Published online Feb 21, 2014. doi: 10.3748/wjg.v20.i7.1777
Revised: November 7, 2013
Accepted: November 28, 2013
Published online: February 21, 2014
Processing time: 150 Days and 21.1 Hours
After the rapid acceptance of laparoscopy to manage multiple benign diseases arising from gastrointestinal districts, some surgeons started to treat malignancies by the same way. However, if the limits of laparoscopy for benign diseases are mainly represented by technical issues, oncologic outcomes remain the foundation of any procedures to cure malignancies. Cancerous patients represent an important group with peculiar aspects including reduced survival expectancy, worsened quality of life due to surgery itself and adjuvant therapies, and challenging psychological impact. All these issues could, potentially, receive a better management with a laparoscopic surgical approach. In order to confirm such aspects, similarly to testing the newest weapons (surgical or pharmacologic) against cancer, long-term follow-up is always recommendable to assess the real benefits in terms of overall survival, cancer-free survival and quality of life. Furthermore, it seems of crucial importance that surgeons will be correctly trained in specific oncologic principles of surgical oncology as well as in modern miniinvasive technologies. Therefore, laparoscopic treatment of gastrointestinal malignancies requires more caution and deep analysis of published evidences, as compared to those achieved for inflammatory bowel diseases, gastroesophageal reflux disease or diverticular disease. This review tries to examine the evidence available to date for the use of laparoscopy and robotics in malignancies arising from the gastrointestinal district.
Core tip: Laparoscopic treatment of gastrointestinal malignancies requires more caution and deep analysis of published evidences, as compared to those achieved for benign diseases. Oncologic outcomes remain the foundation of any procedures to cure malignancies, hence a long-term follow-up is always recommendable in order to asses overall survival, cancer-free survival and quality of life.
- Citation: Bencini L, Bernini M, Farsi M. Laparoscopic approach to gastrointestinal malignancies: Toward the future with caution. World J Gastroenterol 2014; 20(7): 1777-1789
- URL: https://www.wjgnet.com/1007-9327/full/v20/i7/1777.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i7.1777
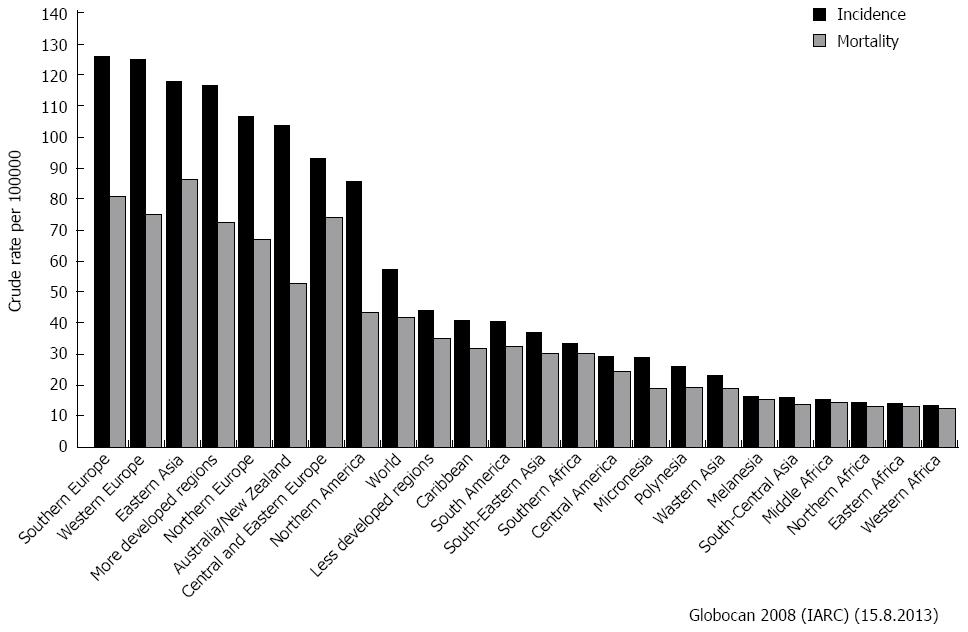
From an epidemiologic point of view, gastrointestinal malignancies represent a vast share of both incidence and mortality for cancer worldwide (Figure 1)[1]. Therefore, the widespread adoption of the minimally invasive (endoscopic, laparoscopic and robotic) approach to cure these malignancies was an attractive and valuable consequence, and many surgeons reported tangible benefits of this technique in terms of morbidity, return to normal activities and mortality.
Nevertheless, the popularity of miniinvasive surgery (MIS) among both surgeons and patients, mixed with some industrial pressure, could have also played a role in the worldwide diffusion and explosion of new technologies.
The proven advantages of laparoscopy are mainly represented by better short-term outcomes including fewer wound complications, less pulmonary impairment, reduction of postoperative pain, shorter length of postoperative stay and, eventually, better cosmetic result. Moreover, there is a well-recognized role of laparoscopy in decreasing the pro-inflammatory and immunologic response to surgery that are, hypothetically, related to an improved immediate or even long-term oncologic result[2]. For all these issues, the laparoscopic technique in oncology seem to be very promising[3].
However, if the limits of MIS are mainly represented by technical issues and patients conformation for benign diseases, oncologic outcomes remain the foundation of any procedure to cure malignancies. Mostly, any laparoscopic procedure should follow the same standard of care of open surgery, including oncologic principles, such as wide margins of resection and extended lymphadenectomy. Such prerequisites often require very good skills that are generally limited to high volume centers with subspecialized teams.
Historically, the first concerns about MIS for cancer were represented by the possibility of port-site metastasis and lower number of lymphnodes retrieved, but none of these issue has been confirmed successively[4,5]. Moreover, advanced laparoscopy for gastrointestinal malignancy requires the whole specimen extraction (often with regional nodes) through a minilaparotomy, thus flawing one of the strongest advantages of MIS represented by short incisions.
Most of the reviewers concluded that, although slowly, colorectal oncologic resections had been recognized to have a non-inferior cancer-free survival and superior short-term results, while the laparoscopic approach to gastric and solid organs malignancies will require further studies with longer follow-up[3,6].
The role of laparoscopy is known to be important in staging gastrointestinal malignancies, limiting the number of unnecessary laparotomies for carcinosis or occult metastasis. Many palliative procedures such as gastrointestinal by-pass, gastrostomy, jejunostomy and colostomy or radiofrequency ablations are easily carried out with MIS[6].
The pure endoscopic treatments of both esophagogastric and colorectal small malignancies (precancerous or T1 lesions) are well accepted among cancer professionals for being as safe and as curative as traditional resections, and they will not be considered further in this review. Therefore, we focused on the full laparoscopic (and robotic) treatment of gastrointestinal malignancies, including those arising from the esophagus, stomach, liver and biliary system, pancreas, small bowel and colorectum. If not otherwise specified, malignancies are intended to be carcinomas: other histopathological subtypes, such as neuroendocrine or sarcomas, will not be considered.
A Pubmed, Embase and Cochrane databases bibliography search was conducted until September 2013, including important cross-matched manual references. References to historical reports and older articles were limited to the minimum. A particular attention was reserved to data arising from randomized controlled clinical trials (RCTs) (or meta-analyses) with long-term follow-up. Oncologic results will be considered of utmost interest, in order to asses overall survival, cancer-free survivals and quality of life, rather than feasibility of the procedure itself and short-term outcomes.
The three-field (Mc Keown procedure) has been the treatment of choice for esophageal cancer for many years. This procedure, as well as the so-called Ivor-Lewis (two-field esophagectomy with esophagogastric intrathoracic anastomosis) and the Orringer procedure (transhiatal esophagectomy) are all feasible by laparoscopy (thoracoscopy) or hybrid (with open surgery combined) technique[7]. MIS for esophageal cancer has spread worldwide, reducing the significative perioperative complications (mainly respiratory). However, many debates still exist on the real efficacy and cost-effectiveness of minimally invasive esophagectomy (MIE). If these complex operations should be performed by the open traditional approaches or carried out by the laparoscopic and thoracoscopic route is still far to be demonstrated by the surgical community[2].
Several technical variables, such as the role of laparoscopic, thoracoscopic or combined steps, the usefulness of the prone or supine position, the choice of stapled or hand-sawn anastomosis and the route of stapled suture are under study. Last, the general poor quality of studies published leads to a great caution, when dealing with outcomes and oncologic results[8]. All of these issues contribute to jeopardize the surgical results and perioperative complications data reporting. Moreover, it should be considered how technical demanding this kind of surgery is, even in the traditional open way, with very few centers having sufficient case-load to gain adequate specific proficiency.
