Published online Feb 14, 2014. doi: 10.3748/wjg.v20.i6.1554
Revised: September 9, 2013
Accepted: September 16, 2013
Published online: February 14, 2014
Processing time: 299 Days and 18.8 Hours
AIM: To investigate the activity and expression of EAAT2 glutamate transporter in both in vitro and in vivo models of cholestasis.
METHODS: This study was conducted on human hepatoblastoma HepG2 cell cultures, the liver of bile duct ligated rats and human specimens from cholestatic patients. EAAT2 glutamate transporter activity and expression were analyzed using a substrate uptake assay, immunofluorescence, reverse transcription-polymerase chain reaction, and immunohistochemistry, respectively.
RESULTS: In HepG2 cells, cholestasis was mimicked by treating cells with the protein kinase C activator, phorbol 12-myristate 13-acetate. Under such conditions, EAAT2 transporter activity was decreased both at the level of substrate affinity and maximal transport velocity. The decreased uptake was correlated with intracellular translocation of EAAT2 molecules as demonstrated using immunofluorescence. In the liver of bile duct ligated rats, an increase in EAAT2 transporter protein expression in hepatocytes was demonstrated using immunohistochemistry. The same findings were observed in human liver specimens of cholestasis in which high levels of γ-glutamyl transpeptidase were documented in patients with biliary atresia and progressive familial intrahepatic cholestasis type 3.
CONCLUSION: This study demonstrates the alteration in glutamate handling by hepatocytes in liver cholestasis and suggests a potential cross-talk between glutamatergic and bile systems.
Core tip: The aim of the current study was to demonstrate the role of glutamate transport, the most abundant intracellular hepatic amino acid, in liver cholestasis. The study was conducted in vitro using HepG2 cells as well as the livers of bile duct ligated rats and human cholestasis specimens. The principal data revealed that the activity and expression of EAAT2-mediated glutamate transport were altered both in vitro and in vivo. This supports the involvement of glutamate transporters, as an indirect liver response and/or as a direct hepatic target, in restoring intracellular pools of this amino acid which are probably altered after cholestasis.
- Citation: Najimi M, Stéphenne X, Sempoux C, Sokal E. Regulation of hepatic EAAT-2 glutamate transporter expression in human liver cholestasis. World J Gastroenterol 2014; 20(6): 1554-1564
- URL: https://www.wjgnet.com/1007-9327/full/v20/i6/1554.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i6.1554
The liver is considered to be the major site of amino acid and protein homeostasis. Of these amino acids, L-glutamate is the most abundant intracellular amino acid. Accordingly, this intermediate is involved in a wide variety of liver metabolic pathways such as ureagenesis, gluconeogenesis and glutathione synthesis. The intracellular availability of glutamate is highly controlled both at the level of synthesis, after conversion of a variety of precursors, and uptake from the extracellular environment. High glutamate concentrations have been documented in liver and bile[1] and can serve as substrate pools for glutamate transporters predominantly expressed at the canalicular side of the hepatic cell[2]. Amongst the glutamate transporters (EAATs 1-5) described so far[3], GLT-1/EAAT2 has been well-documented in the liver[4-6]. However, its role is not yet fully understood, although many reports have demonstrated that glutamate transporters are subject to direct regulation at the transcriptional, translational and posttranslational levels[7]. Glutamate transporters directly or indirectly cross-talk with proteins inserted within the same membrane or with several intracellular signaling pathways[8-11]. Protein kinase C (PKC) represents one of the widely studied intracellular pathways that control both the activity and the expression of glutamate transporters. Such a functional relationship has been demonstrated in several models, particularly of central nervous system origin[9,12-14]. Activation of PKC in the liver is correlated with the appearance of symptoms related to cholestasis. Indeed, a clear positive and negative modulation of bile flow has been reported in the rat liver following PKC activation. Such an effect was correlated with the regulation of both apical exocytosis and sinusoidal bile acid uptake[15]. In vitro, down-regulation of MRP2 transporter expression, a potential condition related to cholestasis development, has been demonstrated in human HepG2 cells treated with PKC activator[16]. Together, these data support the investigation of the potential involvement of glutamate transporters in liver physiopathology.
In the current study, we demonstrate that PMA mediated activation of PKC in HepG2 cells, an experimental condition mimicking liver cholestasis, and modulated both EAAT2 transporter activity and expression. In vivo, EAAT2 expression in the livers of bile duct ligated rats and in human specimens of cholestatic disease was up-regulated. These alterations in GLT-1/EAAT2 expression observed in both experimental in vitro and in vivo models of cholestasis suggest involvement of the glutamate system as part of the liver response or as a direct liver target of cholestasis.
PMA, 4’,6-diamidino-2-phenylindole (DAPI), L-aspartate and phenylarsine oxide (PAO) were obtained from Sigma, Ro-8220 and PD98056 were from Calbiochem, and D-[3H]-aspartate was from Amersham (Amersham Pharmacia Biotech, Roosendaal, Netherlands). Anti-GLT-1/EAAT2 antibody was purchased from Abcam (Cambridge, United Kingdom), and anti-actin and anti-MRP2 antibodies were provided by Sigma and Alexis Biochemicals, respectively. L-trans-pyrrolidine-2,4-dicarboxylic acid (t-PDC) and threo-beta-benzyloxyaspartate (TBOA) were purchased from Tocris (Bristol, United Kingdom) and dihydrokainic acid (DHK) was from Ocean Product International (Nova Scotia, Canada). The culture medium, penicillin, streptomycin, non-essential amino acids, pyruvate sodium, trypsin-EDTA, reverse transcription (RT) kit, elongase and PCR primers were obtained from Invitrogen (Merelbeke, Belgium). Fœtal calf serum was purchased from Perbio Science (Erembodegem, Belgium).
HepG2 cells were cultured in plastic flasks (CELLSTAR®, Greiner Bio One) using DMEM containing 4.5 g/L glucose (Ref number: 41965-039) medium supplemented with 10% fetal calf serum, 1% non-essential amino acids, 1% pyruvate sodium, and 1% penicillin-streptomycin. At confluence, cells were routinely lifted using 0.05% trypsin-EDTA and replated at a 1:10 dilution. All cultures were maintained at 37 °C in a water-saturated atmosphere containing 5% CO2. All culture media and consumables were from Invitrogen.
