Published online Feb 21, 2014. doi: 10.3748/wjg.v20.i7.1694
Revised: December 7, 2013
Accepted: January 3, 2014
Published online: February 21, 2014
Processing time: 134 Days and 11.4 Hours
Gastric cancer (GC) is the second leading cause of cancer-related death. The poor survival rate may reflect the relatively aggressive tumor biology of GC. Recently, the importance of the tumor microenvironment in carcinogenesis has emerged. In the tumor microenvironment, tumor cells and the surrounding stromal cells aberrantly secrete matricellular proteins capable of modulating carcinogenesis and regulating metastasis. The Cyr61/CTGF/Nov (CCN) proteins are a family of matricellular proteins with variable roles in many physiological and pathological processes. The evidence suggests that CCN family proteins contribute to GC carcinogenic processes. Here, we briefly review recent research on the effects of CCN family proteins in GC carcinogenesis and the development of new targeted agents in this field.
Core tip: Cyr61/CTGF/Nov (CCN) proteins are matricellular proteins responsible for many physiological and pathological processes, including carcinogenesis. The prototypical CCN family protein is composed of an N-terminal secretory signal peptide and four structural modules. Several truncated variants participate in the carcinogenesis of gastrointestinal tract cancers. The role of CCNs in carcinogenesis is tumor-type and context-dependent. The evidence suggests that CCN family proteins play important roles in gastric cancer (GC) carcinogenic processes. Recent CCN targeting agents, including monoclonal antibodies, antisense oligonucleotides and RNA interference compounds, may be helpful in future GC therapeutics.
- Citation: Cheng TY, Wu MS, Hua KT, Kuo ML, Lin MT. Cyr61/CTGF/Nov family proteins in gastric carcinogenesis. World J Gastroenterol 2014; 20(7): 1694-1700
- URL: https://www.wjgnet.com/1007-9327/full/v20/i7/1694.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i7.1694
Gastric cancer (GC) is the second leading cause of cancer-related death, accounting for approximately 10% of total cancer deaths worldwide[1]. Despite significant advances in cancer treatment modalities, the prognosis for GC has only modestly improved. The five-year relative survival rate for all stages combined was 28% between 2002 and 2008, compared to 20% between 1987 and 1989[2]. The poor survival rate may reflect the relatively aggressive tumor biology of GC.
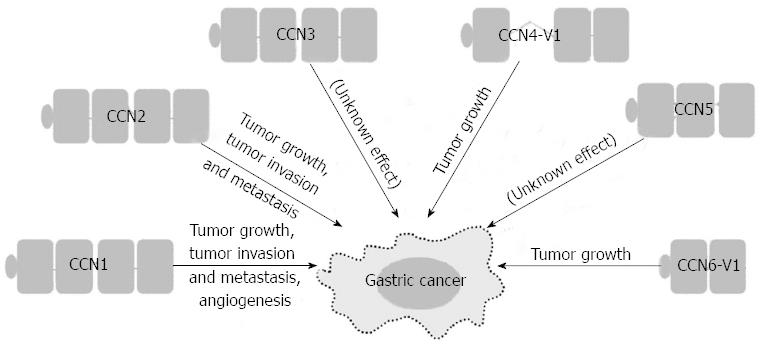
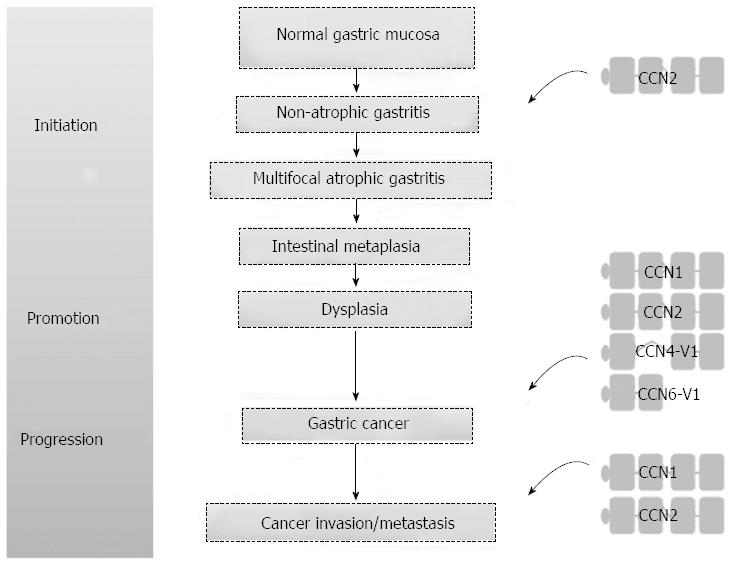
The interplay between the tumor and its microenvironment is crucial for both tumor development and progression. In the tumor microenvironment, tumor cells and the surrounding stromal cells aberrantly secrete matricellular proteins, a group of proteins that function as regulators of cell-cell and cell-matrix interactions that modulate carcinogenesis and the regulatory networks of metastasis[3]. The Cyr61/CTGF/Nov (CCN) proteins are a family of matricellular proteins that play pivotal roles in many physiological and pathological processes, including carcinogenesis[4]. The CCN family proteins include six members, as summarized in Table 1. The expression of CCN family proteins is dependent on cell type and context. CCN family proteins can act both positively and negatively in carcinogenesis for different tumor types. The positive or negative effect depends on whether angiogenic factors are limiting and whether conditions that favor apoptosis or senescence prevail[5,6]. The prototypical CCN family protein is composed of an N-terminal secretory signal peptide and four structural modules: an insulin-like growth factor binding protein-like module, a von Willebrand factor type C repeat (VWC) module, a thrombospondin-homology type 1 repeat (TSP1) module, and a C-terminal cysteine-knot-containing (CT) module[7]. Except for CCN5, which lacks the CT module, all CCN proteins contain the four complete structural