A single-center review[7] of more than 1000 patients (thoraco-laparoscopic McKeown and Ivor-Lewis operations compared) reported global excellent results, with a morbidity and mortality rate of less than 2% and 1%, respectively; with the best approach being the thoraco-laparoscopic Ivor-Lewis. A well-conducted review on MIE concluded that both laparoscopy and thoracoscopy are at least comparable to open surgery in terms of outcomes for non-locally advanced cancers, but the open transthoracic route is superior when considering field exposure[9].
The only prospective, multicentric RCT including few patients[10], and one large retrospective cohort study also confirmed the superiority of MIE in terms of postoperative pulmonary complications (13% in the thoraco-laparoscopic MIE, 38% in the thoracoscopic MIE, and 39% in the open group)[11]. Another ongoing trial was designed to evaluate the benefits of laparoscopic gastric mobilization during Ivor-Lewis intervention in terms of postoperative complications[12]. Moreover, a recent review failed to find any important differences between the two classic stapled anastomosis techniques (transoral anvil introduction and transthoracic) during Ivor-Lewis esophagectomy for cancer[13].
If many review articles report how MIE statistically decreases blood loss, length of stay, and perioperative morbidity at the price of increased operative time and costs, large-scala multicentric trials are still lacking, and few studies had long-term follow-up[14]. Hanna et al[15] selected thirty of the largest and best designed trials concerning MIE for cancer (including only 1 RCT). The author concluded that in most studies a suboptimal lymphadenectomy was carried out, with an average number of nodes retrieved below the standard (over 23), while no homogeneous complications reporting was available. However, the final oncologic outcomes for each stage (disease-free survival and overall survival) were comparable to those achieved by the open traditional surgery.
Lastly, robotic-assisted MIE was also employed in the treatment of esophago-gastric malignancies, but very few studies, even of poor quality, failed to demonstrate real advantages of this method as compared to open surgery[16,17]. A monocentric trial targeted to robotic MIE started recently[18].
In conclusions, due to the relative low frequency of esophageal cancer (especially in Western countries), the technical difficulties, the debated approach (two-field, three-field, transhiatal) and lack of literature evidence, the MIE, although promising, should be reserved to specialized centers within controlled trials.
The standard of care is open gastric resection with complete D2 lymphadenectomy for curable gastric cancer in both Western and Eastern countries, although a debate lasting decades on the extent of lymphadenectomy has been carried on. However, some endoscopic techniques (such as submucosal dissection) are recognized to be appropriate for selected patients with T1 cancers[2]. More controversies still persist regarding laparoscopic gastric resection (LGR) and sentinel node mapping, in those patients who are unfit for endoscopic resection or who have more advanced tumors.
Almost every gastric procedure is feasible by the laparoscopic route, including distal and the more challenging total gastrectomy with intracorporeal anastomosis[19,20] and formal lymphadenectomy[21], but also gastric resections and sentinel node sampling are recommended in selected cases.
The limit of the widespread adoption of the LGR is represented by the technical difficulties (mainly anastomosis) and the oncologic safety. Indeed, the standard D2 lymphadectomy, although feasible with few complications[22,23] is technically demanding, but mandatory for all advanced tumors.
Several review articles and meta-analysis reported that laparoscopy was a safe alternative, if not superior (perioperative outcomes), to open surgery for the treatment of early and advanced gastric cancer[24-30], but data on long-term survival, quality of life and cost effectiveness are still lacking[31,32].
One of the most updated review, including only comparative trials with a pooled cohort of more than 1000 patients[33], and another[34], that included 8 RCTs (more than 700 patients) reached the same conclusions. LGR is better or comparable in the early perioperative results with similar long-term outcomes respect to open surgery, although at the price of longer duration of surgery and technical difficulties. Other two meta-analysis by Wang et al[35] and Chen et al[36] reached the same conclusions in terms of oncologic effectiveness (node dissection) and outcomes. Many benefits of LGR are also confirmed in elderly people suffering for comorbidities according an enormous Chinese database[37].
Large Asian trials with longer follow-up are still ongoing, and only one European study reported data on 10-years follow-up[38]. To the present, one recent RCT (KLASS trial) reported early results: the authors confirmed equivalent outcomes of laparoscopic and open approach to gastrectomy for cancer[39].
A crucial point of concern is represented by the steep learning curve, although a paper reported encouraging results of LGR initiated by experienced surgeons in open gastrectomy and laparoscopy who received adequate training[40].
Most of the papers on LGR come from Eastern countries due to the high volumes of disease, high rates of early cancers and perhaps less diffusion of obesity that can obstacle laparoscopy. Therefore, the reported proportions of LGR rises to more than 20% in Japan in a recent review article[41]. However, some good results are also reported from many Western countries including Europe[42,43] and an international panel published some guidelines for the introduction and diffusion of the technique[44].
More recently, the introduction of the robotic approach to perform very complex operation, including gastric surgery, seems to be promising in order to reduce some of the technical difficulties of laparoscopy[41]. However, very few rigorous studies were published on robotic approach for gastric cancer and a recent meta-analysis[45] ended up selecting only 3 RCTs comparing robotic and laparoscopy. The pooled results showed no significant differences between the two approaches in terms of complications, mortality, conversion, length of stay and number of nodes retrieved. On the other hand, blood loss resulted inferior by robotics, at the price of an increased operative time and costs. If laparoscopic treatment of gastric cancer is still debated, this is even more for robotics, especially in terms of real benefits for patients.
Recent systematic review and meta-analysis of few retrospective comparative trials seem to confirm superiority of LGR as compared to open surgery also when dealing with gastrointestinal stromal tumours (GISTs)[46,47].
Since the advent of advanced laparoscopic techniques and availability of efficacious transection devices, many authors reported the feasibility of liver resections by the key-hole approach, both for malignancies and benign disease. Some retrospective and review studies (including very few comparative trials) provided relative evidence to support further development of case-load and research, to assess safety of laparoscopic hepatectomy for cancer patients or, if any, superiority as compared to standard surgery[48-50].
In 2007, the most acknowledged hepatobiliary surgeons worldwide met in Luisville (United States) to find an international common position on laparoscopic liver surgery (LLS): although few relevant data was available, the experts concluded that this kind of surgery (or hybrid technique, including hand-assisting) is safe and effective, in the hand of trained surgeons and under the control of societies and government. The preferred indications (despite for malignancy) were represented by solitary lesions of less than 5 cm in maximum diameter located in segments 2 to 6[51]. On the other hand, many surgeons began LLS dealing with benign diseases involving left lateral segments[52], while others brought the indications toward upper limits[53]. An international multi-institutional review article proposed the laparoscopic approach to left-sided hepatectomies as the future gold-standard of care[54].
LLS for cancer [including both hepatocellular carcinoma (HCC) and colorectal metastasis (CRM)] seems to offer oncologic results similar to those of laparotomy[55]. Excellent results were also achieved form 3 specialized European centers with large experience in HCC[56].
Laparoscopy seems to add also some benefits in terms of reducing early complications in the subset of patients affected by HCC and cirrhosis[57]. In a case-matched analysis published by Lee et al[58] LLS for HCC showed similar long-term outcomes but some early clinical advantages (complications and hospital stay) as compared to open surgery. Feasibility, less morbidity and shorter hospital stay were also found in patients after hepatectomies carried out for CRM[59,60].
Another very large (300 patients, 103 cancerous) single-center case-matched experience form Chicago (United States)[61] concluded that miniinvasive hepatectomy (including major resections) compared favourably with contemporaneous controls operated by the open approach without any oncological detriment. Positive parameters included blood loss, transfusion requirement, overall complications, postoperative stay and, surprisingly, operative time.
In the most recent and rigorous review of available studies, carried out by Rao et al[62] for the Cochrane Library, the author reported that no conclusion can be drawn on the benefits or harm of laparoscopy versus open technique for liver resection. These unsatisfactory data are consequence of lacking of any published RCT that met strong scientific criteria, although some are still ongoing.
R0 resection represents the main goal of treatment when dealing with hilar cholangiocarcinoma (Klatskin tumor), gallbladder cancer or extrahepatic bile duct cancer. Regional lymphadenectomy should be also performed in order to reduce recurrences[63,64]. Therefore, the laparoscopic approach to hilar structures is very challenging, even for a skilled laparoscopist, although MIS is highly accepted to confirm resectability and avoid unnecessary laparotomies. Some recent retrospective multicentric studies reported encouraging and oncologically acceptable laparoscopic procedures for hilar and gallbladder malignancies, but in the hands of very experienced surgeons working in highly subspecialized surgical units[65].