Experiments performed in this study were approved by the local ethical review board. Male Wistar rats were subjected to a 12-h day-night rhythm with free access to food and water. Four sham and 4 bile duct ligated rats were used for these experiments. Animals were anesthetized with an intraperitoneal injection of xylazine/ketamine, and were randomly assigned to bile duct ligation or sham operation. Briefly, the common bile duct was identified, double-ligated close to the liver hilus and cut between the ligatures. In sham-operated rats, the bile duct was only identified and exposed. Fifteen days later, livers were harvested for analysis. Specimens were fixed in formalin for 48 h and embedded in paraffin.
Cholestatic human liver tissue was obtained from the resected livers of patients undergoing orthotopic liver transplantation for biliary cirrhosis, due to biliary atresia (BA) (n = 4) or progressive familial intrahepatic cholestasis (PFIC) (n = 4). For controls, we used 3 liver specimens from patients transplanted for a non-cholestatic liver disease (2 Crigler Najjar and 1 oxalosis). Use of these tissues for research purposes was approved by the institution review board and the patient’s representative gave approval. Samples of liver tissue were fixed in formalin for 24-48 h and embedded in paraffin.
At 80% confluence, plates with hepG2 cells were placed at the surface of a 37 °C water bath, rinsed twice with preheated Krebs buffer[8] and then treated with drugs or vehicles. For the saturation studies, D-[3H]-aspartate (30 nmol/L) was diluted with unlabeled L-aspartate to achieve final aspartate concentrations of 1-200 μmol/L. Inhibitors were added 15 min before the addition of PMA. Unless stated, uptake was stopped after 6 min by 3 rinses with ice-cold Na+-free Krebs buffer in which NaCl was substituted with equi-osmolar choline chloride. The cells were lysed with 500 μL of 1 N NaOH and the radioactivity of 200 μL of the lysate was determined by liquid scintillation counting. A fraction of the lysate was also used for protein determination. The specific activity of the glutamate transporters (expressed as the uptake velocity per mg of protein) was estimated after subtracting the data obtained using Na+-free Krebs buffer.
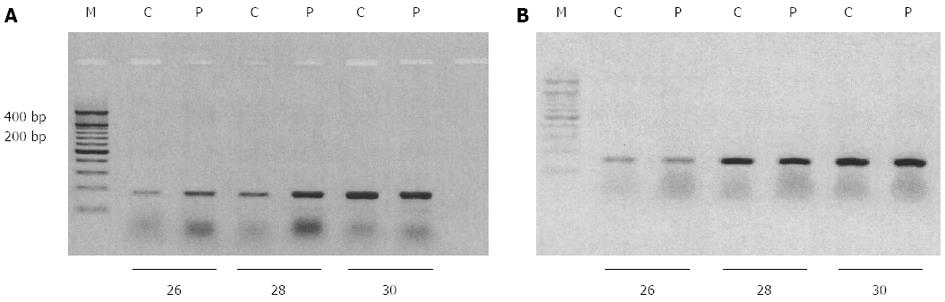
Total RNA was extracted from cells grown in 6 well-plates using the TriPure isolation reagent and cDNA was generated using the reverse transcription kit, according to the manufacturer’s instructions. Polymerase chain reaction (PCR) amplifications targeting EAAT2 and GAPDH were performed using polymerase elongase in a final volume of 25 μL and appropriate primers (EAAT2 sense: 5’-aaatgaatggtgttgtcctgg-3’, EAAT2 antisense: 5’-cttcatgtcatcataaatgg-3’ and GAPDH sense: 5’-cggagtcaacggatttggtcgtat-3’, GAPDH antisense 5’-agccttctccatggtggtgaagac-3’) generating distinct amplification products (341 and 307 base pairs for EAAT2 and GAPDH, respectively). After 26, 28 and 30 cycles of amplification consisting of denaturing at 94 °C for 30 s, annealing at 60 °C for 60 s and extension at 72 °C for 90 s, samples were electrophoresed on a 1% agarose gel and nucleic acids were visualized by ethidium bromide staining.
Total extract samples were mixed with loading buffer (125 mmol/L Tris HCl, 50 mmol/L dithiothreitol, 4% sodium dodecyl sulfate, 20% glycerol, 0.01% bromophenol blue, pH 6.8), boiled for 5 min and loaded onto 5% sodium dodecyl sulfate-polyacrylamide gel according to Laemmli[17]. After electrophoresis, proteins were transferred to nitrocellulose membranes with a Bio-Rad minitransblot electrophoretic transfer cell. Non-specific immunodetection was prevented by incubating the nitrocellulose membranes in TBS buffer (50 mmol/L Tris pH 8.1, 150 mmol/L NaCl) containing 10% dry milk and 0.05% Tween-20 for 1 h with gentle shaking at room temperature. The membranes were thereafter probed with affinity-purified mouse anti-MRP2 (Alexis Biochemicals, 0.5 μg/mL) or rabbit anti-actin (Sigma, 1:1000) diluted in blocking solution for 18 h at 4 °C. The antigen-antibody complex was visualized with a horseradish peroxidase-conjugated anti-mouse (Amersham, 1:6000) or goat anti-rabbit IgG secondary antibody (Sigma, 1:3000). Immunoreactive proteins were detected using enhanced chemiluminescence reagents (Perkin-Elmer).
Cell viability following PMA treatment was evaluated by analyzing HepG2 cell culture supernatants for lactate dehydrogenase (LDH) (Cytotoxicity detection kit, Roche). Intracellular ATP concentrations were also semi-quantitatively measured in HepG2 cell cultures using a commercially available kit (ATP Bioluminescence Assay Kit CLS II, Roche) according to the manufacturer’s instructions.
HepG2 cells were plated on sterile glass coverslips and treated for 4 h with 500 nmol/L PMA or vehicle. Cells were then washed twice in PBS and fixed in 4% paraformaldehyde for 20 min at room temperature. After washing, cells were permeabilized with 0.1% Triton X-100 for 15 min and blocked with 5% dry milk in TBS for 1 h at room temperature. The cells were incubated with anti-EAAT2 primary antibody (1:50) in the blocking solution for 1 h at room temperature. After three washes with TBS, cells were further incubated with a fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit antibody for 1 h at room temperature (1:500, Sigma, Bornem, Belgium). Cells were washed with TBS solution three times, once with PBS and the coverslips were mounted on glass slides in Mowiol with 2.5% DABCO overnight at room temperature and examined with an Axiovert confocal microscope (Zeiss, Oberkochen, Germany) coupled to MRC 1024 confocal scanning equipment (Bio-Rad, Richmond, CA, United States).