modules. However, there are biologically active CCN variants with less than four modules after translational processing or alternative splicing. Some of these truncated variants may participate in the carcinogenesis of gastrointestinal tract cancers[7-10], including GC. More recent evidence suggests that CCN family proteins contribute to GC carcinogenesis (Figure 1). Of the CCN family proteins, only CCN2 has been reported to be involved in Helicobacter pylori-associated chronic gastritis. There is a positive correlation between the density of CCN2-producing mononuclear cells and the severity of chronic gastritis. The actual role of CCN family proteins in the initiation stage of GC carcinogenesis will be clarified with future studies[11]. In this brief review, we focus on the roles that the CCN family proteins play in the promotion and progression of GC, the cell signaling pathways involved in the GC regulatory processes, and the development of new agents in targeted therapy.
| CCN proteins (synonyms) | Chromosomal location | Molecular mass, kDa (number of amino acids) |
| CCN1 (Cyr61; CTGF-2; IGFBP-10/IBP-10; IGFBP-rP4; Cef-10) | 1p22.3 | 42.0 (381) |
| CCN2 (CTGF; IGFBP-8/IBP-8; IGFBP-rP2; hypertrophic chondrocyte-specific protein 24) | 6q23.1 | 38.1 (349) |
| CCN3 (Nov; IGFBP-9/IBP-9; IGFBP-rP3) | 8q24.1 | 39.2 (357) |
| CCN4 (WISP-1; Elm-1) | 8q24.22 | 40.3 (367) |
| CCN5 (WISP-2; CTGF-3; CTGF-L; Cop-1) | 20q13.12 | 26.8 (250) |
| CCN6 (WISP-3; LIBC) | 6q21 | 39.3 (354) |
CCN1 was the first cloned member of the CCN family proteins[12] and has been reported to regulate diverse cellular functions through binding to distinct integrins[13]. CCN1 supports cell adhesion, stimulates cell migration, augments growth factor-induced DNA synthesis, promotes cell survival, inhibits apoptosis, and enhances angiogenesis[14]. Although much more data have been reported from cancer cell lines, CCN1 expression is up-regulated in patients with breast cancer, gliomas, hepatocellular carcinoma, prostate cancer, and oral squamous cell carcinoma[15-20] but is down-regulated in leiomyoma and non-small cell lung cancer[21,22]. The role of CCN1 in carcinogenesis may be cell type- and context-dependent. CCN1 mediates its activities primarily through interaction with cell adhesion receptor integrins and co-receptor heparan sulfate proteoglycans (HSPGs). CCN1 as a ligand of integrins was first demonstrated by the direct binding of CCN1 to integrin αvβ3 to mediate endothelial cell adhesion[23]. Several other integrins, such as α2β1, α6β1, αvβ5, αIIbβ3, αMβ2, and αDβ2, have also been identified as signaling receptors mediating CCN1 functions[5].
In patients with GC, high expression levels of CCN1 correlate with more lymph node metastases, more advanced tumor stage, a histologic diffuse type, and early recurrence[24]. Forced expression of CCN1 can induce angiogenesis, a process essential for nourishing the growing tumor. CCN1 promotes angiogenesis either directly by effects on endothelial cells or indirectly by regulating the angiogenic factors vascular endothelial growth factor (VEGF)-A and VEGF-C[25,26]. However, there are no data illustrating the relationship among CCN1, VEGF-A and VFGF-C in GC. CCN1 promotes tumor growth and increases tumor vascularization upon over-expression in GC cells in the severe combined immunodeficiency mouse model[27]. In addition, in vitro studies have shown that more invasive GC cell lines contain higher levels of CCN1. The forced expression of CCN1 or treatment with recombinant CCN1 in GC cells significantly increases invasive ability. CCN1 regulates GC cell motility/invasion through integrin αvβ3 and induces nuclear factor-κB (NF-κB) activation as well as the subsequent cyclooxygenase-2 (COX-2) up-regulation to promote cell invasion[24]. The importance of COX-2 expression in GC is well established, with its correlation with depth of invasion, lymph node metastasis and advanced stage[28-30].
