectal cancer or large polyps in symptomatic patients (SIGGAR): a multicentre randomised trial. Lancet. 2013;381:1185-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 118] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 10. | Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, Menias CO, Siewert B, Cheema JI, Obregon RG. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 706] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 11. | Kim DH, Pickhardt PJ, Taylor AJ, Leung WK, Winter TC, Hinshaw JL, Gopal DV, Reichelderfer M, Hsu RH, Pfau PR. CT colonography versus colonoscopy for the detection of advanced neoplasia. N Engl J Med. 2007;357:1403-1412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 463] [Article Influence: 25.7] [Reference Citation Analysis (0)] |
| 12. | Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, Wong RK, Nugent PA, Mysliwiec PA, Schindler WR. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1495] [Cited by in RCA: 1284] [Article Influence: 58.4] [Reference Citation Analysis (0)] |
| 13. | Levin B, Lieberman DA, McFarland B, Smith RA, Brooks D, Andrews KS, Dash C, Giardiello FM, Glick S, Levin TR. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin. 2008;58:130-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1169] [Cited by in RCA: 1201] [Article Influence: 70.6] [Reference Citation Analysis (0)] |
| 14. | Lee BI, Hong SP, Kim SE, Kim SH, Kim HS, Hong SN, Yang DH, Shin SJ, Lee SH, Park DI. Korean guidelines for colorectal cancer screening and polyp detection. Clin Endosc. 2012;45:25-43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 15. | Mang T, Carrascosa P, Lefere P, Chawla T, Cadi M, Rogalla P, Morrin M, Sosna J, Laghi A, Iinuma G. Global Implementation of Computed Tomography Colonography. Atlas of Virtual Colonoscopy: Springer 2011; 9-53. |
| 16. | Available from: http://www.vcreimbursement.com. |
| 17. | McFarland EG, Fletcher JG, Pickhardt P, Dachman A, Yee J, McCollough CH, Macari M, Knechtges P, Zalis M, Barish M. ACR Colon Cancer Committee white paper: status of CT colonography 2009. J Am Coll Radiol. 2009;6:756-772.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Mitka M. Virtual colonoscopy dealt setback with rejection for coverage by Medicare. JAMA. 2009;301:1327-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Duszak R, Kim DH, Pickhardt PJ. Expanding utilization and regional coverage of diagnostic CT colonography: early Medicare claims experience. J Am Coll Radiol. 2011;8:235-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Yee J, Keysor KJ, Kim DH. The time has arrived for national reimbursement of screening CT colonography. AJR Am J Roentgenol. 2013;201:73-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 21. | Philip AK, Lubner MG, Harms B. Computed tomographic colonography. Surg Clin North Am. 2011;91:127-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Mulder SA, Kranse R, Damhuis RA, de Wilt JH, Ouwendijk RJ, Kuipers EJ, van Leerdam ME. Prevalence and prognosis of synchronous colorectal cancer: a Dutch population-based study. Cancer Epidemiol. 2011;35:442-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 23. | Adloff M, Arnaud JP, Bergamaschi R, Schloegel M. Synchronous carcinoma of the colon and rectum: prognostic and therapeutic implications. Am J Surg. 1989;157:299-302. [PubMed] |
| 24. | Chong A, Shah JN, Levine MS, Rubesin SE, Laufer I, Ginsberg GG, Long WB, Kochman ML. Diagnostic yield of barium enema examination after incomplete colonoscopy. Radiology. 2002;223:620-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 25. | Winawer SJ, Stewart ET, Zauber AG, Bond JH, Ansel H, Waye JD, Hall D, Hamlin JA, Schapiro M, O’Brien MJ. A comparison of colonoscopy and double-contrast barium enema for surveillance after polypectomy. National Polyp Study Work Group. N Engl J Med. 2000;342:1766-1772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 382] [Cited by in RCA: 327] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 26. | Kahi CJ, Haggstrom DA. In: Waye JD, Rex DK, Williams CB, editors. Colonoscopy after colorectal cancer resection. 2nd ed. Oxford, UK: Wiley-Blackwell 2009; 730-745. |