Immunohistochemistry of both animal and human liver samples was performed on 5 μm-thick liver sections that were deparaffinized and rehydrated in a graded alcohol series. After endogenous peroxidase activity blockade by incubation for 15 min in a 3% hydrogen peroxide methanol solution, the slices were incubated with citric acid monohydrate solution (pH 6.0) at 97 °C for 90 min for antigen retrieval. Non-specific immuno-staining was prevented by incubation in PBS buffer containing 1% normal goat serum for 1 h at room temperature. Slices were thereafter incubated overnight with polyclonal anti-EAAT2 antibody at a dilution of 1/4000 at room temperature.
Staining was visualized by EnVision + System-HRP Labeled Polymer Anti-Rabbit (DAKO) using diaminobenzidine (Sigma, Belgium) as a chromogenic substrate. The nuclei were counterstained using Mayer’s hematoxylin for 10 min. Preparations were then mounted for microscopic analysis.
Data from the glutamate uptake experiments were analyzed by nonlinear regression using the curve-fitting program (GraphPad Software, San Diego, CA, United States). Statistical analysis of the data was performed using GraphPad Prism software. Statistical differences were determined by the Student’s t test for two-group comparison or by One-way ANOVA followed by the Tukey’s test for multiple comparisons between more than two groups. A probability of P < 0.05 was considered significant.
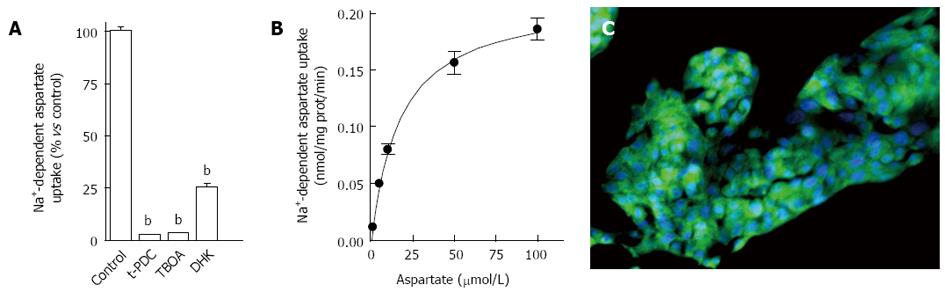
As explained in the Methods section, glutamate transport was evaluated in HepG2 cells following incubation with radiolabeled D-aspartate, a non-metabolizable analog of glutamate, for 6 min. Significant specific Na+-dependent D-[3H]-aspartate uptake was measured and no uptake was detected using Na+-free buffer (data not shown). When HepG2 cells were incubated with the non-selective transportable inhibitor, t-PDC, or the non-transportable inhibitor, TBOA, Na+-dependent aspartate uptake was completely abolished (Figure 1A) indicating the involvement of high affinity glutamate transporters. The use of DHK, a selective inhibitor of EAAT2 mediated uptake, demonstrated that EAAT2 was the predominant glutamate transporter expressed in HepG2 cells (Figure 1A).
Further glutamate transport characterization was performed after incubating HepG2 cells with increasing concentrations of the substrate. Non-linear analysis of D-[3H]-aspartate uptake revealed a maximal velocity of 0.220 ± 0.001 nmol/mg of protein/min and a Km value of 17.1 ± 2.6 μmol/L (Figure 1B). RT-PCR analyses (data not shown) and immunofluorescence confirmed the expression of EAAT2 transporter in HepG2 cells (Figure 1C). Together these data demonstrate that HepG2 cells primarily express functional EAAT2 glutamate transporters.
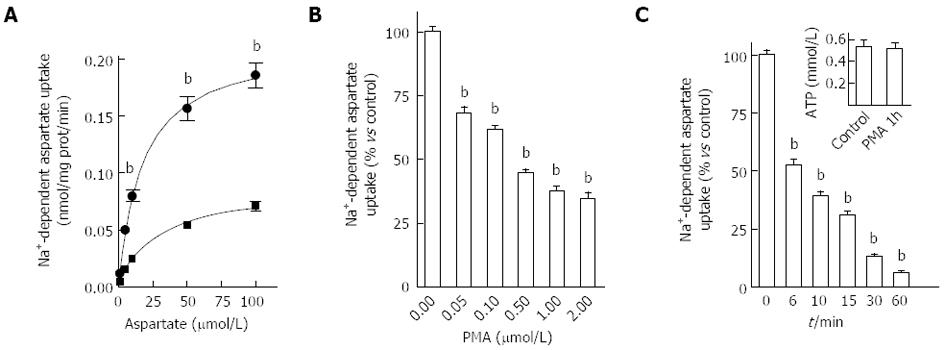
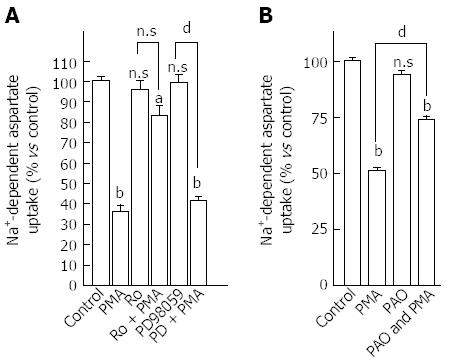
We thereafter examined if EAAT2 transporter activity could be regulated after PKC activation. To do so, HepG2 cells were exposed to 500 nmol/L PMA for 15 min and aspartate uptake was evaluated for 6 min. Non-linear analysis of the specific uptake of D-[3H]-aspartate/L-aspartate isotopic dilutions revealed that PMA treatment decreased EAAT2-mediated uptake capacity by 60% (Bmax values of 0.220 ± 0.001 nmol/mg of protein/min for control cells and 0.088 ± 0.005 nmol/mg of protein/min for PMA-treated cells) as well as EAAT2 affinity for the substrate (Km values of 17.1 ± 2.6 μmol/L for control and 27.1 ± 4.3 μmol/L for PMA-treated cells) (Figure 2A). The effect of PMA on aspartate uptake was concentration-dependent as shown in Figure 2B and was maintained after prolonged exposure (Figure 2C). A 90% decrease in aspartate uptake capacity was reached when HepG2 cells were incubated for 60 min with PMA. This decrease in aspartate uptake was not associated with a decrease in cell viability as no difference in ATP levels was observed between PMA and vehicle-treated cells (Figure 2C, inset). Involvement of the PKC pathway in modulating EAAT2-mediated glutamate uptake in HepG2 cells was confirmed by the complete abolition of the observed effect using the PKC inhibitor Ro-31-8220 (1 μmol/L) (Figure 3A). Besides PKC activation, PMA also indirectly modulated other closely connected pathways. To investigate the involvement of additional mediators in such modulation, HepG2 cells were pretreated with the MEK inhibitor, PD98059 (10 μmol/L). No inhibition of the PMA-mediated decrease in aspartate uptake was observed (Figure 3A).