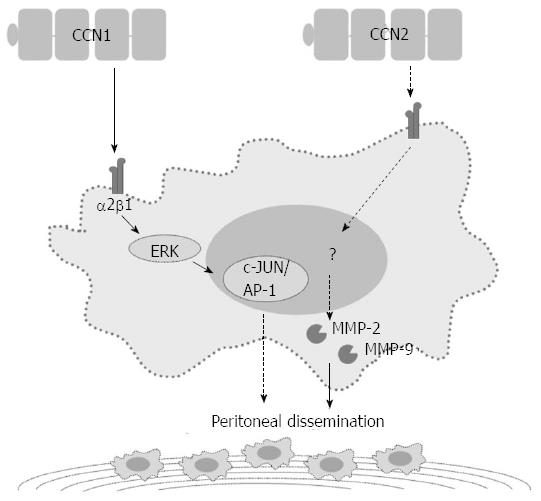
In addition to the NF-κB-dependent pathway, CCN1 regulates GC cell invasiveness by the hypoxia-inducing factor-1α (HIF-1α)-dependent up-regulation of plasminogen activator inhibitor-1 (PAI-1). Both phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin and extracellular signal-regulated kinase 1/2 signaling pathways are essential for HIF-1α accumulation[31]. CCN1 may also contribute to the peritoneal dissemination of GC by promoting tumor-cell adhesion ability. High CCN1 expression levels correlate with peritoneal dissemination in advanced stage GC patients. GC cells over-expressing CCN1 up-regulate integrin α2β1 via an activator protein-1 (AP-1)-dependent pathway (Figure 2)[32].
CCN2 was first recognized as the major platelet-derived growth factor (PDGF)-related mitogen secreted by human vascular endothelial cells[33]. CCN2 is involved in a wide variety of regulatory processes, such as angiogenesis, chondrogenesis, osteogenesis, fibrosis formation, diabetic nephropathy, and tumor development[5]. CCN2 expression is up-regulated in patients with breast cancer, gliomas, esophageal adenocarcinoma, pancreatic cancer, and melanoma[15,17,34-36] but is down-regulated in lung adenocarcinoma and colon cancer[22,37,38]. Similar to CCN1, CCN2 achieves functional versatility through its interaction with different integrins, including αvβ3, α5β1, α6β1, αIIbβ3, and αMβ2. In addition to HSPGs, CCN2 can bind to low-density lipoprotein receptor-related proteins (LRPs), such as LRP-1 and LRP-6, to mediate cell adhesion in some cell types. CCN2 can also interact with neurotrophic tyrosine kinase receptor type 1 (NTRK1/TRKA) in human mesangial cells to enhance the transforming growth factor-β (TGF-β)/Smad signaling pathway and in glioma cells to facilitate NF-κB activation[5,6].
In patients with GC, high CCN2 expression correlates with more lymph node metastases, more peritoneal dissemination, and a shorter five-year survival[39-41]. Down-regulation of CCN2 in GC cells reduces peritoneal dissemination in the nude mouse model. In vitro studies have shown that down-regulation of CCN2 decreases GC cell proliferation and colony formation with a concurrent decrease in cyclin D1 expression[42]. After CCN2 down-regulation, GC cells also show attenuated migration/invasion abilities with decreased protein expression and proteolytic activity of both matrix metalloproteinase (MMP)-2 and MMP-9 (Figure 2)[41].
In GC specimens, CCN2 expression is in agreement with the expression of vascular endothelial growth factor VEGF-C and VEGF-D, as shown by immunohistochemical staining[39]. CCN2 can induce angiogenesis, and it can also regulate VEGF-induced angiogenesis through the TSP1 and CT modules[43]. In addition, CCN2 is transcriptionally induced under hypoxia[44], a condition favoring blood vessel growth by the induction of angiogenic factors such as VEGF. Further studies are necessary to elucidate the complex interaction between CCN2 and the VEGF family proteins in GC.