With the widespread adoption of laparoscopic cholecystectomy, it seems that an increased number of incidental gallbladder cancer could be diagnosticated nowadays. However, no difference in survival was demonstrated, if the surgeon decides to perform a more aggressive resection immediately of during a second look intervention[66,67]. Theoretically, this fact leads to correctly plan the adequate operation and to reach maximum oncologic results by both open delayed resection or immediate laparoscopy. Nevertheless, the experiences of laparoscopic second look resections and lymphadenectomy for gallbladder cancer are almost anecdotical[68]. Some authors reported initial experiences with the use of single-port laparoscopic technique for specific group of selected patients with malignancy and liver dysfunction[69].
Robotics could play a role in development of minimally invasive techniques for hepato-biliary malignancies due to easier dissection in deep and narrow spaces and for the possibility of knot-tying of vascular structures. Good short-term results were reported for robotic-assisted liver resections for HCC and CRM[70,71], while robot-assisted radical resection for gallbladder cancer is both feasible and safe[72] in specialized environments.
A recent paper targeted to a matched comparison of robotic and laparoscopic liver resections failed to show significative differences between the two techniques[73]. Long-term outcomes, larger patient records and comparative studies (with open surgery and pure laparoscopy) are not available yet.
Pancreatic cancer still represents one of the major challenge for the oncologic surgeons due to complex reconstruction, high perioperative morbidity and mortality and poor overall survival. Thus, the advent of laparoscopy was advisable and exciting, in order to minimize operative complications and maximize the early recovery of the patients. On the other hand, the specific technical difficulties and the relative low incidence of pancreatic cancer, have limited the laparoscopic approach to few specialized centers with great experience in both pancreatic surgery and advanced laparoscopy[74]. Moreover, the problem of pancreatic remnant fistula is the same that in open surgery, while some initial and more recent sporadic port-site recurrences were reported in literature[75].
Historically, the first procedure carried out by laparoscopy was distal pancreatectomy for benign disease, because it does not require any anastomosis. However, the preservation of the spleen, when dealing with benign or neuroendocrine tumor, remains challenging[76,77].
A very comprehensive review of the literature by Iacobone et al[78] found more than 300 articles regarding laparoscopic left or distal pancreatectomies (LDP), but most were case-series, with short-term follow-up, different techniques and confused data reporting. Similar findings were reported by Borja-Cacho et al[79]. In addition, the experiences with pancreatic adenocarcinoma or Intraductal Papillary Mucinous Neoplasm (IPMN) were much more limited[80].
One of the largest single-center case series on LDP was published from the Memorial Sloan Kettering Center[81] on more than 300 cases over a 7-year period, resulting in excellent outcomes (27% vs 40% of postoperative complication, P = 0.03, as compared to standard surgery). LDP seems to be almost the standard of care for many centers, in order to achieve a systematic reduction of blood loss and postoperative stay, although a careful patients selection is often advocated[82].
A comparative study demonstrated the cost-effectiveness of LDP as compared to open surgery, when considering the reduction of hospital stay (5 d vs 7 d, P < 0.001)[83], while another[84] reported increasing experience and more complex patients selection although maintaining the same morbidity over a 11-years period.
When limiting literature search to case-matched study, Pericleous[85] identified only 4 articles that fit for quality assessment (but none was a RCT): results were that LDP had a longer operative time, but reduced length of postoperative stay without any differences in perioperative morbidity and mortality, as compared to open surgery. Another similar and more recent meta-analysis[86] found 18 comparative studies including more than 1800 patients. LDP reduced blood loss, length of hospital stay, and overall complications, without increasing the duration of surgery significantly. However, no definitive conclusions were drawn regarding the oncologic safety, although the rate of margins positivity was comparable between open and laparoscopic resection.
As the numbers of laparoscopic advanced procedures increase, some centers began to perform also laparoscopic pancreatodudenectomies (LPD) for malignancies. Some advantages over traditional surgery and comparable oncologic outcomes are reported, although long learning curves limit these initial experiences to subspecialized surgical teams[87-92].
A single center case series (from United Kingdom)[93] with a final review, identified an increasing number of LDP and LPD performed, but almost all were reported in poor quality studies and limited number of patients. The authors concluded that laparoscopic pancreatic procedures should be reserved to selected cases with benign to low grade malignancies. Nonetheless, major vessels resection for malignant involvement have also been reported to be completed by laparoscopy[94].
Few articles reported the oncologic main outcomes, including numbers of nodes harvested, margins of resection, disease-free survival and overall survival. A review by Fischer et al[95] was specifically targeted to laparoscopic pancreatectomies for malignancies to assess those issues. Early results seemed to be oncologically adequate for LDP, while literature, in general, was highly insufficient for LPD. Another recent review of Kudsi et al[96] concluded that, although becoming highly popular, LDP for aggressive tumors may not be appropriate due to the lack of oncologic safety studies.
A very recent single-center series of 200 consecutive laparoscopic pancreatic resections (including LDP, LPD and other more limited procedures) reported excellent result with the use of a robotic controlled laparoscope holder[97].
Some surgeons argue that full robotic surgery could ease many difficult technical maneuvers of the laparoscopic approach, including biliary and pancreatic anastomosis or preservation of the spleen[98-101]. Others[102-104] reported more encouraging early experiences with robotic-assisted pancreatectomies as compared to open approach. A meta-analysis of Zhang et al[105] including 7 trials suggested that robotic pancreatectomy is as safe and efficient as, if not superior to, open surgery but the evidence is highly insufficient.
To the present, those excellent results with robotics are far to be reproducible in most centers worldwide.
Due to the relative low incidence of small bowel carcinomas, most of the laparoscopic resections carried out for malignancy include gastrointestinal stromal tumours (GISTs). According to the well-known peculiar biologic tumoral behaviour, very wide margin and formal lymphadenectomy are unnecessary for GISTs[106]. Therefore, it seems that laparoscopy could be particularly indicated to manage these neoplasms and a variety of endoscopic, laparoscopic, and hybrid techniques are described to surgically excise GISTs of different anatomic locations[107].
However, few papers are specifically targeted to small bowel resections and quality of studies is generally poor (no randomization). Nevertheless, initial experiences reported the laparoscopic treatment of small bowel GISTs to be safe and effective, without oncologic outcome impairment[108,109].
A retrospective comparative study[110] including 9 and 11 patients each arm only, analyzed laparoscopic approach to small bowel tumors compared to open surgery. Despite the insignificant number of patients and the statistical insufficiency of the sample, the authors found how laparoscopic resection favoured short-term outcomes in selected cases. Other similar results were also published[111].
In conclusions, although many of the results advocated for small bowel GISTs are extrapolated from gastric series, it seems that laparoscopic resections of GISTs lead to excellent outcomes in term of perioperative and oncologic outcomes.
To the present, laparoscopic treatment of colorectal cancer has becoming the gold standard of care, and has gained large diffusion worldwide[112-114]. The main reasons are represented by the highest number of good quality studies published, including many RCTs with long follow-up and meta-analysis, the high incidence of colorectal cancer, that permits adequate case-load and the acceptable technical challenge[115,116].
Although laparoscopic colorectal resection (LCR) is feasible in around 90% of elective cancer patients[117] and excellent results are achieved also outside clinical trials[118], many smaller centers still continue to perform routine colorectal operation using the traditional open approach due to the lack of laparoscopic expertise or devices and, probably, some socio-economic disparities[119]. The widespread acceptance of laparoscopic rectal resections, in which some surgeon have demonstrated advantages of robotics, has been slower compared to colon resections.
The most important multicenter RCTs were published in the early 2000’s from the Clinical Outcomes of Surgical Therapy Study Group (COST trial)[120], leaded by Nelson of the Mayo Clinic (Rochester, MN, United States), the Colon Cancer Laparoscopic or Open Resection Study Group (COLOR trial)[121] arisen in Europe, the United Kingdom Medical Research Council (MRC CLASSIC trial)[122]; and the Barcellona[123] and the Australasian (ALCCAS Trial)[124] groups.
All these trials confirmed, in the short-term period, that the use of laparoscopic colon resection maximizes the outcomes without compromising oncological results. Surprisingly, the Barcellona[123] trial showed an increased survival in the stage III patients treated laparoscopically, while the CLASSIC trial[122] reported inferior results for laparoscopic anterior rectal resection that lead the authors to advise against the adoption of this specific procedure.