| 27. | Kim SY, Park SH, Choi EK, Lee SS, Lee KH, Kim JC, Yu CS, Kim HC, Kim AY, Ha HK. Automated carbon dioxide insufflation for CT colonography: effectiveness of colonic distention in cancer patients with severe luminal narrowing. AJR Am J Roentgenol. 2008;190:698-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 28. | Park SH, Lee JH, Lee SS, Kim JC, Yu CS, Kim HC, Ye BD, Kim MJ, Kim AY, Ha HK. CT colonography for detection and characterisation of synchronous proximal colonic lesions in patients with stenosing colorectal cancer. Gut. 2012;61:1716-1722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 29. | Flor N, Mezzanzanica M, Rigamonti P, Rocco EG, Bosari S, Ceretti AP, Soldi S, Peri M, Sardanelli F, Cornalba GP. Contrast-enhanced computed tomography colonography in preoperative distinction between T1-T2 and T3-T4 staging of colon cancer. Acad Radiol. 2013;20:590-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Burling D, Halligan S, Slater A, Noakes MJ, Taylor SA. Potentially serious adverse events at CT colonography in symptomatic patients: national survey of the United Kingdom. Radiology. 2006;239:464-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 135] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 31. | Sosna J, Blachar A, Amitai M, Barmeir E, Peled N, Goldberg SN, Bar-Ziv J. Colonic perforation at CT colonography: assessment of risk in a multicenter large cohort. Radiology. 2006;239:457-463. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 102] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 32. | Whitlock EP, Lin J, Liles E, Beil T, Fu R, O’Connor E, Thompson RN, Cardenas T. Screening for Colorectal Cancer: An Updated Systematic Review. Rockville (MD). 2008;. |
| 33. | Atalla MA, Rozen WM, Niewiadomski OD, Croxford MA, Cheung W, Ho YH. Risk factors for colonic perforation after screening computed tomographic colonography: a multicentre analysis and review of the literature. J Med Screen. 2010;17:99-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 34. | Berrington de Gonzalez A, Kim KP, Yee J. CT colonography: perforation rates and potential radiation risks. Gastrointest Endosc Clin N Am. 2010;20:279-291. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 35. | Fenlon HM, McAneny DB, Nunes DP, Clarke PD, Ferrucci JT. Occlusive colon carcinoma: virtual colonoscopy in the preoperative evaluation of the proximal colon. Radiology. 1999;210:423-428. [PubMed] |
| 36. | Neri E, Giusti P, Battolla L, Vagli P, Boraschi P, Lencioni R, Caramella D, Bartolozzi C. Colorectal cancer: role of CT colonography in preoperative evaluation after incomplete colonoscopy. Radiology. 2002;223:615-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 125] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 37. | Coccetta M, Migliaccio C, La Mura F, Farinella E, Galanou I, Delmonaco P, Spizzirri A, Napolitano V, Cattorini L, Milani D. Virtual colonoscopy in stenosing colorectal cancer. Ann Surg Innov Res. 2009;3:11. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 38. | Galia M, Midiri M, Carcione A, Cusmà S, Bartolotta TV, Angileri T, De Maria M, Lagalla R. Usefulness of CT colonography in the preoperative evaluation of patients with distal occlusive colorectal carcinoma. Radiol Med. 2001;101:235-242. [PubMed] |
| 39. | Kim JH, Kim WH, Kim TI, Kim NK, Lee KY, Kim MJ, Kim KW. Incomplete colonoscopy in patients with occlusive colorectal cancer: usefulness of CT colonography according to tumor location. Yonsei Med J. 2007;48:934-941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 40. | Winawer SJ, Zauber AG. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am. 2002;12:1-9, v. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 253] [Cited by in RCA: 259] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 41. | Benson AB, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fenton MJ. Localized colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2013;11:519-528. [PubMed] |
| 42. | Benson AB, Bekaii-Saab T, Chan E, Chen YJ, Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fuchs CS. Rectal cancer. J Natl Compr Canc Netw. 2012;10:1528-1564. [PubMed] |
