Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18466
Revised: February 24, 2014
Accepted: April 5, 2014
Published online: December 28, 2014
Processing time: 348 Days and 2 Hours
AIM: To conduct a systematic review and meta-analysis of published population-based randomized controlled trials (RCTs).
METHODS: RCTs evaluating the difference in mortality and incidence of colorectal cancer (CRC) between a screening flexible sigmoidoscopy (FS) group and control group (not assigned to screening FS) with a minimum 5 years median follow-up were identified by a search of MEDLINE and EMBASE databases and the Cochrane Central Register for Controlled Trials through August 2013. Random effects model was used for meta-analysis.
RESULTS: Four RCTs with a total of 165659 patients in the FS group and 249707 patients in the control group were included in meta-analysis. Intention-to-treat analysis showed that there was a 22% risk reduction in total incidence of CRC (RR = 0.78, 95%CI: 0.74-0.83), 31% in distal CRC incidence (RR = 0.69, 95%CI: 0.63-0.75), and 9% in proximal CRC incidence (RR = 0.91, 95%CI: 0.83-0.99). Those who underwent screening FS were 18% less likely to be diagnosed with advanced CRC (OR = 0.82, 95%CI: 0.71-0.94). There was a 28% risk reduction in overall CRC mortality (RR = 0.72, 95%CI: 0.65-0.80) and 43% in distal CRC mortality (RR = 0.57, 95%CI: 0.45-0.72).
CONCLUSION: This meta-analysis suggests that screening FS can reduce the incidence of proximal and distal CRC and mortality from distal CRC along with reduction in diagnosis of advanced CRC.
Core tip: This meta-analysis confirms that screening flexible sigmoidoscopy (FS) reduces the overall incidence of and mortality from colorectal cancer (CRC). In addition, FS reduces the incidence of and mortality from distal CRC, incidence of proximal CRC, and decreases the likelihood of subsequent diagnosis of advanced CRC. We believe, based on the proven benefits of FS, lower rates of complications with FS than with colonoscopy and feasibility in clinical practice, FS should be offered as an option for CRC screening, particularly in the population-based CRC screening programs.
- Citation: Shroff J, Thosani N, Batra S, Singh H, Guha S. Reduced incidence and mortality from colorectal cancer with flexible-sigmoidoscopy screening: A meta-analysis. World J Gastroenterol 2014; 20(48): 18466-18476
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18466.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18466
Colorectal cancer (CRC) is one of the most frequently diagnosed malignancies with over 1 million cases diagnosed per year worldwide; it is the third and fourth most frequently diagnosed cancer in women and men, respectively, and accounted for 608000 deaths worldwide in 2008[1]. Five-year survival rates among men were 65% for North America and 54% for Western Europe with improved survival with detection at earlier stage[2]. Given the significant incidence and mortality of CRC, different potential screening tests have been assessed for their ability to identify high risk individuals and for their ability to reduce CRC specific mortality.
Four major screening randomized controlled trials (RCTs) with biennial fecal occult blood testing (FOBT) showed total reduction in CRC mortality of 15% after 12-18 years in one systematic review[3-9]. A recent large prospective study showed that colonoscopy with adenoma polypectomy reduced mortality from CRC which confirmed results of earlier case-control studies[10,11]. Previous case-control studies had shown decreased incidence of CRC all-sites and of distal CRC in those obtaining colonoscopy compared to the general population, with some studies showing significant decreased incidence of proximal CRC as well[12,13]. However, RCTs which are considered the gold standard to determine the efficacy of cancer screening tests have been initiated only recently for colonoscopy and are not expected to publish their first results on CRC incidence and/or mortality for the next ten years or so.
