Published online Feb 14, 2014. doi: 10.3748/wjg.v20.i6.1630
Revised: October 20, 2013
Accepted: December 3, 2013
Published online: February 14, 2014
Processing time: 150 Days and 1.7 Hours
Primary hepatic carcinosarcoma is a rare tumor and is comprised of a mixture of carcinomatous and sarcomatous elements. We present a case of primary carcinosarcoma of the liver in a 59-year-old woman, which was confirmed by pathology following surgical resection. Using contrast-enhanced ultrasonography, the tumor showed peripheral nodular hyperenhancement in the arterial phase with two feeding arterial vessels and a large internal non-enhancing portion in the center. The peripheral nodular portion of the tumor showed hypoenhancement in the later phase.
Core tip: Primary hepatic carcinosarcoma is a rare tumor and is comprised of a mixture of carcinomatous and sarcomatous elements, easily misdiagnosed as other types of tumor or an abscess. This is the first report on the contrast-enhanced ultrasonography characteristics of primary hepatic carcinosarcoma.
- Citation: Liu LP, Yu XL, Liang P, Dong BW. Characterization of primary hepatic carcinosarcoma by contrast-enhanced ultrasonography: A case report. World J Gastroenterol 2014; 20(6): 1630-1634
- URL: https://www.wjgnet.com/1007-9327/full/v20/i6/1630.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i6.1630
Carcinosarcoma of the liver is defined as a tumor containing an intimate mixture of carcinomatous and sarcomatous elements[1]. Primary hepatic carcinosarcoma is rare with less than 30 adequately imaged documented cases reported[2-5]. We present a case of carcinosarcoma of the liver confirmed by pathology following surgical resection. To our knowledge there has been no report of CEUS of primary hepatic carcinosarcoma.
A 59-year-old woman was admitted to our hospital with a liver tumor. She had a high fever with cold symptoms for 1 mo before admission, and had been misdiagnosed with an abscess in a smaller hospital. The main biochemical indices of the liver were: serum alpha-fetoprotein (AFP) level of 221.7 μg/L, γ-glutamyl transpeptidase level of 67 U/L, serum total protein of 81.9 g/L, and total bile acid of 49 μmol/L. Serum markers for hepatitis B and C were negative. A history of hepatitis, tuberculosis, heart disease, hypertension or diabetes was denied.
Conventional ultrasonography (US) and contrast-enhanced ultrasonography (CEUS) were performed using a Technos MPS DU8 sonography system (Esaote Genova, Italy). CEUS software allows real-time imaging at a low mechanical index (MI). The contrast agent used in this study was SonoVue (Bracco, Milan, Italy). The patient gave her full informed consent for SonoVue injection, which was injected intravenously as a bolus of 2.4 mL through the antecubital vein followed by a 5-mL saline flush. The perfusion and enhancement of the lesion were continuously observed until sufficient dynamic information on the lesions was obtained, and the entire liver was then scanned. The MI value was 0.8 on CEUS.
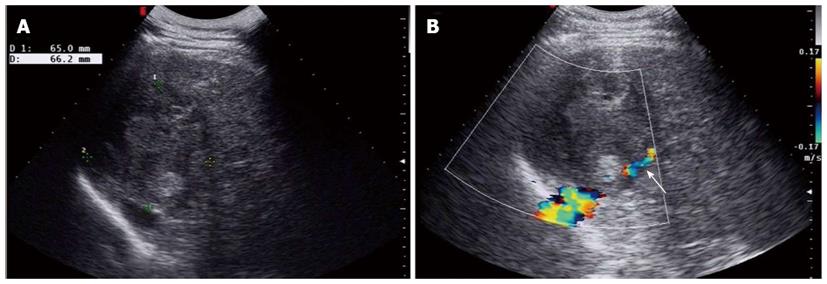
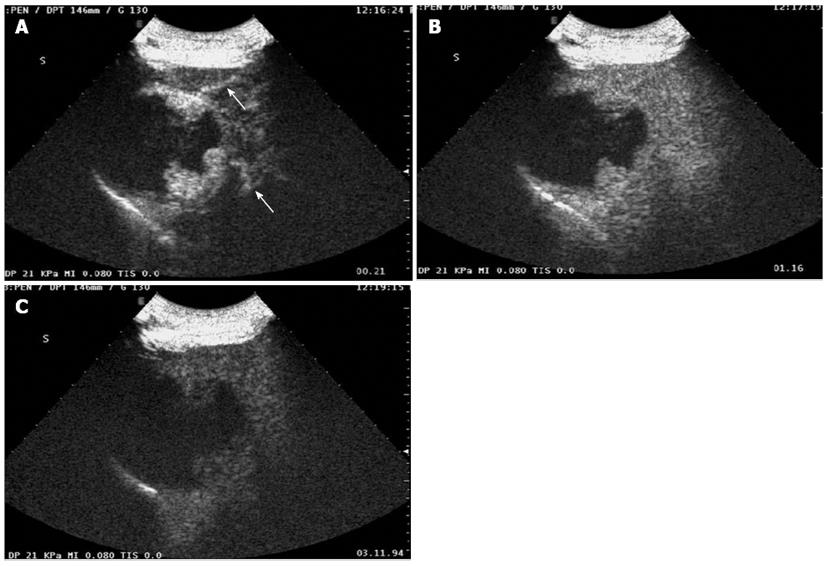
Baseline sonogram of the liver showed both a coarse echo pattern and surface nodularity, which are sonographic signs of cirrhosis. At the same time, an ovoid heterogeneous echogenic mass within the right lobe of the liver was noted (Figure 1A). The tumor was 6.5 cm × 6.6 cm in size. Color Doppler imaging showed that the tumor had compressed the right hepatic vein (Figure 1B). CEUS displayed peripheral nodular enhancement of the tumor in the early arterial phase with two feeding arterial vessels and a large internal non-enhancing portion in the central area (Figure 2A). The peripheral nodular portion of the tumor was hyper-enhancing and iso-enhancing in the portal phase (Figure 2B) and hypo-enhancing in the later phase (Figure 3C). The large internal portion was non-enhancing in all three phases.