| 43. | Chung DJ, Huh KC, Choi WJ, Kim JK. CT colonography using 16-MDCT in the evaluation of colorectal cancer. AJR Am J Roentgenol. 2005;184:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 59] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 44. | Filippone A, Ambrosini R, Fuschi M, Marinelli T, Genovesi D, Bonomo L. Preoperative T and N staging of colorectal cancer: accuracy of contrast-enhanced multi-detector row CT colonography--initial experience. Radiology. 2004;231:83-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 45. | Jin KN, Lee JM, Kim SH, Shin KS, Lee JY, Han JK, Choi BI. The diagnostic value of multiplanar reconstruction on MDCT colonography for the preoperative staging of colorectal cancer. Eur Radiol. 2006;16:2284-2291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 46. | Utano K, Endo K, Togashi K, Sasaki J, Kawamura HJ, Horie H, Nakamura Y, Konishi F, Sugimoto H. Preoperative T staging of colorectal cancer by CT colonography. Dis Colon Rectum. 2008;51:875-881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 47. | Rex DK, Kahi CJ, Levin B, Smith RA, Bond JH, Brooks D, Burt RW, Byers T, Fletcher RH, Hyman N. Guidelines for colonoscopy surveillance after cancer resection: a consensus update by the American Cancer Society and US Multi-Society Task Force on Colorectal Cancer. CA Cancer J Clin. 2006;56:160-167; quiz 185-186;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 48. | Pickhardt PJ, Hassan C, Halligan S, Marmo R. Colorectal cancer: CT colonography and colonoscopy for detection--systematic review and meta-analysis. Radiology. 2011;259:393-405. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 356] [Cited by in RCA: 303] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 49. | Farrell JJ. Preoperative colonic stenting: how, when and why? Curr Opin Gastroenterol. 2007;23:544-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 50. | Sebastian S, Johnston S, Geoghegan T, Torreggiani W, Buckley M. Pooled analysis of the efficacy and safety of self-expanding metal stenting in malignant colorectal obstruction. Am J Gastroenterol. 2004;99:2051-2057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 504] [Cited by in RCA: 411] [Article Influence: 19.6] [Reference Citation Analysis (1)] |
| 51. | Vitale MA, Villotti G, d’Alba L, Frontespezi S, Iacopini F, Iacopini G. Preoperative colonoscopy after self-expandable metallic stent placement in patients with acute neoplastic colon obstruction. Gastrointest Endosc. 2006;63:814-819. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 52. | Lim SG, Lee KJ, Suh KW, Oh SY, Kim SS, Yoo JH, Wi JO. Preoperative colonoscopy for detection of synchronous neoplasms after insertion of self-expandable metal stents in occlusive colorectal cancer: comparison of covered and uncovered stents. Gut Liver. 2013;7:311-316. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 53. | Cha EY, Park SH, Lee SS, Kim JC, Yu CS, Lim SB, Yoon SN, Shin YM, Kim AY, Ha HK. CT colonography after metallic stent placement for acute malignant colonic obstruction. Radiology. 2010;254:774-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 54. | Bhasin DK, Rana SS. Malignant colorectal obstruction: looking for synchronous lesions with the scope through a metal stent...! Gastrointest Endosc. 2006;63:820-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 55. | Rodríguez-Moranta F, Saló J, Arcusa A, Boadas J, Piñol V, Bessa X, Batiste-Alentorn E, Lacy AM, Delgado S, Maurel J. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J Clin Oncol. 2006;24:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 205] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 56. | Rosen M, Chan L, Beart RW, Vukasin P, Anthone G. Follow-up of colorectal cancer: a meta-analysis. Dis Colon Rectum. 1998;41:1116-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 125] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 57. | Jeffery M, Hickey BE, Hider PN. Follow-up strategies for patients treated for non-metastatic colorectal cancer. Cochrane Database Syst Rev. 2007;CD002200. [PubMed] |