CCN3 was first discovered as an over-expressed gene in a myeloblastosis-associated virus type-1-induced nephroblastoma in chickens[45]. CCN3 is implicated in many diverse biological processes, such as proliferation, differentiation, and angiogenesis, as well as some pathological conditions, including fibrosis and cancer[46]. CCN3 is up-regulated in patients with Wilms’ tumor with predominantly stromal elements and metastatic Ewing’s sarcoma[47,48] but is down-regulated in malignant adrenocortical tumors and poorly differentiated prostate cancer[49,50]. CCN3 can mediate its various activities through interacting with integrins, such as αvβ3, αvβ5, α5β1 and α6β1[5,6]. CCN3 expression has not been reported in GC samples.
CCN4 was first identified in low-metastatic cells by comparing mRNA differential display data from high- and low-metastatic murine melanoma cells[51]. CCN4 is involved in regulating morphological transformation, cell growth, and tumor growth[52,53]. Although CCN4 over-expression suppresses the growth of melanoma tumors in a mouse model, CCN4 is up-regulated in patients with breast cancer, non-small cell lung cancer, colorectal cancer, esophageal squamous cell carcinoma, endometrial endometrioid adenocarcinoma, and prostate cancer[15,22,54-59].
In patients with GC, a truncated variant of CCN4-V1 completely lacking the VWC module is up-regulated in scirrhous GC. In vitro experiments have shown that forced expression of CCN4-V1 in fibroblast cells induces cellular transformation and a rapid growth characterized by cell piling. CCN4-V1 transfectants can enhance the invasive abilities of co-cultured GC cells[8].
The rat homologue of CCN5 was first reported to be down-regulated in rat embryo fibroblasts transformed by the cooperation of the activated H-ras oncogene and the inactivated p53 tumor suppressor gene[60]. CCN5 is involved in regulating cell growth, morphological transformation, and attenuating cell migration[61,62]. CCN5 is down-regulated in patients with colon cancer, pancreatic cancer, and invasive breast cancer[53,61,62]. CCN5 expression has not been reported in cases of GC.
CCN6 was first identified as an expressed sequence tag after database screening for differentially expressed cDNAs after Wnt1-induction in mouse mammary epithelial cells[54]. CCN6 is involved in regulating morphological transformation, inhibiting cell growth, attenuating cell migration, and inhibiting tumor-induced angiogenesis[63,64]. CCN6 is down-regulated in patients with inflammatory breast cancer[65].
In patients with GC, the truncated variant CCN6-V1 lacking TSP1 and CT modules is noted in 11%-20% of microsatellite unstable GCs. A frameshift mutation in the (A)9 repeat in exon 4 of CCN6 leads to a premature stop codon in exon 4 and consequently to truncated CCN6-V1. Forced expression of CCN6 in GC cells can inhibit cell invasive abilities, but CCN6-V1 transfectants lose the inhibitory effect[9,66].
With the greater understanding of the molecular biology of carcinogenesis, more targeted agents have been developed and are associated with improved outcomes in some advanced cancers. Trastuzumab, the first and only targeted agent approved for the treatment of GC, has shown clinical benefits in response rates and survival in combination treatment with chemotherapy for HER-2 positive advanced GC[67]. Because CCN family proteins are implicated in many processes of carcinogenesis, it is reasonable to develop treatment strategies for these potential targets. For this family of secreted proteins, monoclonal antibodies are good therapeutic candidates. Among the six family members, CCN2 has received the most attention because of previous detailed studies and its strong clinical association with fibrosis. Blocking CCN2 with FG-3019, a CCN2 monoclonal antibody, inhibits pancreatic tumor growth and metastases in both xenograft and orthotopic mouse models[68,69]. A phase I study that assessed the safety and tolerance of FG-3019 has been performed in patients with idiopathic pulmonary fibrosis, and FG-3019 was shown to be safe and well-tolerated[70]. Further phase II clinical trials for evaluating its efficacy are underway. For cancer therapy, there is only one ongoing phase I study evaluating FG-3019 therapy in combination with gemcitabine and erlotinib for patients with locally advanced or metastatic pancreatic cancer (ClinicalTrials.gov identifier: NCT01181245). There are currently no clinical trials of CCN-targeted therapy in GC.
In addition to monoclonal antibodies, antisense oligonucleotides (EXC 001) and RNA interference compounds (RXI-109) have been recently developed to reduce scar formation by inhibiting CCN2 expression[6]. Further application of these targeting agents as cancer therapeutics may be helpful for patients with GC.
In summary, CCN family proteins play important roles in mediating GC carcinogenesis (Figure 3), including their involvement in cell signaling pathways, angiogenesis, tumor formation, tumor invasion and metastasis. The recognition of the matricellular protein nature of CCNs with a corresponding biological niche in GC carcinogenesis, as illustrated in this review, may allow oncologic issues to be considered in a new way that will have a positive impact on the future management of GC.