A meta-analisys[125] of the first four randomized trials (COST, COLOR, CLASSIC and Barcellona, involving 1765 patients overall), with at least 3 years of complete follow-up, confirmed that laparoscopy for colon cancer was oncologically safe (3-year disease-free survival rates in the laparoscopically assisted and open arms were 75.8% and 75.3%, respectively; the 3-year overall survival rates 82.2% and 83.5%; without any difference between stages).
In addition, a very comprehensive review and meta-analysis from the Cochrane Group[126] including the best 25 RCTs (3526 patients) stated that, although operative time was longer in the laparoscopic surgery, many parameters such as the intraoperative blood loss, postoperative pain and ileus, hospital stay and quality of life at the 30th day were superior in comparison to open surgery. Therefore, total morbidity and local (surgical) morbidity were decreased in the laparoscopic groups. General (non-surgical) morbidity and mortality were not different between both groups. Some benefits of LCR for cancer rather than the oncologic outcome, could be stronger in the elderly people[127] or in the long-term period, including reduction of adhesions and incisional hernias[128].
On the other hand, when considering the absolute values (rather than statistic difference) of same of the short-terms advantages of the trials, only very few benefits were detectable (for example 5-9 vs 6-12 postoperative days)[126], with comparable overall morbidity and mortality, while some trials reported increased duration of surgery for LCR[129].
The oncologic long-term results were also tested in the COST trial, demonstrating the non-inferiority of LCR in terms of disease-free 5-year survival, overall 5-year survival and sites of recurrence[130]. Similarly, the long-term outcomes of the COLOR[131] trial found a statistically insignificant difference in favour of open colectomy, while the Barcellona[132] trial confirmed how LCR was associated with a reduced risk of tumor relapse. Also the CLASSIC trial[133] confirmed, after a 5-year analysis, the oncological safety of laparoscopic surgery for both colonic and rectal cancer. A more recent Australasian RCT reported similar long-term oncologic outcomes (recurrence and survival) between open and LCR, although it found some short-term surrogate oncological markers (smaller distal resection margin) to be worst in the laparoscopic group[134].
Another specific meta-analysis from the Cochrane Group[4], including 33 RCTs and 3346 patients, concluded that laparoscopic resection of carcinoma of the colon is associated with a long-term outcome not different from that of open colectomy, although more RCTs are needed to confirm long-term outcomes for rectal cancer and the real incidence of incisional hernias and adhesions.
Recent pioneeristic experiences begin to report the application of NOTES (Natural Orifice Transluminal Endoscopic Surgery)[135,136] or SILC (Single Incision Laparoscopic Colectomy)[137] for colon cancer, but they should be considered absolutely insufficient to be proposed in routine clinical practice.
Data supporting the routine laparoscopic approach to rectal cancer are still incomplete, and the first experiences failed to confirm oncologic safety (CLASSIC trial, not statistically significant increase of positive margins)[122], while a specific Cochrane review[138] including 80 poor quality studies and only 1 RCT did not assess safety of the procedure. However, many data come from patients operated at the end of the nineties or beginning of the twenties, thus justifying some technical mistake in the hands of surgeons without great experience. Moreover, there is also a generalized scientific confusion in the definition of rectal cancer, the distinction between low and medium rectal cancer, the standardization of total mesorectal excision (TME) and the need of perioperative radiochemotherapy. All these issues contribute to jeopardize results and increase difficulties in data reporting.
A very recent RCT from the same group (European centers) of COLOR trial[139] was targeted to laparoscopic treatment of rectal cancer (LRR) with more encouraging results in terms of similar safety, excision margins, and completeness of resection to that of open surgery. Indeed, completeness of the resection was not different between laparoscopy and open surgery (88% vs 92%; P = 0.250), while a positive circumferential resection margin (< 2 mm) and a median distal resection margin were of 10% and 3 cm in both groups respectively. Recovery was confirmed to be improved after laparoscopic surgery, although the results for the primary endpoint - locoregional recurrence - are expected by the end of 2013.
Similar early good results were reached by another recent Korean trial[140] (170 patients each arm of study), when considering blood loss, pain and recovery, that were superior in the laparoscopic group without differences in the margin of resection. Moreover, a very large (more than 4000 patients) non-randomised Spanish trial[141] concluded that laparoscopic surgery is the best option for the surgical treatment of rectal cancer, with similar rates of local recurrence and survival.
Despite the lack of any evidence to support its use, some surgeons began to perform colorectal surgery by the robotic-assisted technique[142-144]. A large retrospective review of colorectal operation in the United States found the percentage of robotic operations to be less than 3%, without any tangible advantages over conventional laparoscopy (except for decreased conversion rates) and higher rate of postoperative bleeding[145].
To resolve the intrinsic difficulty of performing a formal laparoscopic TME, many centers with the available technology and expertise, introduced the use of robot to perform LRR[146]. However, robotic rectal surgery is at least more expensive than laparoscopy[147] and probably equivalent in terms of short term results[148]. Nevertheless, oncologic early results (number of harvested nodes, distal and circumferential margins, port-site recurrence) lead to consider robotic rectal resections safe[149,150]. A prospective, international, multicenter, RCT was recently designed to test robotic versus standard LRR[151].
The dramatic widespread popularity of laparoscopy has significantly changed the surgical approach to gastrointestinal malignancy toward less invasive, miniinvasive, laparoscopic, hybrid and robotic interventions. Excellent results in terms of reduction of postoperative “stress” (including immunologic response, pain, overall morbidity, length of stay, self-corporeal appearance and mortality) have been reported form North America, Europe and Eastern countries.
Laparoscopy is now accepted and, probably, recognized as the gold standard in the management of colorectal malignancy in most of hospitals worldwide. GISTs should also been treated by laparoscopy whenever feasible, and very good result have recently been reported for gastric (mainly distal stomach), esophageal and pancreatic (mostly tail) cancers as well. Total gastrectomy, pancreaticoduodenectomy and major hepatic resections (except for left lateral segments) should be considered pioneeristic operations, reserved to few surgeons within rigorous clinical protocol studies (Table 1).
| District | Open surgery | Laparoscopic surgery | Robotic | Level of Evidence1 |
| Esophagus | Standard | Accepted | Pioneeristic | LE 2 |
| Stomach (proximal) | Standard | Being accepted | Pioneeristic | LE 3 |
| Stomach (distal) | Standard | Accepted | Pioneeristic | LE 2 |
| Liver (major resection) | Standard | Pioneeristic | Pioneeristic | LE 4 |
| Liver (minor resection) | Standard | Being standard | Pioneeristic | LE 3 |
| Gallbladder | Standard | Pioneeristic | Pioneeristic | LE 5 |
| Biliary tree | Standard | Pioneeristic | Pioneeristic | LE 5 |
| Pancreas (head) | Standard | Pioneeristic | Pioneeristic | LE 4 |
| Pancreas (body-tail) | Standard | Being standard | Pioneeristic | LE 3 |
| Small bowel | Standard | Being standard | Pioneeristic | LE 5 |
| Colon | Accepted | Standard | Pioneeristic | LE 1 |
| Rectum | Standard | Being standard | Pioneeristic | LE 2 |
However, the lack (and the intrinsic difficulty of techniques) of RCTs still leaves many important unresolved issues. The cornerstone of oncologic safety, the real benefits for the cancerous patients and the cost-effectiveness, in the setting of limiting heath care resources, are the principal ones. It is also well established that advanced laparoscopic techniques, especially for malignant disease, should be initiated and carried out only in selected tertiary centers with the greatest surgical experience in both laparoscopy and surgical oncology. Moreover, every new laparoscopic program should be tutored, monitored and validated by a final and a long-term oncologic follow-up.