| 58. | Manfredi S, Bouvier AM, Lepage C, Hatem C, Dancourt V, Faivre J. Incidence and patterns of recurrence after resection for cure of colonic cancer in a well defined population. Br J Surg. 2006;93:1115-1122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 213] [Cited by in RCA: 235] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 59. | Renehan AG, Egger M, Saunders MP, O’Dwyer ST. Impact on survival of intensive follow up after curative resection for colorectal cancer: systematic review and meta-analysis of randomised trials. BMJ. 2002;324:813. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 469] [Cited by in RCA: 449] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 60. | Tsikitis VL, Malireddy K, Green EA, Christensen B, Whelan R, Hyder J, Marcello P, Larach S, Lauter D, Sargent DJ. Postoperative Surveillance Recommendations for Early Stage Colon Cancer Based on Results From the Clinical Outcomes of Surgical Therapy Trial. J Clin Oncol. 2009;27:3671-3676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 61. | Desch CE, Benson AB, Somerfield MR, Flynn PJ, Krause C, Loprinzi CL, Minsky BD, Pfister DG, Virgo KS, Petrelli NJ. Colorectal cancer surveillance: 2005 update of an American Society of Clinical Oncology practice guideline. J Clin Oncol. 2005;23:8512-8519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 391] [Cited by in RCA: 381] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 62. | Choi YJ, Park SH, Lee SS, Choi EK, Yu CS, Kim HC, Kim JC. CT colonography for follow-up after surgery for colorectal cancer. AJR Am J Roentgenol. 2007;189:283-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Almond LM, Bowley DM, Karandikar SS, Roy-Choudhury SH. Role of CT colonography in symptomatic assessment, surveillance and screening. Int J Colorectal Dis. 2011;26:959-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 64. | Amitai MM, Fidder H, Avidan B, Portnoy O, Apter S, Konen E, Hertz M. Contrast-enhanced CT colonography with 64-slice MDCT compared to endoscopic colonoscopy in the follow-up of patients after colorectal cancer resection. Clin Imaging. 2009;33:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 65. | Fletcher JG, Johnson CD, Krueger WR, Ahlquist DA, Nelson H, Ilstrup D, Harmsen WS, Corcoran KE. Contrast-enhanced CT colonography in recurrent colorectal carcinoma: feasibility of simultaneous evaluation for metastatic disease, local recurrence, and metachronous neoplasia in colorectal carcinoma. AJR Am J Roentgenol. 2002;178:283-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 66. | Laghi A, Iannaccone R, Bria E, Carbone I, Trasatti L, Piacentini F, Lauro S, Vecchione A, Passariello R. Contrast-enhanced computed tomographic colonography in the follow-up of colorectal cancer patients: a feasibility study. Eur Radiol. 2003;13:883-889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 67. | You YT, Chang Chien CR, Wang JY, Ng KK, Chen JS, Tang R, Chiang JM, Yeh CY, Hsieh PS. Evaluation of contrast-enhanced computed tomographic colonography in detection of local recurrent colorectal cancer. World J Gastroenterol. 2006;12:123-126. [PubMed] |
| 68. | Kim HJ, Park SH, Pickhardt PJ, Yoon SN, Lee SS, Yee J, Kim DH, Kim AY, Kim JC, Yu CS. CT colonography for combined colonic and extracolonic surveillance after curative resection of colorectal cancer. Radiology. 2010;257:697-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 69. | Singh A, Kuo YF, Goodwin JS. Many patients who undergo surgery for colorectal cancer receive surveillance colonoscopies earlier than recommended by guidelines. Clin Gastroenterol Hepatol. 2013;11:65-72.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Pickhardt PJ, Taylor AJ, Gopal DV. Surface visualization at 3D endoluminal CT colonography: degree of coverage and implications for polyp detection. Gastroenterology. 2006;130:1582-1587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 71. | Weinstock LB, Shatz BA. Endoscopic abnormalities of the anastomosis following resection of colonic neoplasm. Gastrointest Endosc. 1994;40:558-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 72. | Rossini FP, Waye JD. Colonoscopy after colon cancer resection. Colonoscopy: principles and practice. 1st ed. Malden, MA: Blackwell Publishing 2003; 468-477. |