P- Reviewers: Aoyagi K, Ding SZ, Higgins PJ, Lee HC, Stanojevic GZ S- Editor: Qi Y L- Editor: A E- Editor: Wu HL
| 1. | Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23762] [Cited by in RCA: 25540] [Article Influence: 1824.3] [Reference Citation Analysis (7)] |
| 2. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9856] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 3. | Chong HC, Tan CK, Huang RL, Tan NS. Matricellular proteins: a sticky affair with cancers. J Oncol. 2012;2012:351089. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 81] [Cited by in RCA: 93] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 4. | Perbal B. CCN proteins: A centralized communication network. J Cell Commun Signal. 2013;7:169-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 5. | Chen CC, Lau LF. Functions and mechanisms of action of CCN matricellular proteins. Int J Biochem Cell Biol. 2009;41:771-783. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 457] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 6. | Jun JI, Lau LF. Taking aim at the extracellular matrix: CCN proteins as emerging therapeutic targets. Nat Rev Drug Discov. 2011;10:945-963. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 425] [Cited by in RCA: 522] [Article Influence: 37.3] [Reference Citation Analysis (0)] |
| 7. | Bork P. The modular architecture of a new family of growth regulators related to connective tissue growth factor. FEBS Lett. 1993;327:125-130. [PubMed] |
| 8. | Tanaka S, Sugimachi K, Saeki H, Kinoshita J, Ohga T, Shimada M, Maehara Y, Sugimachi K. A novel variant of WISP1 lacking a Von Willebrand type C module overexpressed in scirrhous gastric carcinoma. Oncogene. 2001;20:5525-5532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Thorstensen L, Diep CB, Meling GI, Aagesen TH, Ahrens CH, Rognum TO, Lothe RA. WNT1 inducible signaling pathway protein 3, WISP-3, a novel target gene in colorectal carcinomas with microsatellite instability. Gastroenterology. 2001;121:1275-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Tanaka S, Sugimachi K, Shimada M, Maehara Y, Sugimachi K. Variant WISPs as targets for gastrointestinal carcinomas. Gastroenterology. 2002;123:392-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Li Z, Li J. Local expressions of TGF-beta1, TGF-beta1RI, CTGF, and Smad-7 in Helicobacter pylori-associated gastritis. Scand J Gastroenterol. 2006;41:1007-1012. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Lau LF, Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985;4:3145-3151. [PubMed] |
| 13. | Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6323] [Cited by in RCA: 6566] [Article Influence: 285.5] [Reference Citation Analysis (0)] |
| 14. | Lau LF. CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci. 2011;68:3149-3163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 266] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 15. | Xie D, Nakachi K, Wang H, Elashoff R, Koeffler HP. Elevated levels of connective tissue growth factor, WISP-1, and CYR61 in primary breast cancers associated with more advanced features. Cancer Res. 2001;61:8917-8923. [PubMed] |
| 16. | Tsai MS, Hornby AE, Lakins J, Lupu R. Expression and function of CYR61, an angiogenic factor, in breast cancer cell lines and tumor biopsies. Cancer Res. 2000;60:5603-5607. [PubMed] |
| 17. | Xie D, Yin D, Wang HJ, Liu GT, Elashoff R, Black K, Koeffler HP. Levels of expression of CYR61 and CTGF are prognostic for tumor progression and survival of individuals with gliomas. Clin Cancer Res. 2004;10:2072-2081. [PubMed] |
| 18. | Zeng ZJ, Yang LY, Ding X, Wang W. Expressions of cysteine-rich61, connective tissue growth factor and Nov genes in hepatocellular carcinoma and their clinical significance. World J Gastroenterol. 2004;10:3414-3418. [PubMed] |
| 19. | D’Antonio KB, Toubaji A, Albadine R, Mondul AM, Platz EA, Netto GJ, Getzenberg RH. Extracellular matrix associated protein CYR61 is linked to prostate cancer development. J Urol. 2010;183:1604-1610. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Kok SH, Chang HH, Tsai JY, Hung HC, Lin CY, Chiang CP, Liu CM, Kuo MY. Expression of Cyr61 (CCN1) in human oral squamous cell carcinoma: An independent marker for poor prognosis. Head Neck. 2010;32:1665-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Sampath D, Zhu Y, Winneker RC, Zhang Z. Aberrant expression of Cyr61, a member of the CCN (CTGF/Cyr61/Cef10/NOVH) family, and dysregulation by 17 beta-estradiol and basic fibroblast growth factor in human uterine leiomyomas. J Clin Endocrinol Metab. 