P- Reviewers: Hsiao KCW, Jani K S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet]. Lyon: International Agency for Research on Cancer 2010; Available from: http://globocan.iarc.fr, accessed on day/month/year. |
| 2. | Goldfarb M, Brower S, Schwaitzberg SD. Minimally invasive surgery and cancer: controversies part 1. Surg Endosc. 2010;24:304-334. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 3. | Sharma B, Baxter N, Grantcharov T. Outcomes after laparoscopic techniques in major gastrointestinal surgery. Curr Opin Crit Care. 2010;16:371-376. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. 2008;CD003432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 250] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 5. | Zanghì A, Cavallaro A, Piccolo G, Fisichella R, Di Vita M, Spartà D, Zanghì G, Berretta S, Palermo F, Cappellani A. Dissemination metastasis after laparoscopic colorectal surgery versus conventional open surgery for colorectal cancer: a metanalysis. Eur Rev Med Pharmacol Sci. 2013;17:1174-1184. [PubMed] |
| 6. | Torab FC, Bokobza B, Branicki F. Laparoscopy in gastrointestinal malignancies. Ann N Y Acad Sci. 2008;1138:155-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Luketich JD, Pennathur A, Awais O, Levy RM, Keeley S, Shende M, Christie NA, Weksler B, Landreneau RJ, Abbas G. Outcomes after minimally invasive esophagectomy: review of over 1000 patients. Ann Surg. 2012;256:95-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 647] [Cited by in RCA: 621] [Article Influence: 47.8] [Reference Citation Analysis (0)] |
| 8. | Uttley L, Campbell F, Rhodes M, Cantrell A, Stegenga H, Lloyd-Jones M. Minimally invasive oesophagectomy versus open surgery: is there an advantage? Surg Endosc. 2013;27:724-731. [PubMed] |
| 9. | Butler N, Collins S, Memon B, Memon MA. Minimally invasive oesophagectomy: current status and future direction. Surg Endosc. 2011;25:2071-2083. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Biere SS, van Berge Henegouwen MI, Maas KW, Bonavina L, Rosman C, Garcia JR, Gisbertz SS, Klinkenbijl JH, Hollmann MW, de Lange ES. Minimally invasive versus open oesophagectomy for patients with oesophageal cancer: a multicentre, open-label, randomised controlled trial. Lancet. 2012;379:1887-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1067] [Cited by in RCA: 1205] [Article Influence: 92.7] [Reference Citation Analysis (0)] |
| 11. | Kinjo Y, Kurita N, Nakamura F, Okabe H, Tanaka E, Kataoka Y, Itami A, Sakai Y, Fukuhara S. Effectiveness of combined thoracoscopic-laparoscopic esophagectomy: comparison of postoperative complications and midterm oncological outcomes in patients with esophageal cancer. Surg Endosc. 2012;26:381-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 12. | Briez N, Piessen G, Bonnetain F, Brigand C, Carrere N, Collet D, Doddoli C, Flamein R, Mabrut JY, Meunier B. Open versus laparoscopically-assisted oesophagectomy for cancer: a multicentre randomised controlled phase III trial - the MIRO trial. BMC Cancer. 2011;11:310. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 114] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 13. | Maas KW, Biere SS, Scheepers JJ, Gisbertz SS, Turrado Rodriguez VT, van der Peet DL, Cuesta MA. Minimally invasive intrathoracic anastomosis after Ivor Lewis esophagectomy for cancer: a review of transoral or transthoracic use of staplers. Surg Endosc. 2012;26:1795-1802. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 14. | Schumer E, Perry K, Melvin WS. Minimally invasive esophagectomy for esophageal cancer: evolution and review. Surg Laparosc Endosc Percutan Tech. 2012;22:383-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Hanna GB, Arya S, Markar SR. Variation in the standard of minimally invasive esophagectomy for cancer--systematic review. Semin Thorac Cardiovasc Surg. 2012;24:176-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Clark J, Sodergren MH, Purkayastha S, Mayer EK, James D, Athanasiou T, Yang GZ, Darzi A. The role of robotic assisted laparoscopy for oesophagogastric oncological resection; an appraisal of the literature. Dis Esophagus. 2011;24:240-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 59] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 17. | de la Fuente SG, Weber J, Hoffe SE, Shridhar R, Karl R, Meredith KL. Initial experience from a large referral center with robotic-assisted Ivor Lewis esophagogastrectomy for oncologic purposes. Surg Endosc. 2013;27:3339-3347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 18. | van der Sluis PC, Ruurda JP, van der Horst S, Verhage RJ, Besselink MG, Prins MJ, Haverkamp L, Schippers C, Rinkes IH, Joore HC. Robot-assisted minimally invasive thoraco-laparoscopic esophagectomy versus open transthoracic esophagectomy for resectable esophageal cancer, a randomized controlled trial (ROBOT trial). Trials. 2012;13:230. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 125] [Cited by in RCA: 120] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 19. | Liao GQ, Ou XW, Liu SQ, Zhang SR, Huang W. Laparoscopy-assisted total gastrectomy with trans-orally inserted anvil (OrVil™): a single institution experience. World J Gastroenterol. 2013;19:755-760. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 26] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Chen K, Xu XW, Zhang RC, Pan Y, Wu D, Mou YP. Systematic review and meta-analysis of laparoscopy-assisted and open total gastrectomy for gastric cancer. World J Gastroenterol. 2013;19:5365-5376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 59] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Cui M, Xing JD, Yang W, Ma YY, Yao ZD, Zhang N, Su XQ. D2 dissection in laparoscopic and open gastrectomy for gastric cancer. World J Gastroenterol. 2012;18:833-839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 22. | Shinohara T, Kanaya S, Taniguchi K, Fujita T, Yanaga K, Uyama I. Laparoscopic total gastrectomy with D2 lymph node dissection for gastric cancer. Arch Surg. 2009;144:1138-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 97] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Zhao XF, Jeong O, Jung MR, Ryu SY, Park YK. A propensity score-matched case-control comparative study of laparoscopic and open extended (D2) lymph node dissection for distal gastric carcinoma. Surg Endosc. 2013;27:2792-2800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Yakoub D, Athanasiou T, Tekkis P, Hanna GB. Laparoscopic assisted distal gastrectomy for early gastric cancer: is it an alternative to the open approach? Surg Oncol. 2009;18:322-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 76] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Kodera Y, Fujiwara M, Ohashi N, Nakayama G, Koike M, Morita S, Nakao A. Laparoscopic surgery for gastric cancer: a collective review with meta-analysis of randomized trials. J Am Coll Surg. 2010;211:677-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 104] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 26. | Wei HB, Wei B, Qi CL, Chen TF, Huang Y, Zheng ZH, Huang JL, Fang JF. Laparoscopic versus open gastrectomy with D2 lymph node dissection for gastric cancer: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2011;21:383-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 27. | Sun J, Li J, Wang J, Pan T, Zhou J, Fu X, Zhang S. Meta-analysis of randomized controlled trials on laparoscopic gastrectomy vs. open gastrectomy for distal gastric cancer. Hepatogastroenterology. 2012;59:1699-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Bracale U, Rovani M, Bracale M, Pignata G, Corcione F, Pecchia L. Totally laparoscopic gastrectomy for gastric cancer: meta-analysis of short-term outcomes. Minim Invasive Ther Allied Technol. 2012;21:150-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 29. | Pavlidis TE, Pavlidis ET, Sakantamis AK. The role of laparoscopic surgery in gastric cancer. J Minim Access Surg. 2012;8:35-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 30. | Phillips JD, Nagle AP, Soper NJ. Laparoscopic gastrectomy for cancer. Surg Oncol Clin N Am. 2013;22:39-57, v-vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 31. | Lee JH, Son SY, Lee CM, Ahn SH, Park do J, Kim HH. Morbidity and mortality after laparoscopic gastrectomy for advanced gastric cancer: results of a phase II clinical trial. Surg Endosc. 2013;27:2877-2885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 32. | Wang W, Chen K, Xu XW, Pan Y, Mou YP. Case-matched comparison of laparoscopy-assisted and open distal gastrectomy for gastric cancer. World J Gastroenterol. 2013;19:3672-3677. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Lee HJ, Yang HK. Laparoscopic gastrectomy for gastric cancer. Dig Surg. 2013;30:132-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 31] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Liang Y, Li G, Chen P, Yu J, Zhang C. Laparoscopic versus open gastrectomy for early distal gastric cancer: a meta-analysis. ANZ J Surg. 2011;81:673-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 35. | Wang W, Li Z, Tang J, Wang M, Wang B, Xu Z. Laparoscopic versus open total gastrectomy with D2 dissection for gastric cancer: a meta-analysis. J Cancer Res Clin Oncol. 2013;139:1721-1734. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 36. | Chen K, Xu XW, Mou YP, Pan Y, Zhou YC, Zhang RC, Wu D. Systematic review and meta-analysis of laparoscopic and open gastrectomy for advanced gastric cancer. World J Surg Oncol. 2013;11:182. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 37. | Yu J, Hu J, Huang C, Ying M, Peng X, Wei H, Jiang Z, Du X, Liu Z, Liu H. The impact of age and comorbidity on postoperative complications in patients with advanced gastric cancer after laparoscopic D2 gastrectomy: results from the Chinese laparoscropic gastrointestinal surgery study (CLASS) group. Eur J Surg Oncol. 2013;39:1144-1149. [PubMed] |