With regard to the use of flexible sigmoidoscopy (FS) for CRC screening, the sensitivity of FS for detecting CRC in the entire colon was 58%-75% in the community setting in small studies[14,15]. In addition, FS has been shown to be a very low risk procedure with a serious harm rate of about 3.4 per 10000 procedures, which is much lower than that reported with colonoscopy[8,14,16], and can be performed by many non-physician health care providers. Four large population-based RCTs have been published recently which evaluated the effect of FS screening on CRC incidence and mortality. Review of the these trials evaluating the effect of FS screening (intervention group) compared to those not assigned screening with FS (control group) with regard to CRC incidence and mortality shows differing conclusions. The PLCO (United States) trial intention to treat (ITT) analysis showed that incidence and mortality of CRC were significantly reduced in the FS group compared to the control group[17]. Incidence of proximal and distal CRC was significantly decreased while mortality from only distal CRC (not proximal) was significantly reduced in the intervention group. The United Kingdom trial ITT analysis also showed significant reduction in the incidence and mortality of CRC in the intervention group over controls. There was evidence of decreased incidence of distal CRC but not proximal CRC[18]. ITT analysis in the SCORE (Italy) trial showed a statistically significant reduction in incidence of CRC in the intervention group but no statistically significant reduction in CRC mortality all-sites, proximal CRC, or distal CRC. The incidence of distal CRC but not proximal CRC was reduced significantly[19]. Finally, the NORCCAP (Norway) trial did not show a statistically significant difference in incidence of CRC and only a non-significant trend toward reduced CRC mortality in the intervention group compared to the controls[20]. We therefore performed this meta-analysis to synthesize the evidence and derive summative conclusions regarding the effect of FS screening on incidence and mortality from CRC.
This systematic review was performed by using the developed guidelines for conducting systematic reviews. We performed a literature search up to July 2013 using Medline and Embase databases and the Cochrane Central Register for Controlled Trials without language restriction and including articles ahead of publications. The following key words were used in the searching: “flexible sigmoidoscopy” and “incidence” and “mortality” of “CRC or colon cancer or rectal cancer”. We also performed a manual search of references cited in the selected articles and published reviews to capture additional relevant studies.
Studies were included in the meta-analysis if they met the following criteria: (1) population-based RCTs; (2) the screening test of interest was FS; (3) the outcome of interest was incidence and/or mortality due to colorectal, colon, or rectal cancer; and (4) relative risk (RR), OR or HR estimated with 95%CI (or sufficient data to calculate these) were reported.
The following data were extracted from each study: the first author’s last name, publication year, country where the study was performed, study population database, study period, participant age and sex, sample size, variables adjusted for in the analysis, and RR or HR with corresponding 95%CI for main analysis and each category of outcomes (incidence and mortality of CRC). We extracted the RRs or HRs that reflected the greatest degree of control for potential confounders for use in the main analyses. Data extraction was conducted independently by 3 authors (J.S., S.B., and N.T.) with disagreements resolved by consensus and discussion with fourth author (S.G.). The major disagreement was inclusion of Telemark Polyp Study I[21], which was dropped from our analysis after further discussion as it didn’t satisfy our eligibility criteria for a RCT.
This was performed independently by two authors (J.S. and N.T.), with disagreements resolved by discussion with senior investigators (H.S. and S.G.). Overall study quality and risk of bias was assessed as described in the Cochrane handbook[22], by recording the method used to generate the randomization schedule, the method used to conceal allocation, whether blinding was implemented, what proportion of patients completed follow-up, whether an intention-to-treat analysis was extractable, and whether there was evidence of selective reporting of outcomes.
From the original study data, we recalculated the study specific ITT RRs for incidence and mortality from CRC for patients who were randomized to receive at least one screening FS (intervention group) against patients who did not receive FS screening (control group). We used DerSimonian-Laird random effect model to obtain overall estimates for the effect of screening FS on CRC incidence and mortality by combining study-specific RR estimates. The random-effects model is more robust than the fixed effect model and incorporates into the weighing scheme both within-study and between-study variations[23]. We performed further risk stratification analysis for incidence and mortality of distal and proximal CRC in the intervention and control groups.
Statistical heterogeneity among studies was evaluated by using the Cochran Q statistic and quantified by I2 statistics[24]. We considered low, moderate, and high heterogeneity as I2 values of 25%, 50%, and 75%, respectively. These cut-offs are arbitrary and generally used for descriptive purposes only[25]. Sensitivity analysis was performed by removing each study in the meta-analysis one at a time to determine its influence on pooled RR. The robustness of the meta-analysis to the publication bias was assessed by various bias indicators, including the Egger regression asymmetry test[26], Fail-safe N tests, and the trim-and-fill method[27]. Funnel plot was constructed to evaluate the publication bias using the standard error and diagnostic OR[28]. All statistical tests were performed with the STATA v12 Data Analysis and Statistical Software (StataCorp LP, College Station, TX). P < 0.05 was considered statistically significant for this meta-analysis.
The literature review using the search criteria described above produced 1738 articles. After removal of duplicates and screening the titles for relevance to the objectives and outcomes of the meta-analysis, 75 articles were considered for abstract and or full article search (Figure 1). Finally, after excluding the articles not fulfilling inclusion criteria, summary measures of the outcomes from 4 articles were used to perform this meta-analysis.