2001;86:1707-1715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 22. | Chen PP, Li WJ, Wang Y, Zhao S, Li DY, Feng LY, Shi XL, Koeffler HP, Tong XJ, Xie D. Expression of Cyr61, CTGF, and WISP-1 correlates with clinical features of lung cancer. PLoS One. 2007;2:e534. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 105] [Cited by in RCA: 124] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 23. | Kireeva ML, Lam SC, Lau LF. Adhesion of human umbilical vein endothelial cells to the immediate-early gene product Cyr61 is mediated through integrin alphavbeta3. J Biol Chem. 1998;273:3090-3096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 172] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 24. | Lin MT, Zuon CY, Chang CC, Chen ST, Chen CP, Lin BR, Wang MY, Jeng YM, Chang KJ, Lee PH. Cyr61 induces gastric cancer cell motility/invasion via activation of the integrin/nuclear factor-kappaB/cyclooxygenase-2 signaling pathway. Clin Cancer Res. 2005;11:5809-5820. [PubMed] |
| 25. | Chen CC, Mo FE, Lau LF. The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts. J Biol Chem. 2001;276:47329-47337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 226] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 26. | Mo FE, Muntean AG, Chen CC, Stolz DB, Watkins SC, Lau LF. CYR61 (CCN1) is essential for placental development and vascular integrity. Mol Cell Biol. 2002;22:8709-8720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 322] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 27. | Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci USA. 1998;95:6355-6360. [PubMed] |
| 28. | Ohno R, Yoshinaga K, Fujita T, Hasegawa K, Iseki H, Tsunozaki H, Ichikawa W, Nihei Z, Sugihara K. Depth of invasion parallels increased cyclooxygenase-2 levels in patients with gastric carcinoma. Cancer. 2001;91:1876-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Murata H, Kawano S, Tsuji S, Tsuji M, Sawaoka H, Kimura Y, Shiozaki H, Hori M. Cyclooxygenase-2 overexpression enhances lymphatic invasion and metastasis in human gastric carcinoma. Am J Gastroenterol. 1999;94:451-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 191] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 30. | Yu HG, Li JY, Yang YN, Luo HS, Yu JP, Meier JJ, Schrader H, Bastian A, Schmidt WE, Schmitz F. Increased abundance of cyclooxygenase-2 correlates with vascular endothelial growth factor-A abundance and tumor angiogenesis in gastric cancer. Cancer Lett. 2003;195:43-51. [PubMed] |
| 31. | Lin MT, Kuo IH, Chang CC, Chu CY, Chen HY, Lin BR, Sureshbabu M, Shih HJ, Kuo ML. Involvement of hypoxia-inducing factor-1alpha-dependent plasminogen activator inhibitor-1 up-regulation in Cyr61/CCN1-induced gastric cancer cell invasion. J Biol Chem. 2008;283:15807-15815. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 32. | Lin MT, Chang CC, Lin BR, Yang HY, Chu CY, Wu MH, Kuo ML. Elevated expression of Cyr61 enhances peritoneal dissemination of gastric cancer cells through integrin alpha2beta1. J Biol Chem. 2007;282:34594-34604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285-1294. [PubMed] |
| 34. | Koliopanos A, Friess H, di Mola FF, Tang WH, Kubulus D, Brigstock D, Zimmermann A, Büchler MW. Connective tissue growth factor gene expression alters tumor progression in esophageal cancer. World J Surg. 2002;26:420-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 35. | Hartel M, Di Mola FF, Gardini A, Zimmermann A, Di Sebastiano P, Guweidhi A, Innocenti P, Giese T, Giese N, Büchler MW. Desmoplastic reaction influences pancreatic cancer growth behavior. World J Surg. 2004;28:818-825. [PubMed] |
| 36. | Braig S, Wallner S, Junglas B, Fuchshofer R, Bosserhoff AK. CTGF is overexpressed in malignant melanoma and promotes cell invasion and migration. Br J Cancer. 2011;105:231-238. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 65] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Chang CC, Shih JY, Jeng YM, Su JL, Lin BZ, Chen ST, Chau YP, Yang PC, Kuo ML. Connective tissue growth factor and its role in lung adenocarcinoma invasion and metastasis. J Natl Cancer Inst. 2004;96:364-375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 133] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 38. | Lin BR, Chang CC, Che TF, Chen ST, Chen RJ, Yang CY, Jeng YM, Liang JT, Lee PH, Chang KJ. Connective tissue growth factor inhibits metastasis and acts as an independent prognostic marker in colorectal cancer. Gastroenterology. 2005;128:9-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 39. | Liu L, Li Z, Feng G, You W, Li J. Expression of connective tissue growth factor is in agreement with the expression of VEGF, VEGF-C, -D and associated with shorter survival in gastric cancer. Pathol Int. 2007;57:712-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 40. | Liu LY, Han YC, Wu SH, Lv ZH. Expression of connective tissue growth factor in tumor tissues is an independent predictor of poor prognosis in patients with gastric cancer. World J Gastroenterol. 2008;14:2110-2114. [PubMed] |