As the inhibition of aspartate uptake observed in HepG2 cells after PMA treatment could be correlated with internalization of transporter molecules from the plasma membrane, cells were pretreated with PAO in order to block both internalization and membrane insertion processes[18-20]. In PAO pre-treated cells, a partial inhibition of the PMA effect was observed (Figure 3B) suggesting that internalization of glutamate transporters is part of the mechanism (in addition to decreased affinity) behind the decrease in HepG2 cells’ ability to take up aspartate. Together these data suggest the potential of PKC to modulate both EAAT2-mediated transport activity and membrane localization.
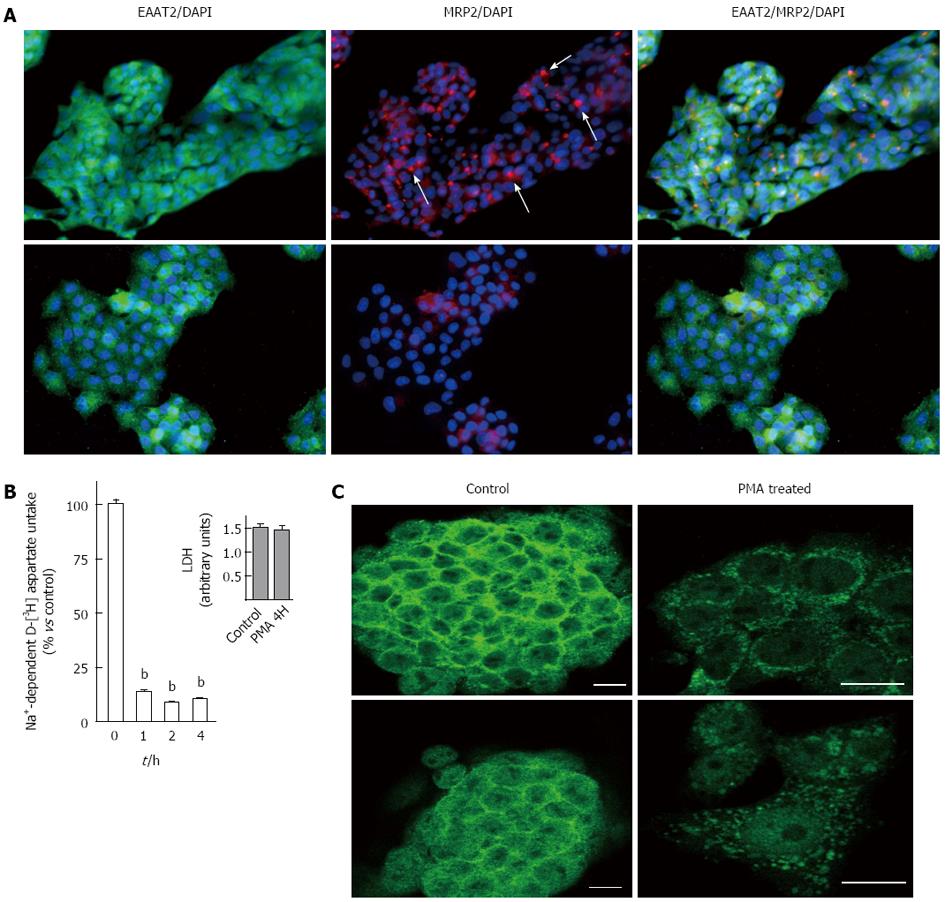
We then focused on the behavior of the EAAT2 transporter after long-term PKC activation. These experimental conditions have been shown to mimic liver cholestasis by inducing down-regulation of MRP2 transporter expression from apical membranes in these cells and in perfused rat liver[15]. In our study, we first confirmed the dramatic loss of MRP2 canalicular immunoreactivity in 4 h PMA-treated HepG2 cells using immunofluorescence (Figure 4A). We further investigated EAAT2 glutamate transporter activity after 1, 2 and 4 h by evaluating uptake after incubation with radiolabeled aspartate for 6 min. As shown in Figure 4B, a 90% decrease in aspartate uptake was observed in PMA-treated HepG2 cells as compared to controls, an effect that was observed after 1 h and was unchanged up to 4 h. The decreased uptake activity was not correlated with altered cell viability as demonstrated using the LDH assay at the longest period of PMA treatment (Figure 4B inset). This suggests that chronic PKC activation maintained decreased EAAT2 activity.
Confocal microscopy was used to evaluate whether decreased aspartate uptake observed after PMA treatment was correlated with internalization of EAAT2 membranous transporters. After immunostaining, labeled cells were scanned under 488 nm wavelength excitation and images were acquired as single transcellular optical sections. As shown in Figure 4C, under control conditions, EAAT2 immunoreactivity appeared predominantly at the cell surface with concomitant intracellular staining (Figure 4C). After PMA treatment, immunoreactivity was concentrated in the peri-nuclear region, whereas very low immunostaining was observed at the membrane. EAAT2 transporter molecules were particularly redistributed into large vesicular compartments within the cell suggesting a possible transfer into late endosomal or lysosomal pathways. These data suggest that internalization of EAAT2 cell surface transporters is involved in the inhibition of aspartate uptake observed after chronic PKC activation. We further examined if EAAT2 genetic expression was affected. Using RT-PCR, analysis of EAAT2 transporter mRNA level revealed that PMA stimulated the expression of this transporter as compared to control cells (Figure 5A). The effect of PMA was observed after 26 and 28 cycles of amplification and was specific to the EAAT2 transporter as the amplification of GAPDH cDNA appeared similar in the controls and PMA-treated cells (Figure 5B).