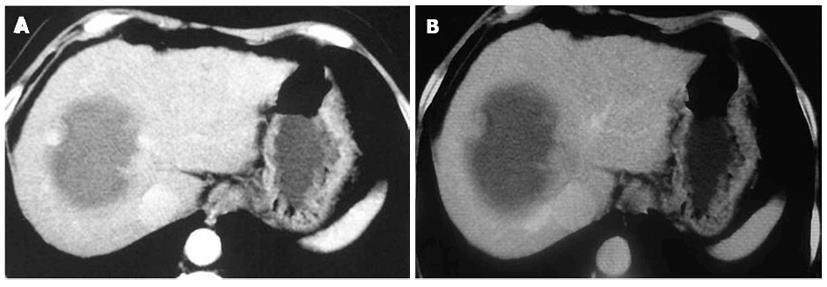
Computed tomographic (CT) scan of the tumor revealed peripheral nodular enhancing portions in the arterial phase with large internal non-enhancing necrotic portions (Figure 3A). The peripheral nodular portion of the tumor was iso-enhancing in the portal phase (Figure 3B). Enhanced-CT imaging features were the same as contrast-enhanced sonography.
The patient underwent surgical resection. The liver was found to be cirrhotic with a nodular surface. It was a large mass without daughter nodules. However, the middle hepatic vein was impinged by a thrombus, which was resected with complete removal of the hepatic venous thrombus.
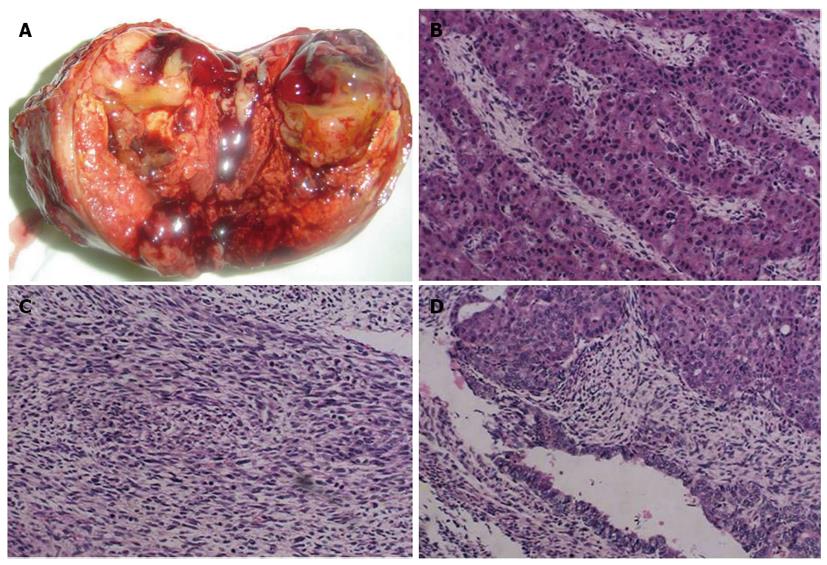
Macropathology of the tumor showed that it was grayish-white, grayish-red and soft, with hemorrhagic necrosis (Figure 4A). The surrounding organizational boundaries were unclear, with central gray, gelatinous tissue. The mass measured 8 cm × 7 cm × 6 cm with no capsule. The diagnosis was carcinosarcoma containing both hepatocellular and sarcomatous elements (Figure 4B-D). Furthermore, the thrombus in the middle hepatic vein was metastasis.