| 38. | Huscher CG, Mingoli A, Sgarzini G, Brachini G, Binda B, Di Paola M, Ponzano C. Totally laparoscopic total and subtotal gastrectomy with extended lymph node dissection for early and advanced gastric cancer: early and long-term results of a 100-patient series. Am J Surg. 2007;194:839-844; discussion 844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 39. | Kim HH, Hyung WJ, Cho GS, Kim MC, Han SU, Kim W, Ryu SW, Lee HJ, Song KY. Morbidity and mortality of laparoscopic gastrectomy versus open gastrectomy for gastric cancer: an interim report--a phase III multicenter, prospective, randomized Trial (KLASS Trial). Ann Surg. 2010;251:417-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 619] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 40. | Yoshikawa T, Cho H, Rino Y, Yamamoto Y, Kimura M, Fukunaga T, Hasegawa S, Yamada T, Aoyama T, Tsuburaya A. A prospective feasibility and safety study of laparoscopy-assisted distal gastrectomy for clinical stage I gastric cancer initiated by surgeons with much experience of open gastrectomy and laparoscopic surgery. Gastric Cancer. 2013;16:126-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 41. | Uyama I, Suda K, Satoh S. Laparoscopic surgery for advanced gastric cancer: current status and future perspectives. J Gastric Cancer. 2013;13:19-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 42. | Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 650] [Cited by in RCA: 655] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 43. | Rosin D, Goldes Y, Bar Zakai B, Shabtai M, Ayalon A, Zmora O. Laparoscopic subtotal gastrectomy for gastric cancer. JSLS. 2009;13:318-322. [PubMed] |
| 44. | Bracale U, Pignata G, Lirici MM, Hüscher CG, Pugliese R, Sgroi G, Romano G, Spinoglio G, Gualtierotti M, Maglione V. Laparoscopic gastrectomies for cancer: The ACOI-IHTSC national guidelines. Minim Invasive Ther Allied Technol. 2012;21:313-319. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 45. | Xiong B, Ma L, Zhang C. Robotic versus laparoscopic gastrectomy for gastric cancer: a meta-analysis of short outcomes. Surg Oncol. 2012;21:274-280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 46. | Koh YX, Chok AY, Zheng HL, Tan CS, Chow PK, Wong WK, Goh BK. A systematic review and meta-analysis comparing laparoscopic versus open gastric resections for gastrointestinal stromal tumors of the stomach. Ann Surg Oncol. 2013;20:3549-3560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 98] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 47. | Liang JW, Zheng ZC, Zhang JJ, Zhang T, Zhao Y, Yang W, Liu YQ. Laparoscopic versus open gastric resections for gastric gastrointestinal stromal tumors: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2013;23:378-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 48. | Laurence JM, Lam VW, Langcake ME, Hollands MJ, Crawford MD, Pleass HC. Laparoscopic hepatectomy, a systematic review. ANZ J Surg. 2007;77:948-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 49. | Pilgrim CH, To H, Usatoff V, Evans PM. Laparoscopic hepatectomy is a safe procedure for cancer patients. HPB (Oxford). 2009;11:247-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 50. | Mizuguchi T, Kawamoto M, Meguro M, Shibata T, Nakamura Y, Kimura Y, Furuhata T, Sonoda T, Hirata K. Laparoscopic hepatectomy: a systematic review, meta-analysis, and power analysis. Surg Today. 2011;41:39-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 51. | Buell JF, Cherqui D, Geller DA, O’Rourke N, Iannitti D, Dagher I, Koffron AJ, Thomas M, Gayet B, Han HS. The international position on laparoscopic liver surgery: The Louisville Statement, 2008. Ann Surg. 2009;250:825-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1249] [Cited by in RCA: 1153] [Article Influence: 72.1] [Reference Citation Analysis (0)] |
| 52. | Hasegawa Y, Nitta H, Sasaki A, Takahara T, Ito N, Fujita T, Kanno S, Nishizuka S, Wakabayashi G. Laparoscopic left lateral sectionectomy as a training procedure for surgeons learning laparoscopic hepatectomy. J Hepatobiliary Pancreat Sci. 2013;20:525-530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 53. | Doughtie CA, Egger ME, Cannon RM, Martin RC, McMasters KM, Scoggins CR. Laparoscopic hepatectomy is a safe and effective approach for resecting large colorectal liver metastases. Am Surg. 2013;79:566-571. [PubMed] |
| 54. | Belli G, Gayet B, Han HS, Wakabayashi G, Kim KH, Cannon R, Kaneko H, Gamblin T, Koffron A, Dagher I. Laparoscopic left hemihepatectomy a consideration for acceptance as standard of care. Surg Endosc. 2013;27:2721-2726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 55. | Vibert E, Perniceni T, Levard H, Denet C, Shahri NK, Gayet B. Laparoscopic liver resection. Br J Surg. 2006;93:67-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 273] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 56. | Dagher I, Belli G, Fantini C, Laurent A, Tayar C, Lainas P, Tranchart H, Franco D, Cherqui D. Laparoscopic hepatectomy for hepatocellular carcinoma: a European experience. J Am Coll Surg. 2010;211:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 57. | Kaneko H, Tsuchiya M, Otsuka Y, Yajima S, Minagawa T, Watanabe M, Tamura A. Laparoscopic hepatectomy for hepatocellular carcinoma in cirrhotic patients. J Hepatobiliary Pancreat Surg. 2009;16:433-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 49] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 58. | Lee KF, Chong CN, Wong J, Cheung YS, Wong J, Lai P. Long-term results of laparoscopic hepatectomy versus open hepatectomy for hepatocellular carcinoma: a case-matched analysis. World J Surg. 2011;35:2268-2274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 59. | Qiu J, Chen S, Pankaj P, Wu H. Laparoscopic hepatectomy for hepatic colorectal metastases -- a retrospective comparative cohort analysis and literature review. PLoS One. 2013;8:e60153. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 60. | Zhang L, Chen YJ, Shang CZ, Zhang HW, Huang ZJ. Total laparoscopic liver resection in 78 patients. World J Gastroenterol. 2009;15:5727-5731. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 61. | Koffron AJ, Auffenberg G, Kung R, Abecassis M. Evaluation of 300 minimally invasive liver resections at a single institution: less is more. Ann Surg. 2007;246:385-392; discussion 392-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 380] [Article Influence: 21.1] [Reference Citation Analysis (0)] |
| 62. | Rao AM, Ahmed I. Laparoscopic versus open liver resection for benign and malignant hepatic lesions in adults. Cochrane Database Syst Rev. 2013;5:CD010162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 63. | Ito F, Cho CS, Rikkers LF, Weber SM. Hilar cholangiocarcinoma: current management. Ann Surg. 2009;250:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 169] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 64. | Lau SH, Lau WY. Current therapy of hilar cholangiocarcinoma. Hepatobiliary Pancreat Dis Int. 2012;11:12-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | Gumbs AA, Jarufe N, Gayet B. Minimally invasive approaches to extrapancreatic cholangiocarcinoma. Surg Endosc. 2013;27:406-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 66. | Shih SP, Schulick RD, Cameron JL, Lillemoe KD, Pitt HA, Choti MA, Campbell KA, Yeo CJ, Talamini MA. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg. 2007;245:893-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 67. | Goetze TO, Paolucci V. Prognosis of incidental gallbladder carcinoma is not influenced by the primary access technique: analysis of 837 incidental gallbladder carcinomas in the German Registry. Surg Endosc. 2013;27:2821-2828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 68. | de Aretxabala X, Leon J, Hepp J, Maluenda F, Roa I. Gallbladder cancer: role of laparoscopy in the management of potentially resectable tumors. Surg Endosc. 2010;24:2192-2196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 69. | Aikawa M, Miyazawa M, Okamoto K, Toshimitsu Y, Okada K, Ueno Y, Yamaguchi S, Koyama I. Single-port laparoscopic hepatectomy: technique, safety, and feasibility in a clinical case series. Surg Endosc. 2012;26:1696-1701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 70. | Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic liver resection for hepatocellular carcinoma: short-term outcome. Am J Surg. 2013;205:697-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 71. | Ho CM, Wakabayashi G, Nitta H, Ito N, Hasegawa Y, Takahara T. Systematic review of robotic liver resection. Surg Endosc. 2013;27:732-739. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 72. | Shen BY, Zhan Q, Deng XX, Bo H, Liu Q, Peng CH, Li HW. Radical resection of gallbladder cancer: could it be robotic? Surg Endosc. 2012;26:3245-3250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 73. | Tsung A, Geller DA, Sukato DC, Sabbaghian S, Tohme S, Steel J, Marsh W, Reddy SK, Bartlett DL. Robotic Versus Laparoscopic Hepatectomy: A Matched Comparison. Ann Surg. 2013;Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 257] [Article Influence: 23.4] [Reference Citation Analysis (0)] |