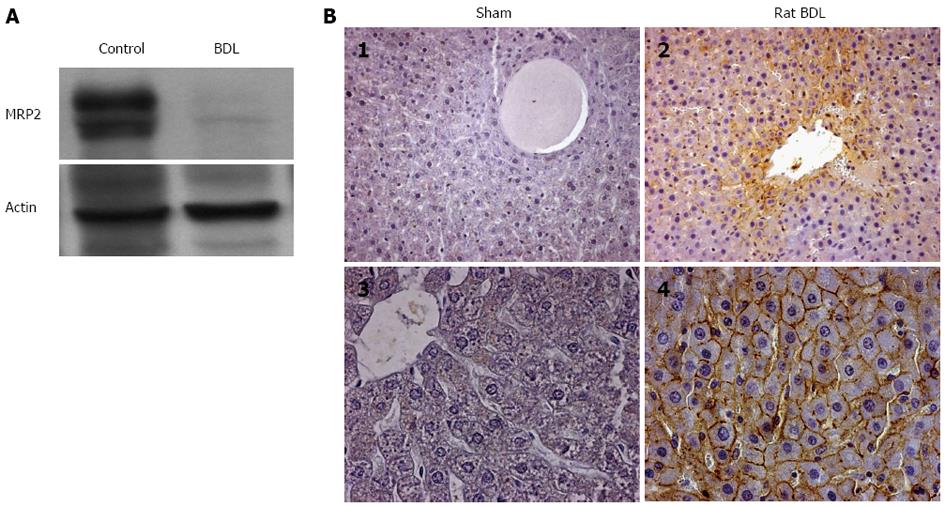
Based on the data obtained in HepG2 cells, it may be surmised that in vivo GLT-1/EAAT2 transporter expression is modulated in liver cholestasis. Therefore, GLT-1 immunoreactivity was analyzed in rat liver 15 d after bile duct ligation (BDL). Using immunoblotting, we first evaluated the effect of this surgical intervention on the expression of MRP2 transporter. As shown in Figure 6A, a marked loss in MRP2 protein expression was observed after BDL, whereas no changes were seen in actin expression, used as the internal control, between the sham-operated and BDL rats. We then analyzed the expression of GLT-1 transporter in paraffin-embedded liver sections using immunohistochemistry. At the anti-GLT-1 antibody concentration used, no staining was observed in sham-operated rats (Figures 6B1 and 6B3), whereas strong up-regulation of cell surface GLT-1 transporter expression was observed in all the membranes of hepatocytes in BDL rats (Figures 6B2 and 6B4). These results, although contrary to the results obtained in the HepG2 cell line, confirm that the expression of EAAT2/GLT-1 transporter is altered during in vivo cholestasis.
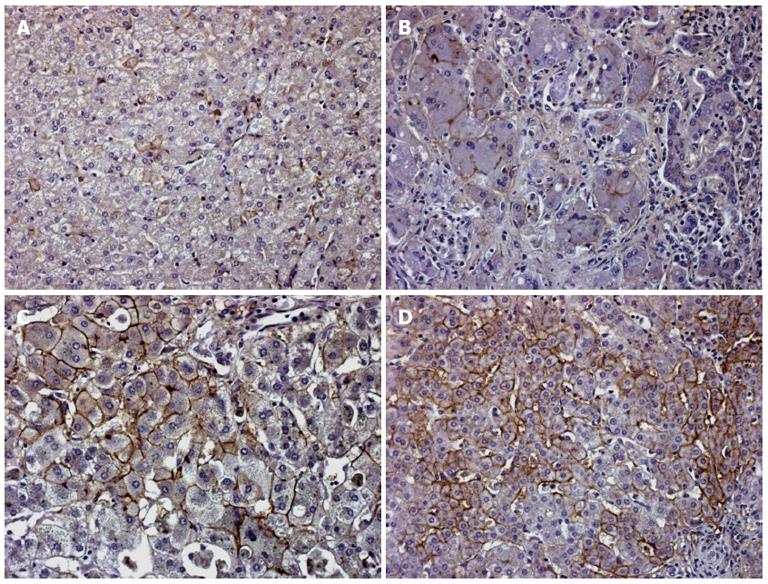
Analysis of human liver disease specimens revealed that control non-cholestatic liver specimens showed rare focal cell surface immunostaining of hepatocytes without any specific topography in the liver lobule (Figure 7A). In cholestatic liver slices, plasma membrane expression of EAAT2 transporter, as in BDL rats, increased in all membranes of the diseased hepatocytes. In genetically proven progressive familial intrahepatic cholestasis type 2 (PFIC 2) samples, the immunostaining remained focal (Figure 7B), whereas in BA (Figure 7C) and PFIC 3 (Figure 7D) strong membranous labeling was present in numerous hepatocytes. In PFIC 3, this labeling was concentrated in the periportal tract.
Using PMA-treated human hepatoblastoma HepG2 cells, the current study demonstrated that EAAT2 glutamate transporter expression and activity were modulated under experimental cholestasis conditions. In vivo, we showed an up-regulation of EAAT2/GLT-1 glutamate transporter protein expression in the membranes of hepatocytes of both bile duct-ligated rats and cholestatic human liver samples in which high levels of GGT were noted. Although divergent, the in vitro and in vivo data from the current study confirm the involvement of glutamate transport systems in liver physiopathology.
Liver is a major site of glutamate synthesis and displays all the related enzymatic pathways. Hepatic tissue concentrations of glutamate are approximately 30 times higher than plasmatic concentrations[2,21,22] suggesting that sinusoidal glutamate uptake activity is low. High glutamate concentrations are also found in bile and do not originate from direct secretion of free glutamate. These high levels are generated within the biliary tree by the action of GGT on glutathione secreted into bile[2]. The glutamate transport system detected in the canalicular membrane may then serve to reclaim some of this amino acid.