| 41. | Jiang CG, Lv L, Liu FR, Wang ZN, Liu FN, Li YS, Wang CY, Zhang HY, Sun Z, Xu HM. Downregulation of connective tissue growth factor inhibits the growth and invasion of gastric cancer cells and attenuates peritoneal dissemination. Mol Cancer. 2011;10:122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 42. | Walker JL, Assoian RK. Integrin-dependent signal transduction regulating cyclin D1 expression and G1 phase cell cycle progression. Cancer Metastasis Rev. 2005;24:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 43. | Inoki I, Shiomi T, Hashimoto G, Enomoto H, Nakamura H, Makino K, Ikeda E, Takata S, Kobayashi K, Okada Y. Connective tissue growth factor binds vascular endothelial growth factor (VEGF) and inhibits VEGF-induced angiogenesis. FASEB J. 2002;16:219-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 285] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 44. | Higgins DF, Biju MP, Akai Y, Wutz A, Johnson RS, Haase VH. Hypoxic induction of Ctgf is directly mediated by Hif-1. Am J Physiol Renal Physiol. 2004;287:F1223-F1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 226] [Cited by in RCA: 227] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 45. | Joliot V, Martinerie C, Dambrine G, Plassiart G, Brisac M, Crochet J, Perbal B. Proviral rearrangements and overexpression of a new cellular gene (nov) in myeloblastosis-associated virus type 1-induced nephroblastomas. Mol Cell Biol. 1992;12:10-21. [PubMed] |
| 46. | Ouellet V, Siegel PM. CCN3 modulates bone turnover and is a novel regulator of skeletal metastasis. J Cell Commun Signal. 2012;6:73-85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 47. | Martinerie C, Huff V, Joubert I, Badzioch M, Saunders G, Strong L, Perbal B. Structural analysis of the human nov proto-oncogene and expression in Wilms tumor. Oncogene. 1994;9:2729-2732. [PubMed] |
| 48. | Manara MC, Perbal B, Benini S, Strammiello R, Cerisano V, Perdichizzi S, Serra M, Astolfi A, Bertoni F, Alami J. The expression of ccn3(nov) gene in musculoskeletal tumors. Am J Pathol. 2002;160:849-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 90] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Martinerie C, Gicquel C, Louvel A, Laurent M, Schofield PN, Le Bouc Y. Altered expression of novH is associated with human adrenocortical tumorigenesis. J Clin Endocrinol Metab. 2001;86:3929-3940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 50. | Wu L, Runkle C, Jin HJ, Yu J, Li J, Yang X, Kuzel T, Lee C, Yu J. CCN3/NOV gene expression in human prostate cancer is directly suppressed by the androgen receptor. Oncogene. 2013;Jan 14; Epub ahead of print. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 51. | Hashimoto Y, Shindo-Okada N, Tani M, Takeuchi K, Toma H, Yokota J. Identification of genes differentially expressed in association with metastatic potential of K-1735 murine melanoma by messenger RNA differential display. Cancer Res. 1996;56:5266-5271. [PubMed] |
| 52. | Xu L, Corcoran RB, Welsh JW, Pennica D, Levine AJ. WISP-1 is a Wnt-1- and beta-catenin-responsive oncogene. Genes Dev. 2000;14:585-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 151] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 53. | Hashimoto Y, Shindo-Okada N, Tani M, Nagamachi Y, Takeuchi K, Shiroishi T, Toma H, Yokota J. Expression of the Elm1 gene, a novel gene of the CCN (connective tissue growth factor, Cyr61/Cef10, and neuroblastoma overexpressed gene) family, suppresses In vivo tumor growth and metastasis of K-1735 murine melanoma cells. J Exp Med. 1998;187:289-296. [PubMed] |
| 54. | Pennica D, Swanson TA, Welsh JW, Roy MA, Lawrence DA, Lee J, Brush J, Taneyhill LA, Deuel B, Lew M. WISP genes are members of the connective tissue growth factor family that are up-regulated in wnt-1-transformed cells and aberrantly expressed in human colon tumors. Proc Natl Acad Sci USA. 1998;95:14717-14722. [PubMed] |