The patient was discharged after 31 d of hospitalization. She then received chemotherapy. Seventeen days after discharge, she was re-admitted with hepatic decompensation due to hepatorenal syndrome, portal hypertension and ascites. Subsequently, peritoneal thickening and enlarged abdominal lymph nodes were found. She died of recurrence 50 d after surgery.
Carcinosarcoma is a miscellaneous tumor[1]. The World Health Organization (WHO) defined carcinosarcoma as a tumor containing both carcinomatous (either hepatocellular or cholangiocellular) and sarcomatous elements. It is a malignant mixed tumor. Including the present case, less than 30 cases of carcinosarcoma fulfilling the WHO criteria have been reported in PubMed. Hepatic carcinosarcoma generally has a highly aggressive and malignant biological course with rapid growth and local infiltration[6].
Kwon et al[7] reported a case of carcinosarcoma of the liver with cholangiocarcinomatous elements and osteosarcomatous and chondrosarcomatous elements. CT analysis of this tumor revealed peripheral enhancing viable portions, large internal non-enhancing necrotic portions, and a dense radiopaque lesion. On magnetic resonance imaging, this tumor was observed, on T2-weighted images, to have slightly hyperintense peripheral viable portions, moderately hyperintense internal necrotic portions, and very hyperintense small central cystic portions. In addition, calcification and ossification had low or dark signal intensity on T1- and T2-weighted images.
Sumiyoshi et al[6] observed one case of hepatic carcinosarcoma which was demonstrated to be embryonal sarcoma in cholangiocarcinoma. This carcinosarcoma on magnetic resonance imaging revealed hypointensity on T1-weighted images and heterogeneous hyperintensity on T2-weighted images. Shu et al[5] reported one case of liver carcinosarcoma, and triple-phase contrast CT revealed a mixed density mass with inhomogeneous enhancement, multiple cystic nodules and irregular rim enhancement. They demonstrated that the hepatic carcinosarcoma CT presentation was unspecific and the pre-operative diagnosis was difficult.
Deng et al[8] described one case that was misdiagnosed as liver abscess by baseline sonography. Contrast-enhanced CT revealed a hepatic malignant tumor (AFP, 202 μg/L). After surgery, the tumor was diagnosed as primary hepatic carcinosarcoma with necrotic and cystic elements based on pathology.
Low mechanical index real-time CEUS is a new US technique which improves the differential diagnosis of focal liver lesions. Hepatic focal nodular hyperplasia (FNH) appears spoke wheel-shaped with a centrifugal filling contrast enhancement pattern in the early artery phase on CEUS[9]. In the portal or later phase, some FNHs may show central scars[10]. Hemangiomas show peripheral enhancement that gradually fills in the arterial phase[11]. However, the present case demonstrated peripheral nodular enhancement with two feeding artery vessels in the arterial phase without gradual fill-in. Furthermore, there was slight hypoenhancement of the peripheral nodular area in the later phase with a large non-enhanced center in the three phases, which is different from typical hepatocellular carcinoma (HCC) and liver metastatic tumors. Typically, HCC appears with homogeneous hyperenhancement during the arterial phase and hypoenhancement during the portal or/and late phases[12]. Typically, hepatic metastasis shows peripheral rim-like or variable intralesional enhancement during the arterial phase, and often marks hypoenhancement during the portal and late phases[13]. The CEUS enhancement patterns of different hepatic tumors are summarized in Table 1.
| Tumor | In the artery phase | In the portal phase | In the late phase |
| FNH | Spoked-wheel enhancement | Hyperenhancing/or with scar | Iso/hyperenhancing/or with scar |
| Hemangiomas | Peripheral nodular enhancement and centripetal fill-in | Partial/complete centripetal fill-in | Partial/complete centripetal fill-in |
| HCC | Hyperenhancing | Iso/hypoenhancement | Hypoenhancing |
| Metastatic tumor | Rim-like or variable intralesional enhancement | Homogeneous or heterogeneous hypoenhancement | Hypoenhancement (often marked hypoenhancement) |
| Hepatic carcinosarcoma | Peripheral nodular or irregular rim enhancement with large internal non-enhancing area | Peripheral isoenhancing with large internal non-enhancement area | Peripheral hypoenhancing with large internal non-enhancement area |
The imaging findings of hepatic carcinosarcoma presented in our case showed a large mass with a large necrotic portion. It is difficult to establish an accurate preoperative diagnosis. However, if a primary hepatic tumor exhibits a large necrotic mass, carcinosarcoma should be considered. Conventional sonography is usually the first imaging technique used in patients with carcinosarcoma, and CEUS plays an important role in demonstrating primary liver carcinosarcoma enhancement patterns.
The patient had high fever with cold symptoms for 1 mo.
The patient had primary hepatic tumor with cirrhosis and an alpha-fetoprotein (AFP) level of 221.7 μg/L.