| 74. | Rosales-Velderrain A, Bowers SP, Goldberg RF, Clarke TM, Buchanan MA, Stauffer JA, Asbun HJ. National trends in resection of the distal pancreas. World J Gastroenterol. 2012;18:4342-4349. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 72] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 75. | Young S, Abbitt P, Hughes SJ. Port-site recurrence of pancreatic adenocarcinoma following laparoscopic pancreaticoduodenectomy. J Gastrointest Surg. 2012;16:2294-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 76. | Salky BA, Edye M. Laparoscopic pancreatectomy. Surg Clin North Am. 1996;76:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 77. | Mabrut JY, Fernandez-Cruz L, Azagra JS, Bassi C, Delvaux G, Weerts J, Fabre JM, Boulez J, Baulieux J, Peix JL. Laparoscopic pancreatic resection: results of a multicenter European study of 127 patients. Surgery. 2005;137:597-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 346] [Cited by in RCA: 312] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 78. | Iacobone M, Citton M, Nitti D. Laparoscopic distal pancreatectomy: up-to-date and literature review. World J Gastroenterol. 2012;18:5329-5337. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 32] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 79. | Borja-Cacho D, Al-Refaie WB, Vickers SM, Tuttle TM, Jensen EH. Laparoscopic distal pancreatectomy. J Am Coll Surg. 2009;209:758-765; quiz 800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 80. | Gumbs AA, Chouillard EK. Laparoscopic distal pancreatectomy and splenectomy for malignant tumors. J Gastrointest Cancer. 2012;43:83-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 81. | Jayaraman S, Gonen M, Brennan MF, D’Angelica MI, DeMatteo RP, Fong Y, Jarnagin WR, Allen PJ. Laparoscopic distal pancreatectomy: evolution of a technique at a single institution. J Am Coll Surg. 2010;211:503-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 82. | Nakamura M, Nakashima H. Laparoscopic distal pancreatectomy and pancreatoduodenectomy: is it worthwhile? A meta-analysis of laparoscopic pancreatectomy. J Hepatobiliary Pancreat Sci. 2013;20:421-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 108] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 83. | Fox AM, Pitzul K, Bhojani F, Kaplan M, Moulton CA, Wei AC, McGilvray I, Cleary S, Okrainec A. Comparison of outcomes and costs between laparoscopic distal pancreatectomy and open resection at a single center. Surg Endosc. 2012;26:1220-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 84. | Kneuertz PJ, Patel SH, Chu CK, Fisher SB, Maithel SK, Sarmiento JM, Weber SM, Staley CA, Kooby DA. Laparoscopic distal pancreatectomy: trends and lessons learned through an 11-year experience. J Am Coll Surg. 2012;215:167-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 85. | Pericleous S, Middleton N, McKay SC, Bowers KA, Hutchins RR. Systematic review and meta-analysis of case-matched studies comparing open and laparoscopic distal pancreatectomy: is it a safe procedure? Pancreas. 2012;41:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 91] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 86. | Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg. 2012;255:1048-1059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 429] [Cited by in RCA: 388] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 87. | Dulucq JL, Wintringer P, Mahajna A. Laparoscopic pancreaticoduodenectomy for benign and malignant diseases. Surg Endosc. 2006;20:1045-1050. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 123] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 88. | Palanivelu C, Jani K, Senthilnathan P, Parthasarathi R, Rajapandian S, Madhankumar MV. Laparoscopic pancreaticoduodenectomy: technique and outcomes. J Am Coll Surg. 2007;205:222-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 176] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 89. | Pugliese R, Scandroglio I, Sansonna F, Maggioni D, Costanzi A, Citterio D, Ferrari GC, Di Lernia S, Magistro C. Laparoscopic pancreaticoduodenectomy: a retrospective review of 19 cases. Surg Laparosc Endosc Percutan Tech. 2008;18:13-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 87] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 90. | Cho A, Yamamoto H, Nagata M, Takiguchi N, Shimada H, Kainuma O, Souda H, Gunji H, Miyazaki A, Ikeda A. Comparison of laparoscopy-assisted and open pylorus-preserving pancreaticoduodenectomy for periampullary disease. Am J Surg. 2009;198:445-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 91. | Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg. 2010;145:19-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 254] [Article Influence: 16.9] [Reference Citation Analysis (0)] |
| 92. | Corcione F, Pirozzi F, Cuccurullo D, Piccolboni D, Caracino V, Galante F, Cusano D, Sciuto A. Laparoscopic pancreaticoduodenectomy: experience of 22 cases. Surg Endosc. 2013;27:2131-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 55] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 93. | Ammori BJ, Ayiomamitis GD. Laparoscopic pancreaticoduodenectomy and distal pancreatectomy: a UK experience and a systematic review of the literature. Surg Endosc. 2011;25:2084-2099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 94. | Kendrick ML, Sclabas GM. Major venous resection during total laparoscopic pancreaticoduodenectomy. HPB (Oxford). 2011;13:454-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 85] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 95. | Fisher SB, Kooby DA. Laparoscopic pancreatectomy for malignancy. J Surg Oncol. 2013;107:39-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 96. | Kudsi OY, Gagner M, Jones DB. Laparoscopic distal pancreatectomy. Surg Oncol Clin N Am. 2013;22:59-73, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 97. | Moore PS, Porter PE. Nursing deans in small, liberal arts colleges and universities: roles, challenges, and opportunities. J Prof Nurs. 1987;3:20-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 98. | Giulianotti PC, Sbrana F, Bianco FM, Elli EF, Shah G, Addeo P, Caravaglios G, Coratti A. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc. 2010;24:1646-1657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 266] [Article Influence: 17.7] [Reference Citation Analysis (0)] |
| 99. | Choi SH, Kang CM, Lee WJ, Chi HS. Robot-assisted spleen-preserving laparoscopic distal pancreatectomy. Ann Surg Oncol. 2011;18:3623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 100. | Horiguchi A, Uyama I, Miyakawa S. Robot-assisted laparoscopic pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2011;18:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 101. | Zeh HJ, Zureikat AH, Secrest A, Dauoudi M, Bartlett D, Moser AJ. Outcomes after robot-assisted pancreaticoduodenectomy for periampullary lesions. Ann Surg Oncol. 2012;19:864-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 114] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 102. | Narula VK, Mikami DJ, Melvin WS. Robotic and laparoscopic pancreaticoduodenectomy: a hybrid approach. Pancreas. 2010;39:160-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 103. | Lai EC, Yang GP, Tang CN. Robot-assisted laparoscopic pancreaticoduodenectomy versus open pancreaticoduodenectomy--a comparative study. Int J Surg. 2012;10:475-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 157] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 104. | Daouadi M, Zureikat AH, Zenati MS, Choudry H, Tsung A, Bartlett DL, Hughes SJ, Lee KK, Moser AJ, Zeh HJ. Robot-assisted minimally invasive distal pancreatectomy is superior to the laparoscopic technique. Ann Surg. 2013;257:128-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 230] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 105. | Zhang J, Wu WM, You L, Zhao YP. Robotic versus open pancreatectomy: a systematic review and meta-analysis. Ann Surg Oncol. 2013;20:1774-1780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 106. | Grover S, Ashley SW, Raut CP. Small intestine gastrointestinal stromal tumors. Curr Opin Gastroenterol. 2012;28:113-123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 107. | Dholakia C, Gould J. Minimally invasive resection of gastrointestinal stromal tumors. Surg Clin North Am. 2008;88:1009-118, vi. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 108. | Tabrizian P, Nguyen SQ, Divino CM. Laparoscopic management and longterm outcomes of gastrointestinal stromal tumors. J Am Coll Surg. 2009;208:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 109. | Arolfo S, Teggia PM, Nano M. Gastrointestinal stromal tumors: thirty years experience of an institution. World J Gastroenterol. 2011;17:1836-1839. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 110. | Tsui DK, Tang CN, Ha JP, Li MK. Laparoscopic approach for small bowel tumors. Surg Laparosc Endosc Percutan Tech. 2008;18:556-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 111. | Chen YH, Liu KH, Yeh CN, Hsu JT, Liu YY, Tsai CY, Chiu CT, Jan YY, Yeh TS. Laparoscopic resection of gastrointestinal stromal tumors: safe, efficient, and comparable oncologic outcomes. J Laparoendosc Adv Surg Tech A. 2012;22:758-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 112. | Lee SW. Laparoscopic procedures for colon and rectal cancer surgery. Clin Colon Rectal Surg. 2009;22:218-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 113. | Martel G, Crawford A, Barkun JS, Boushey RP, Ramsay CR, Fergusson DA. Expert opinion on laparoscopic surgery for colorectal cancer parallels evidence from a cumulative meta-analysis of randomized controlled trials. PLoS One. 2012;7:e35292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 114. | Aly EH. Colorectal surgery: current practice & amp; future developments. Int J Surg. 2012;10:182-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 115. | Stocchi L, Nelson H. Laparoscopic colon resection for cancer. Adv Surg. 2006;40:59-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 116. | Martel G, Boushey RP. Laparoscopic colon surgery: past, present and future. Surg Clin North Am. 2006;86:867-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 57] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 117. | Buchanan GN, Malik A, Parvaiz A, Sheffield JP, Kennedy RH. Laparoscopic resection for colorectal cancer. Br J Surg. 2008;95:893-902. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 118. | Aslani N, Lobo-Prabhu K, Heidary B, Phang T, Raval MJ, Brown CJ. Outcomes of laparoscopic colon cancer surgery in a population-based cohort in British Columbia: are they as good as the clinical trials? Am J Surg. 2012;204:411-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 119. | Robinson CN, Balentine CJ, Sansgiry S, Berger DH. Disparities in the use of minimally invasive surgery for colorectal disease. J Gastrointest Surg. 2012;16:897-903; discussion 903-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 120. | Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2606] [Cited by in RCA: 2512] [Article Influence: 119.6] [Reference Citation Analysis (0)] |