According to its important intermediary metabolic role in liver ammonia detoxification, gluconeogenesis and acid-base balance, the control of hepatic glutamate transport may significantly modulate its availability and these related intrahepatic metabolic processes. Indeed, glutamate transport across the liver sinusoidal membrane is currently documented to be important for controlling liver nitrogen flux by modulating perivenous glutamine synthesis[23]. The activity of glutamate transporters in liver sinusoidal membranes is influenced by a variety of factors in vivo including those which alter plasma amino acid composition or the Na+ electrochemical gradient. Modulation of both the degree and the distribution of glutamate transporter expression within the liver may enhance the range of hepatic response to a variety of physiological and physio-pathological challenges. This is the case for managing whole-body nitrogen metabolism, as demonstrated after starvation, diabetes and glucocorticoids treatment[24].
Several studies have reported the involvement of PKC in the regulation of glutamate transport, especially in cell models of central nervous system origin[9]. In the liver, activation of this kinase leads to the induction of hepatic cholestasis as demonstrated in HepG2 cells and perfused rat liver[15,16]. Our results show that HepG2 cells specifically take up radiolabeled D-aspartate in a sodium-dependent manner. To our knowledge, this is the first report of the presence of functional glutamate transporters in this human cell line, although other studies have demonstrated the presence of such systems in rat hepatoma cells[25,26]. After demonstrating the predominance of EAAT2 expression in this cell line, we showed that acute activation of PKC decreased both EAAT2 affinity for the substrate and maximal transport velocity. These data were in part correlated with rapid internalization of EAAT2 cell surface transporter molecules from all membranes. In previous studies, the decrease in GLT-1/EAAT2 activity documented after PKC activation has been associated only with an internalization process[14] or with a decrease in the affinity for the substrate as demonstrated in Y-79 human retinoblastoma cells[13]. In our study, the modulation of the above uptake parameters of the EAAT2 transporter may be related to the PKC subtype or to the GLT-1 isoform eventually expressed in HepG2 cells.
We subsequently revealed that long-term PKC activation maintained a marked decrease in EAAT2 transporter activity and expression with no loss in cell viability. Such experimental conditions have been shown to induce cellular symptoms of cholestasis (down-regulation of MRP2 transporter expression as confirmed by our group in PMA-treated HepG2 cells). Decreased EAAT2 expression due mainly to an internalization of cell surface transporter molecules towards the intracellular compartment was demonstrated using confocal microscopy. Further studies are needed to determine if these transporter molecules will be targeted for degradation or recycling in the plasma membrane. We were unable to study the immunoreactivity of the EAAT2 transporter at the cell membrane and intracellular fractions as no specific signal was detected in western blotting experiments using the commercially available anti-EAAT2 antibody.
By analyzing EAAT2 mRNA expression using RT-PCR, we demonstrated that chronic activation of PKC increased EAAT2 mRNA levels suggesting that this signaling pathway is involved in the stimulation of EAAT2 gene transcription or the decrease in its mRNA destabilization. This stipulates that cis-acting responsive elements for this kinase may be present in the 5’ or 3’ untranslated EAAT2 gene regions and that the PKC pathway may indirectly control the long-term level of the availability of extracellular glutamate in the liver. Furthermore, EAAT2 mRNA up-regulation may suggest that EAAT2 molecules internalized after chronic activation are targeted to degradation.
We determined whether EAAT2 modulated expression was observed in vivo. Thus, EAAT2 protein expression was analyzed in the livers of rats following BDL for 2 wk. Using immunohistochemistry, our data revealed a marked up-regulation in hepatic GLT-1/EAAT2 expression after BDL, an experimental condition of extrahepatic obstructive cholestasis. The discrepancy in the data obtained in vivo and in vitro may be related to the model of cholestasis, which is of intrahepatic origin in HepG2 cells and extrahepatic in bile duct ligated rats. The predominant cell surface expression of GLT-1 observed in hepatocytes after BDL revealed that transporters are oriented at all membranes of hepatocytes to take up extracellular glutamate.
Using transport measurements, it was proposed that sodium gradient-dependent glutamate transport was localized only in the canalicular domain[2]. In our experimental conditions, no immunostaining for GLT-1 was observed in control rat livers at the canalicular or the sinusoidal side. Nevertheless, it was clear that BDL stimulated the cell surface expression of this transporter in all hepatocyte membranes. This suggests a need for this amino acid, due to the low energetic pool that can occur after BDL. In addition, the high extracellular concentrations of glutamate at the plasmatic and biliary levels, as previously demonstrated in patients with high levels of GGT[27], force the hepatocytes to reclaim this amino acid. No intracellular staining was observed in hepatocytes indicating that these cells are not able to synthesize new transporter molecules.
The presence of free radicals, oxidative stress, and lipid peroxidation have been confirmed during cholestasis[28-32]. This was attributed to the pro-oxidant potential of hydrophobic bile acids highly accumulated inside the hepatocytes[33] and a consequent strong inflammatory response[34]. The up-regulation of glutamate transport via the EAAT2/GLT-1 subtype could also be indirectly associated with liver glutathione synthesis, which is altered after cholestasis[35].
The antioxidant defence will activate the maintenance of normal intracellular levels of glutathione. Its de novo synthesis is closely related to an adequate supply of precursor amino acids such as glutamate which may be supplied via transporter activity[36]. Indeed, the free glutamate found in excreted bile is formed from the intra-biliary hydrolysis of GSH due to the hydrolytic reaction of GGT[37]. Glutamate formed in the canalicular bile may be transported back into the liver via the identified canalicular glutamate transport systems[2]. The significance of this is still unknown, although substantial intra-hepatic cycling of specific biliary constituents was proposed. This complex interacting pathway (GSH-GGT-Glutamate) may be altered in HepG2 cells according to the potential divergent role of GGT such as in tumour progression[36].
EAAT2 transporters are able to take up cysteine, one of the three amino acid precursors of glutathione, and synthesis can also be reduced if glutamate transport is inhibited[38]. In this way, the demonstrated increase in EAAT2/GLT-1 transporter activity may help hepatocytes to avoid liver oxidative damage.
A relationship between PKC and GSH has also been documented[39]. Indeed, negative regulation of PKC has been reported consequent to GSH depletion as in oxidative stress. Such depletion has been reported to remove the negative modulation of PKC and to provide a permissive environment for its activity[40]. A decrease in liver GSH, induced by L-buthionine sulfoximine or diethylmaleate treatment, is accompanied by the inactivation of classic PKC isoforms and increased activity of novel PKC isoforms, in particular PKC-δ[41].