| 55. | Tian C, Zhou ZG, Meng WJ, Sun XF, Yu YY, Li L, Luo HZ, Yang L, Zhou B, Gu J. Overexpression of connective tissue growth factor WISP-1 in Chinese primary rectal cancer patients. World J Gastroenterol. 2007;13:3878-3882. [PubMed] |
| 56. | Davies SR, Davies ML, Sanders A, Parr C, Torkington J, Jiang WG. Differential expression of the CCN family member WISP-1, WISP-2 and WISP-3 in human colorectal cancer and the prognostic implications. Int J Oncol. 2010;36:1129-1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 57. | Nagai Y, Watanabe M, Ishikawa S, Karashima R, Kurashige J, Iwagami S, Iwatsuki M, Baba Y, Imamura Y, Hayashi N. Clinical significance of Wnt-induced secreted protein-1 (WISP-1/CCN4) in esophageal squamous cell carcinoma. Anticancer Res. 2011;31:991-997. [PubMed] |
| 58. | Tang Q, Jiang X, Li H, Lin Z, Zhou X, Luo X, Liu L, Chen G. Expression and prognostic value of WISP-1 in patients with endometrial endometrioid adenocarcinoma. J Obstet Gynaecol Res. 2011;37:606-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 59. | Ono M, Inkson CA, Sonn R, Kilts TM, de Castro LF, Maeda A, Fisher LW, Robey PG, Berendsen AD, Li L. WISP1/CCN4: a potential target for inhibiting prostate cancer growth and spread to bone. PLoS One. 2013;8:e71709. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 56] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 60. | Zhang R, Averboukh L, Zhu W, Zhang H, Jo H, Dempsey PJ, Coffey RJ, Pardee AB, Liang P. Identification of rCop-1, a new member of the CCN protein family, as a negative regulator for cell transformation. Mol Cell Biol. 1998;18:6131-6141. [PubMed] |
| 61. | Dhar G, Mehta S, Banerjee S, Gardner A, McCarty BM, Mathur SC, Campbell DR, Kambhampati S, Banerjee SK. Loss of WISP-2/CCN5 signaling in human pancreatic cancer: a potential mechanism for epithelial-mesenchymal-transition. Cancer Lett. 2007;254:63-70. [PubMed] |
| 62. | Banerjee S, Dhar G, Haque I, Kambhampati S, Mehta S, Sengupta K, Tawfik O, Phillips TA, Banerjee SK. CCN5/WISP-2 expression in breast adenocarcinoma is associated with less frequent progression of the disease and suppresses the invasive phenotypes of tumor cells. Cancer Res. 2008;68:7606-7612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 63. | Kleer CG, Zhang Y, Pan Q, van Golen KL, Wu ZF, Livant D, Merajver SD. WISP3 is a novel tumor suppressor gene of inflammatory breast cancer. Oncogene. 2002;21:3172-3180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 64. | Huang W, Zhang Y, Varambally S, Chinnaiyan AM, Banerjee M, Merajver SD, Kleer CG. Inhibition of CCN6 (Wnt-1-induced signaling protein 3) down-regulates E-cadherin in the breast epithelium through induction of snail and ZEB1. Am J Pathol. 2008;172:893-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 65. | van Golen KL, Davies S, Wu ZF, Wang Y, Bucana CD, Root H, Chandrasekharappa S, Strawderman M, Ethier SP, Merajver SD. A novel putative low-affinity insulin-like growth factor-binding protein, LIBC (lost in inflammatory breast cancer), and RhoC GTPase correlate with the inflammatory breast cancer phenotype. Clin Cancer Res. 1999;5:2511-2519. [PubMed] |
| 66. | Tanaka S, Sugimachi K, Maehara S, Shimada M, Maehara Y. A loss of function mutation in WISP3 derived from microsatellite unstable gastric carcinoma. Gastroenterology. 2003;125:1563-1564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 67. | Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687-697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5541] [Cited by in RCA: 5317] [Article Influence: 354.5] [Reference Citation Analysis (3)] |
| 68. | Mageto Y, Flaherty K, Brown K, Fong A, Raghu G. Safety and tolerability of human monoclonal antibody FG-3019, anti-connective tissue growth factor, in patients with idiopathic pulmonary fibrosis. Chest. 2004;126:773S. [RCA] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 69. | Dornhöfer N, Spong S, Bennewith K, Salim A, Klaus S, Kambham N, Wong C, Kaper F, Sutphin P, Nacamuli R. Connective tissue growth factor-specific monoclonal antibody therapy inhibits pancreatic tumor growth and metastasis. Cancer Res. 2006;66:5816-5827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 122] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 70. | Aikawa T, Gunn J, Spong SM, Klaus SJ, Korc M. Connective tissue growth factor-specific antibody attenuates tumor growth, metastasis, and angiogenesis in an orthotopic mouse model of pancreatic cancer. Mol Cancer Ther. 2006;5:1108-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 128] [Article Influence: 6.7] [Reference Citation Analysis (0)] |