A total of 4 studies[17-20] were published between 2009-2012, focusing on screening FS and incidence and mortality from CRC. The study characteristics are shown in detail in Table 1. The studies were conducted in the United States[17], United Kingdom[18], Italy[19], and Norway[20]. Study participants were randomized to the control group where no screening FS was assigned or to the intervention group where at least one screening FS was performed with a total of 165659 patients in the FS group and 249707 patients in the control group. The control group received usual care given to the general population. The randomization occurred before invitation for participation in the NORCAAP[20] study and after invitation and enrollment in the other three[17-19]. Follow up time was different amongst the studies. The median follow up for CRC incidence in the PLCO[17] and United Kingdom[18] trials was similar at 11.9 and 11.2 years, respectively. Median follow up for CRC incidence in the SCORE[19] trial was 10.5 years and 7 years in the NORCCAP[20] study. The age range in each study was also similar, with participants being 55-64 years old in all the studies except for in the United States[17] where the age range was 55-74 years. The ratio of male to female participants in each study was approximately 1:1. It should be noted that the definition of “distal” colonic lesion was different depending on the study. A distal lesion was defined as any CRC in and distal to the splenic flexure in the PLCO[17] trial, any CRC in or distal to the descending colon in the SCORE[19] trial, and any CRC in the rectum and sigmoid colon in the United Kingdom[18] and NORCCAP[20] trials.
| Ref. | Location | Study period (median follow-up time) | Age range (yr) | No. of patients per group | Study design | Total number of CRC cases | Incidence rate1(95%CI) | Number of deaths due to CRC | CRC Mortality rate1 | Study quality |
| Atkin et al[18], 2010 | United Kingdom | 11.2 yr | 55-64 | (C): 113195 | FS | (C): 1818 | (C): 149 (143 -156) | (C): 538 | (C): 52 (48-56) | 7 |
| (S): 57237 | Colonoscopy on detection of high risk polyps | (S): 706 | (S): 114 (106-123) | (S): 189 | (S): 36 (31-41) | |||||
| Hoff et al[20], 2009 | Norway | 7 yr | 55-64 | (C): 41913 | FS/FS + FIT | (C): 362 | (C): 134 | (C): 99 | 7 | |
| Colonoscopy on detection of high risk polyps | (262 advanced CRC) | |||||||||
| (S): 13823 | (S): 123 | (S): 132 | (S): 24 | |||||||
| (78 advanced CRC) | ||||||||||
| Schoen et al[17], 2012 | United States | 11.9 yr | 55-74 | (C): 77455 | FS | (C): 1287 | (C): 152 (144-160) | (C): 341 | (C): 39 (35-43) | 8 |
| Follow-up FS at 3-5 yr | (537 advanced CRC) | |||||||||
| (S): 77445 | Colonoscopy on detection of any polyp | (S): 1012 | (S): 119 (112-127) | (S): 252 | (S): 29 (25-32) | |||||
| (381 advanced CRC) | ||||||||||
| Segnan et al[19], 2011 | Italy | 10.5 yr | 55-64 | (C): 17144 | FS | (C): 306 | (C): 176 (158-197) | (C): 83 | (C): 44 (36-65) | 7 |
| Colonoscopy for high risk Polyps | (152 advanced CRC) | |||||||||
| (S): 17148 | (S): 251 | (S): 144 (127-163) | (S): 65 | (S): 35 (37 -55) | ||||||
| (112 advanced CRC) |
In the PLCO trial[17], participants were recruited by indicating on questionnaires that they would be interested in obtaining at least one screening FS. Only those who responded were enrolled between 1993 through 2001 and included in the ITT analysis. The participants who were randomized to the intervention group were offered FS at baseline and a repeat at 3-5 years (3 years for those who underwent randomization before April 1995 and 5 years for the rest). A positive FS was defined by presence of any polyp or mass, and colonoscopy was recommended for these patients. Repeat screening in participants diagnosed with CRC or colorectal adenoma was discouraged. Follow up was done through mailed questionnaire.
In the United Kingdom trial[18], participants responded to questionnaires that were sent out between 1994 and 1999 and indicated if they were interested in obtaining a single screening FS. Only those who responded to the questionnaire were included in the ITT analysis. The participants of this study were followed on the National Health Service Central Registrar. A colonoscopy was recommended for those with any polyp ≥ 10 mm, ≥ 3 adenomas, any polyp with villous component or severe dysplasia, any cancer or ≥ 20 hyperplastic polyps proximal to the distal rectum.