The cell surface expression of EAAT2 transporter was confirmed in cholestatic human liver samples especially in BA and PFIC 3 patients, characterized by high levels of GGT, as compared to non-cholestatic patients or PFIC 2. Hepatocytes are the principal bile producing liver cells (a complex fluid containing salts and phospholipids). Bile is delivered to the intestine via the bile ducts and acts like a detergent in order to dissolve fat and aid the absorption of vitamins. An alteration in PFIC gene expression causes poor bile flow including salts (in PFIC-1 deficiency) or phospholipids (PFIC-3 deficiency). The consequent accumulation of such bile substances in the liver leads to hepatic damage and cholestasis. In PFIC3 (MDR3 deficiency), GGT is present in large quantities in the canalicular and bile-duct membranes. When these are damaged, GGT is released into the bile. GGT then leaks across the wall of the biliary tract into the blood and the concentrations of GGT in serum are augmented. GGT is lacking in the canalicular membranes in PFIC-1 patients. In PFIC-2, bile acids are not normally pumped into bile, although GGT is present in the canalicular membranes. Without the detergent action of bile acids, GGT cannot be released. The involvement of this enzyme in modulating membrane transport has already been demonstrated in vitro. Indeed, in GGT-implanted human erythrocytes[42], an increase in amino acid translocation across cell membranes has been proposed to be the result of an increase in the transporter cell surface expression. The results of this study support the potential role of GGT as an intracellular pathway regulating glutamate transport through modulation of its membrane localization of transporters.
In summary, the current study demonstrates the involvement of the glutamate transport system in liver cholestasis both in cellular and animal models as well as in humans. This may help in understanding the role of glutamate and acidic amino acid transport in liver diseases.
The authors wish to thank Philippe Camby for the immunohistochemical detection of EAAT2 transporter in paraffin-embedded liver sections. MN is a researcher of IREC.
L-glutamate, the most abundant intracellular amino acid in the liver, is involved in a wide variety of important hepatic metabolic pathways such as ureagenesis, gluconeogenesis and glutathione synthesis. Glutamate uptake is involved in controlling intracellular glutamate availability and is clearly implicated in diseases of other organs such as the central nervous system. Although many of the metabolic pathways associated with glutamate are altered in several liver diseases, few studies on the role of glutamate uptake in liver physiopathology are available.
High glutamate concentrations are found both in the liver and bile as compared to plasma. Few data are available regarding the potential role of glutamate transporters in the liver, although this is well documented for other organs including the brain. In the current study, the authors demonstrate the involvement of EAAT2-mediated glutamate transport in liver cholestasis. Altered hepatocytes, the major metabolic liver cells, modulate cell surface EAAT2 transporter molecule expression probably to normalize extracellular glutamate level and recover basal intracellular metabolic activities.
This is the first study to investigate the modulation of EAAT2 mediated glutamate transport in human liver cholestasis.
Glutamate availability is very important for the execution of several intrahepatic pathways. Pharmacological modulation of its transport activity at the hepatic and/or extra-hepatic level should be taken into account as it may indirectly control/perturbate these hepatic metabolic pathways.
The authors clearly demonstrated the alteration of glutamate handling by EAAT2 in cholestatic liver disease in humans both at the intrahepatic level as in progressive familial intrahepatic cholestasis and extrahepatic level as in biliary atresia. The study design is clear and the methodology used is appropriate and clearly described. Figures are clearly expressing the results in logical sequence that is in accordance with scientific thinking. The conclusion is strongly based on the obtained results from the experiments.
P- Reviewers: Mottino AD, Romani A, Sira MM S- Editor: Wen LL L- Editor: Webster JR E- Editor: Liu XM
| 1. | Hems R, Stubbs M, Krebs HA. Restricted permeability of rat liver for glutamate and succinate. Biochem J. 1968;107:807-815. [PubMed] |
| 2. | Ballatori N, Moseley RH, Boyer JL. Sodium gradient-dependent L-glutamate transport is localized to the canalicular domain of liver plasma membranes. Studies in rat liver sinusoidal and canalicular membrane vesicles. J Biol Chem. 1986;261:6216-6221. [PubMed] |
| 3. | Kanai Y. [Na+ -dependent amino acid transporters: their structure and function]. Nihon Rinsho. 1996;54:638-645. [PubMed] |
| 4. | Utsunomiya-Tate N, Endou H, Kanai Y. Tissue specific variants of glutamate transporter GLT-1. FEBS Lett. 1997;416:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 5. | Kim SY, Chao W, Choi SY, Volsky DJ. Cloning and characterization of the 3’-untranslated region of the human excitatory amino acid transporter 2 transcript. J Neurochem. 2003;86:1458-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Jean C, Rome S, Mathé V, Huneau JF, Aattouri N, Fromentin G, Achagiotis CL, Tomé D. Metabolic evidence for adaptation to a high protein diet in rats. J Nutr. 2001;131:91-98. [PubMed] |
| 7. | Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3344] [Cited by in RCA: 3514] [Article Influence: 146.4] [Reference Citation Analysis (0)] |
| 8. | Najimi M, Maloteaux JM, Hermans E. Pertussis toxin-sensitive modulation of glutamate transport by endothelin-1 type A receptors in glioma cells. Biochim Biophys Acta. 2005;1668:195-202. [PubMed] |
| 9. | González MI, Robinson MB. Protein kinase C-dependent remodeling of glutamate transporter function. Mol Interv. 2004;4:48-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Trotti D, Rossi D, Gjesdal O, Levy LM, Racagni G, Danbolt NC, Volterra A. Peroxynitrite inhibits glutamate transporter subtypes. J Biol Chem. 1996;271:5976-5979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 248] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 11. | Zerangue N, Arriza JL, Amara SG, Kavanaugh MP. Differential modulation of human glutamate transporter subtypes by arachidonic acid. J Biol Chem. 1995;270:6433-6435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 134] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Davis KE, Straff DJ, Weinstein EA, Bannerman PG, Correale DM, Rothstein JD, Robinson MB. Multiple signaling pathways regulate cell surface expression and activity of the excitatory amino acid carrier 1 subtype of Glu transporter in C6 glioma. J Neurosci. 1998;18:2475-2485. [PubMed] |