Contrast-enhanced ultrasonography, computed tomographic scan and serum AFP determination helped in the differential diagnosis.
Serum markers for hepatitis B and C were negative, but serum AFP level was 221.7 μg/L.
On contrast-enhanced ultrasonography (CEUS), the tumor showed peripheral nodular hyperenhancement in the arterial phase with two feeding arterial vessels and a large internal non-enhancing portion in the center.
The patient underwent surgical resection of the tumor and received chemotherapy.
The tumor revealed a large internal non-enhancing portion in the center, easily misdiagnosed as a liver abscess, however, peripheral nodular hyperenhancement in the arterial phase and AFP level helped in the diagnosis of malignancy.
The tumor showed a large internal non-enhancing portion in the center, easily misdiagnosed as a liver abscess in patients with high fever. Furthermore, peripheral nodular hyperenhancement in the arterial phase may easily be misdiagnosed as a hemangioma, but without gradual fill-in and the presence of cirrhosis, and elevated AFP level helped in the diagnosis of malignancy.
The authors reported on the rare CEUS characteristics of a primary hepatic carcinosarcoma, and the contrast enhanced-computed tomographic imaging, pathologic and surgical specimen findings. The paper has interesting information about a rare disease with new technology for the diagnosis.
P- Reviewers: Tellez-Avila F, Yan SL S- Editor: Zhai HH L- Editor: A E- Editor: Wang CH
| 1. | Ishak KG, Anthony PP, Sobin LH. Histological typing of tumours of the liver (WHO. World Health Organization. International Histological Classification of Tumours). 2nd ed. Berlin: Springer-Verlag 1994; 27-28. |
| 2. | Lin YS, Wang TY, Lin JC, Wang HY, Chou KF, Shih SC, Chen MJ. Hepatic carcinosarcoma: clinicopathologic features and a review of the literature. Ann Hepatol. 2013;12:495-500. [PubMed] |
| 3. | Aparicio MA, Esteban C, Bengoechea O, Muñoz-Bellvís L. Primary carcinosarcoma of the liver: an unusual case with clearly separated epithelial and mesenchymal components. Rev Esp Enferm Dig. 2011;103:336-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Celikbilek M, Deniz K, Torun E, Artis T, Ozaslan E, Karahan OI, Patiroglu TE, Ozbakir O. Primary hepatic carcinosarcoma. Hepatobiliary Pancreat Dis Int. 2011;10:101-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Shu RY, Ye M, Yu WY. A case of primary liver carcinosarcoma: CT findings. Chin J Cancer. 2010;29:346-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Sumiyoshi S, Kikuyama M, Matsubayashi Y, Kageyama F, Ide Y, Kobayashi Y, Nakamura H. Carcinosarcoma of the liver with mesenchymal differentiation. World J Gastroenterol. 2007;13:809-812. [PubMed] |
| 7. | Kwon JH, Kang YN, Kang KJ. Carcinosarcoma of the liver: a case report. Korean J Radiol. 2007;8:343-347. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 30] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Deng HQ, Li W. Cystic degeneration in primary hepatic carcinosarcoma misdiagnosed as liver abscess. Linchuang Wuzhen Wuzhi Zazhi. 2004;17:28. |
| 9. | Wang W, Chen LD, Lu MD, Liu GJ, Shen SL, Xu ZF, Xie XY, Wang Y, Zhou LY. Contrast-enhanced ultrasound features of histologically proven focal nodular hyperplasia: diagnostic performance compared with contrast-enhanced CT. Eur Radiol. 2013;23:2546-2554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Liu LP, Dong BW, Yu XL, Liang P, Zhang DK, An LC. Focal hypoechoic tumors of Fatty liver: characterization of conventional and contrast-enhanced ultrasonography. J Ultrasound Med. 2009;28:1133-1142. [PubMed] |
| 11. | Quaia E, Bartolotta TV, Midiri M, Cernic S, Belgrano M, Cova M. Analysis of different contrast enhancement patterns after microbubble-based contrast agent injection in liver hemangiomas with atypical appearance on baseline scan. Abdom Imaging. 2006;31:59-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 12. | Jang JY, Kim MY, Jeong SW, Kim TY, Kim SU, Lee SH, Suk KT, Park SY, Woo HY, Kim SG. Current consensus and guidelines of contrast enhanced ultrasound for the characterization of focal liver lesions. Clin Mol Hepatol. 2013;19:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Xu HX, Liu GJ, Lu MD, Xie XY, Xu ZF, Zheng YL, Liang JY. Characterization of focal liver lesions using contrast-enhanced sonography with a low mechanical index mode and a sulfur hexafluoride-filled microbubble contrast agent. J Clin Ultrasound. 2006;34:261-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.3] [Reference Citation Analysis (0)] |