In the SCORE trial[19], participants were selected randomly between 1995 and 1999 from the National Health Service register and general practice registry to partake in the study. Only patients who expressed interest in participating in the study on a questionnaire were included in the ITT analysis. A positive screen resulting in referral for colonoscopy included: larger distal polyps (> 5 mm), inadequate bowel preparation and harboring at least one polyp, invasive CRC, high-risk adenoma (one adenoma > 10 mm, high-grade dysplasia, or villous component of > 20%), or three or more adenomas of any type or five or more hyperplastic polyps located proximal to the rectum.
In the NORCCAP trial[20], participants were selected from a national population registry in a process meant to simulate invitation procedures for national screening programs. Follow up was registry based. The ITT analysis included data from all people who were invited, not just those who agreed to participate. Of note, approximately half of the participants in the intervention group received a fecal immunochemical test (FIT) (a newer version of FOBT) in addition to a single screening FS (performed in 1999 and 2000) to further examine compliance effect of adding another supplementary screening modality. A positive screen which would make the participant eligible for colonoscopy included: having any polyp greater than or equal to 10 mm in size, any histologically verified adenoma irrespective of size, carcinoma, or a positive FIT.
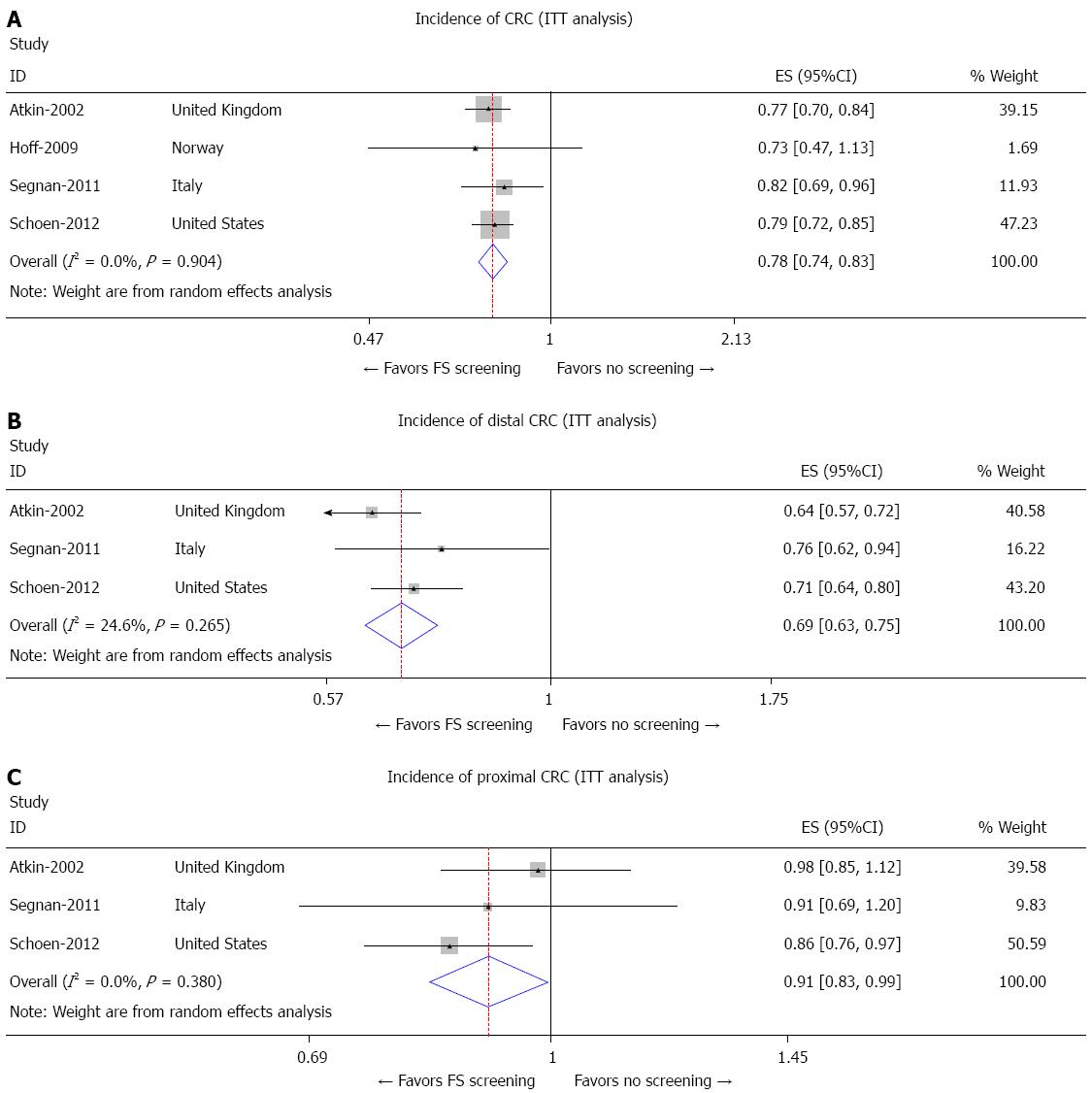
The PLCO[17], United Kingdom[18], and SCORE[19] trials all showed significantly reduced incidence of CRC in patients undergoing at least one screening FS compared to those not formally assigned to receive FS (RR = 0.79, 95%CI: 0.72-0.85; RR = 0.77, 95%CI: 0.70-0.84; and RR = 