| 13. | Ganel R, Crosson CE. Modulation of human glutamate transporter activity by phorbol ester. J Neurochem. 1998;70:993-1000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Kalandadze A, Wu Y, Robinson MB. Protein kinase C activation decreases cell surface expression of the GLT-1 subtype of glutamate transporter. Requirement of a carboxyl-terminal domain and partial dependence on serine 486. J Biol Chem. 2002;277:45741-45750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 121] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 15. | Kubitz R, Saha N, Kühlkamp T, Dutta S, vom Dahl S, Wettstein M, Häussinger D. Ca2+-dependent protein kinase C isoforms induce cholestasis in rat liver. J Biol Chem. 2004;279:10323-10330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 16. | Kubitz R, Huth C, Schmitt M, Horbach A, Kullak-Ublick G, Häussinger D. Protein kinase C-dependent distribution of the multidrug resistance protein 2 from the canalicular to the basolateral membrane in human HepG2 cells. Hepatology. 2001;34:340-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 17. | Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185380] [Cited by in RCA: 188763] [Article Influence: 3432.1] [Reference Citation Analysis (0)] |
| 18. | Gibson AE, Noel RJ, Herlihy JT, Ward WF. Phenylarsine oxide inhibition of endocytosis: effects on asialofetuin internalization. Am J Physiol. 1989;257:C182-C184. [PubMed] |
| 19. | Bruneau EG, Akaaboune M. The dynamics of recycled acetylcholine receptors at the neuromuscular junction in vivo. Development. 2006;133:4485-4493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 59] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Smit MJ, Timmerman H, Alewijnse AE, Punin M, van den Nieuwenhof I, Blauw J, van Minnen J, Leurs R. Visualization of agonist-induced internalization of histamine H2 receptors. Biochem Biophys Res Commun. 1995;214:1138-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Berl S, Takagaki G, Clarke DD, WAELSCH H. Metabolic compartments in vivo. Ammonia and glutamic acid metabolism in brain and liver. J Biol Chem. 1962;237:2562-2569. [PubMed] |
| 22. | Schimassek H, Gerok W. Control of the levels of free amino acids in plasma by the liver. Biochem Z. 1965;343:407-415. [PubMed] |
| 23. | Häussinger D, Lang F. Exposure of perfused liver to hypotonic conditions modifies cellular nitrogen metabolism. J Cell Biochem. 1990;43:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 33] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 24. | Low SY, Taylor PM, Hundal HS, Pogson CI, Rennie MJ. Transport of L-glutamine and L-glutamate across sinusoidal membranes of rat liver. Effects of starvation, diabetes and corticosteroid treatment. Biochem J. 1992;284:333-340. [PubMed] |
| 25. | McGivan JD. Rat hepatoma cells express novel transport systems for glutamine and glutamate in addition to those present in normal rat hepatocytes. Biochem J. 1998;330:255-260. [PubMed] |
| 26. | Pollard M, McGivan J. The rat hepatoma cell line H4-II-E-C3 expresses high activities of the high-affinity glutamate transporter GLT-1A. FEBS Lett. 2000;484:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 27. | Vermeulen T, Marquardt T, Häberle J. Pseudodeficiency of glutamine in infant liver disease. Amino Acids. 2009;37:435-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Sokol RJ, Devereaux M, Khandwala RA. Effect of dietary lipid and vitamin E on mitochondrial lipid peroxidation and hepatic injury in the bile duct-ligated rat. J Lipid Res. 1991;32:1349-1357. [PubMed] |
| 29. | Sokol RJ, Devereaux MW, Khandwala R. Effect of oxypurinol, a xanthine oxidase inhibitor, on hepatic injury in the bile duct-ligated rat. Pediatr Res. 1998;44:397-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Parola M, Leonarduzzi G, Biasi F, Albano E, Biocca ME, Poli G, Dianzani MU. Vitamin E dietary supplementation protects against carbon tetrachloride-induced chronic liver damage and cirrhosis. Hepatology. 1992;16:1014-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 146] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 31. | Pastor A, Collado PS, Almar M, González-Gallego J. Antioxidant enzyme status in biliary obstructed rats: effects of N-acetylcysteine. J Hepatol. 1997;27:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 114] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 32. | Svegliati Baroni G, D’Ambrosio L, Ferretti G, Casini A, Di Sario A, Salzano R, Ridolfi F, Saccomanno S, Jezequel AM, Benedetti A. Fibrogenic effect of oxidative stress on rat hepatic stellate cells. Hepatology. 1998;27:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 220] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 33. | Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677-1689. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 469] [Cited by in RCA: 515] [Article Influence: 32.2] [Reference Citation Analysis (3)] |
| 34. | Jaeschke H. Reactive oxygen and mechanisms of inflammatory liver injury: Present concepts. J Gastroenterol Hepatol. 2011;26 Suppl 1:173-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 410] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 35. | Purucker E, Winograd R, Roeb E, Matern S. Glutathione status in liver and plasma during development of biliary cirrhosis after bile duct ligation. Res Exp Med (Berl). 1998;198:167-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 36. | Paolicchi A, Tongiani R, Tonarelli P, Comporti M, Pompella A. gamma-Glutamyl transpeptidase-dependent lipid peroxidation in isolated hepatocytes and HepG2 hepatoma cells. Free Radic Biol Med. 1997;22:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 76] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 37. | McIntyre TM, Curthoys NP. The interorgan metabolism of glutathione. Int J Biochem. 1980;12:545-551. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 97] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 38. | Chen Y, Swanson RA. The glutamate transporters EAAT2 and EAAT3 mediate cysteine uptake in cortical neuron cultures. J Neurochem. 2003;84:1332-1339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 151] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 39. | Nitti M, Pronzato MA, Marinari UM, Domenicotti C. PKC signaling in oxidative hepatic damage. Mol Aspects Med. 2008;29:36-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 40. | Ward NE, Pierce DS, Chung SE, Gravitt KR, O’Brian CA. Irreversible inactivation of protein kinase C by glutathione. J Biol Chem. 1998;273:12558-12566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 67] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 41. | Domenicotti C, Paola D, Vitali A, Nitti M, Cottalasso D, Pronzato MA, Poli G, Melloni E, Marinari UM. Ethanol-induced effects on expression level, activity, and distribution of protein kinase C isoforms in rat liver Golgi apparatus. Chem Biol Interact. 1998;114:33-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 42. | Kalra VK, Sikka SC, Sethi GS. Transport of amino acids in gamma-glutamyl transpeptidase-implanted human erythrocytes. J Biol Chem. 1981;256:5567-5571. [PubMed] |