| 121. | Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1677] [Article Influence: 83.9] [Reference Citation Analysis (0)] |
| 122. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2292] [Article Influence: 114.6] [Reference Citation Analysis (0)] |
| 123. | Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, Visa J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224-2229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1901] [Cited by in RCA: 1816] [Article Influence: 79.0] [Reference Citation Analysis (0)] |
| 124. | Hewett PJ, Allardyce RA, Bagshaw PF, Frampton CM, Frizelle FA, Rieger NA, Smith JS, Solomon MJ, Stephens JH, Stevenson AR. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg. 2008;248:728-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 271] [Article Influence: 15.9] [Reference Citation Analysis (1)] |
| 125. | Bonjer HJ, Hop WC, Nelson H, Sargent DJ, Lacy AM, Castells A, Guillou PJ, Thorpe H, Brown J, Delgado S. Laparoscopically assisted vs open colectomy for colon cancer: a meta-analysis. Arch Surg. 2007;142:298-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 397] [Cited by in RCA: 388] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 126. | Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005;CD003145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 293] [Cited by in RCA: 431] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 127. | Allardyce RA, Bagshaw PF, Frampton CM, Frizelle FA, Hewett PJ, Rieger NA, Smith JS, Solomon MJ, Stevenson AR. Australasian Laparoscopic Colon Cancer Study shows that elderly patients may benefit from lower postoperative complication rates following laparoscopic versus open resection. Br J Surg. 2010;97:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 128. | Taylor GW, Jayne DG, Brown SR, Thorpe H, Brown JM, Dewberry SC, Parker MC, Guillou PJ. Adhesions and incisional hernias following laparoscopic versus open surgery for colorectal cancer in the CLASICC trial. Br J Surg. 2010;97:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 132] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 129. | Neudecker J, Klein F, Bittner R, Carus T, Stroux A, Schwenk W. Short-term outcomes from a prospective randomized trial comparing laparoscopic and open surgery for colorectal cancer. Br J Surg. 2009;96:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 112] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 130. | Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW, Hellinger M, Flanagan R, Peters W, Nelson H. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655-662; discussion 662-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 798] [Article Influence: 44.3] [Reference Citation Analysis (0)] |
| 131. | Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 965] [Cited by in RCA: 1053] [Article Influence: 61.9] [Reference Citation Analysis (0)] |
| 132. | Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, Pique JM. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 436] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 133. | Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 737] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 134. | Bagshaw PF, Allardyce RA, Frampton CM, Frizelle FA, Hewett PJ, McMurrick PJ, Rieger NA, Smith JS, Solomon MJ, Stevenson AR. Long-term outcomes of the australasian randomized clinical trial comparing laparoscopic and conventional open surgical treatments for colon cancer: the Australasian Laparoscopic Colon Cancer Study trial. Ann Surg. 2012;256:915-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 136] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 135. | Leroy J, Diana M, Wall J, Costantino F, D’Agostino J, Marescaux J. Laparo-endoscopic single-site (LESS) with transanal natural orifice specimen extraction (NOSE) sigmoidectomy: a new step before pure colorectal natural orifices transluminal endoscopic surgery (NOTES®). J Gastrointest Surg. 2011;15:1488-1492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 136. | Diana M, Perretta S, Wall J, Costantino FA, Leroy J, Demartines N, Marescaux J. Transvaginal specimen extraction in colorectal surgery: current state of the art. Colorectal Dis. 2011;13:e104-e111. [PubMed] [DOI] [Full Text] |
| 137. | Egi H, Hattori M, Hinoi T, Takakura Y, Kawaguchi Y, Shimomura M, Tokunaga M, Adachi T, Urushihara T, Itamoto T. Single-port laparoscopic colectomy versus conventional laparoscopic colectomy for colon cancer: a comparison of surgical results. World J Surg Oncol. 2012;10:61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 138. | Breukink S, Pierie J, Wiggers T. Laparoscopic versus open total mesorectal excision for rectal cancer. Cochrane Database Syst Rev. 2006;CD005200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 127] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 139. | van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1206] [Article Influence: 100.5] [Reference Citation Analysis (0)] |
| 140. | Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 687] [Cited by in RCA: 750] [Article Influence: 50.0] [Reference Citation Analysis (0)] |
| 141. | Lujan J, Valero G, Biondo S, Espin E, Parrilla P, Ortiz H. Laparoscopic versus open surgery for rectal cancer: results of a prospective multicentre analysis of 4,970 patients. Surg Endosc. 2013;27:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 142. | Patel CB, Ragupathi M, Ramos-Valadez DI, Haas EM. A three-arm (laparoscopic, hand-assisted, and robotic) matched-case analysis of intraoperative and postoperative outcomes in minimally invasive colorectal surgery. Dis Colon Rectum. 2011;54:144-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 143. | Deutsch GB, Sathyanarayana SA, Gunabushanam V, Mishra N, Rubach E, Zemon H, Klein JD, Denoto G. Robotic vs. laparoscopic colorectal surgery: an institutional experience. Surg Endosc. 2012;26:956-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 144. | Fung AK, Aly EH. Robotic colonic surgery: is it advisable to commence a new learning curve? Dis Colon Rectum. 2013;56:786-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 145. | Halabi WJ, Kang CY, Jafari MD, Nguyen VQ, Carmichael JC, Mills S, Stamos MJ, Pigazzi A. Robotic-assisted colorectal surgery in the United States: a nationwide analysis of trends and outcomes. World J Surg. 2013;37:2782-2790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 134] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 146. | Kim SH, Kwak JM. Robotic total mesorectal excision: operative technique and review of the literature. Tech Coloproctol. 2013;17 Suppl 1:S47-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 147. | Baek SJ, Kim SH, Cho JS, Shin JW, Kim J. Robotic versus conventional laparoscopic surgery for rectal cancer: a cost analysis from a single institute in Korea. World J Surg. 2012;36:2722-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 148. | Helvind NM, Eriksen JR, Mogensen A, Tas B, Olsen J, Bundgaard M, Jakobsen HL, Gögenür I. No differences in short-term morbidity and mortality after robot-assisted laparoscopic versus laparoscopic resection for colonic cancer: a case-control study of 263 patients. Surg Endosc. 2013;27:2575-2580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 149. | Fernandez R, Anaya DA, Li LT, Orcutt ST, Balentine CJ, Awad SA, Berger DH, Albo DA, Artinyan A. Laparoscopic versus robotic rectal resection for rectal cancer in a veteran population. Am J Surg. 2013;206:509-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 150. | Baek JH, McKenzie S, Garcia-Aguilar J, Pigazzi A. Oncologic outcomes of robotic-assisted total mesorectal excision for the treatment of rectal cancer. Ann Surg. 2010;251:882-886. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 151. | Collinson FJ, Jayne DG, Pigazzi A, Tsang C, Barrie JM, Edlin R, Garbett C, Guillou P, Holloway I, Howard H. An international, multicentre, prospective, randomised, controlled, unblinded, parallel-group trial of robotic-assisted versus standard laparoscopic surgery for the curative treatment of rectal cancer. Int J Colorectal Dis. 2012;27:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 181] [Article Influence: 13.9] [Reference Citation Analysis (0)] |