0.82, 95%CI: 0.69-0.96, respectively) while the NORCCAP[20] trial did not find significant difference in the risk for incidence of CRC between the two groups (Figure 2A). Overall, there was a 22% risk reduction in total incidence of CRC (RR = 0.78, 95%CI: 0.74-0.83). Furthermore, no difference in overall risk reduction of CRC incidence was observed after exclusion of the NORCCAP[20] trial.
The PLCO[17], United Kingdom[18], and SCORE[19] trials all showed a reduction in the incidence of distal CRC between the screening FS and control groups as shown in Figure 2B (RR = 0.71, 95%CI: 0.64-0.80; RR = 0.64, 95%CI: 0.57-0.72; and RR = 0.76, 95%CI: 0.62-0.94, respectively). The NORCCAP[20] trial did not comment on incidence of distal CRC (Figure 2B).
The PLCO[17] trial was the only study to show a reduced incidence of proximal CRC between the intervention and control group (RR = 0.86, 95%CI: 0.76-0.97), while the United Kingdom[18] and SCORE[19] trials did not show significant reduction in incidence of proximal CRC (RR = 0.98, 95%CI: 0.85-1.12 and RR = 0.91, 95%CI: 0.69-1.20, respectively) as shown in Figure 2C. The incidence of proximal CRC was not commented on in the NORCCAP[20] trial (Figure 2C).
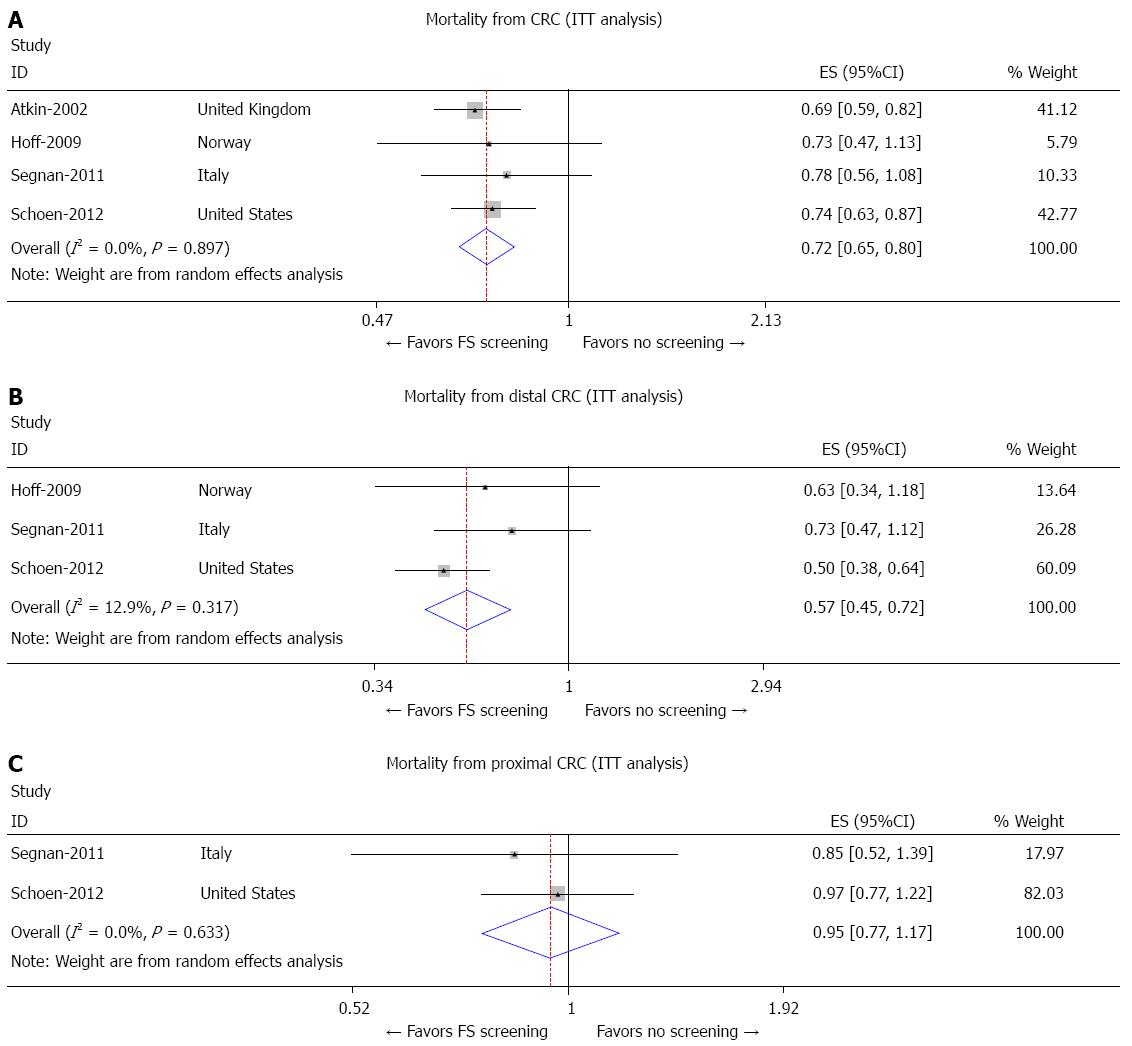
With regard to mortality from CRC, the PLCO[17] and United Kingdom[18] trials showed a significant reduction in CRC mortality in the screening FS group compared to the control group (RR = 0.74, 95%CI: 0.63-0.87 and RR = 0.69, 95%CI: 0.59-0.82, respectively) while the NORCCAP[20] and SCORE[19] trials showed non-statistically significant trend toward reduced CRC mortality (RR = 0.73; 95%CI: 0.47-1.13 and RR = 0.78; 95%CI: 0.56-1.08, respectively) between the two groups (Figure 3A). Overall, there was a 28% risk reduction in CRC mortality (RR = 0.72, 95%CI: 0.65-0.80). Furthermore, no difference was observed in CRC mortality during sensitivity analysis after exclusion of the NORCCAP[20] trial; however, there was a 22% risk reduction in CRC mortality with exclusion of the SCORE[19] trial (RR = 0.78, 95%CI: 0.73-0.83).
The PLCO[17] trial was the only study to show reduction in mortality from distal CRC in the screening FS group (RR = 0.50, 95%CI: 0.38-0.64), while the SCORE[19] and NORCCAP[20] trials did not show a significant reduction in mortality risk from distal CRC between the two groups (RR = 0.73, 95%CI: 0.47-1.12 and RR = 0.63, 95%CI: 0.34-1.18, respectively) as shown in Figure 3B. The United Kingdom[18] trial did not comment on mortality risk from distal CRC.
In both the PLCO[17] and SCORE[19] trials, the mortality risk from proximal CRC was not significantly different between the screening FS group and the control group (RR = 0.97, 95%CI: 0.77-1.22 and RR = 0.85, 95%CI: 0.52-1.39, respectively) as shown in Figure 3C. The United Kingdom[18] and NORCCAP[20] trials did not comment on mortality risk from proximal CRC between the intervention and control groups.
Pooled analysis from all 4 included studies using random effect meta-analysis[22,23] showed that FS screening reduced the overall incidence of CRC by 22% using ITT analysis (Figure 2A) (Pooled RR for CRC incidence in the FS screening group: 0.78; 95%CI: 0.74-0.83). FS screening reduced the incidence of distal CRC by 31% in the ITT analysis (Pooled RR for distal CRC incidence in the FS group: 0.69; 95%CI: 0.63-0.75) as shown in Figure 2B. Three studies reported the incidence of proximal CRC and random effect meta-analysis from these 3 trials showed about 9% reduction in the incidence of proximal CRC with the use of FS screening (Pooled RR for incidence of proximal CRC: 0.91; 95%CI: 0.83-0.99) as shown in Figure 2C.
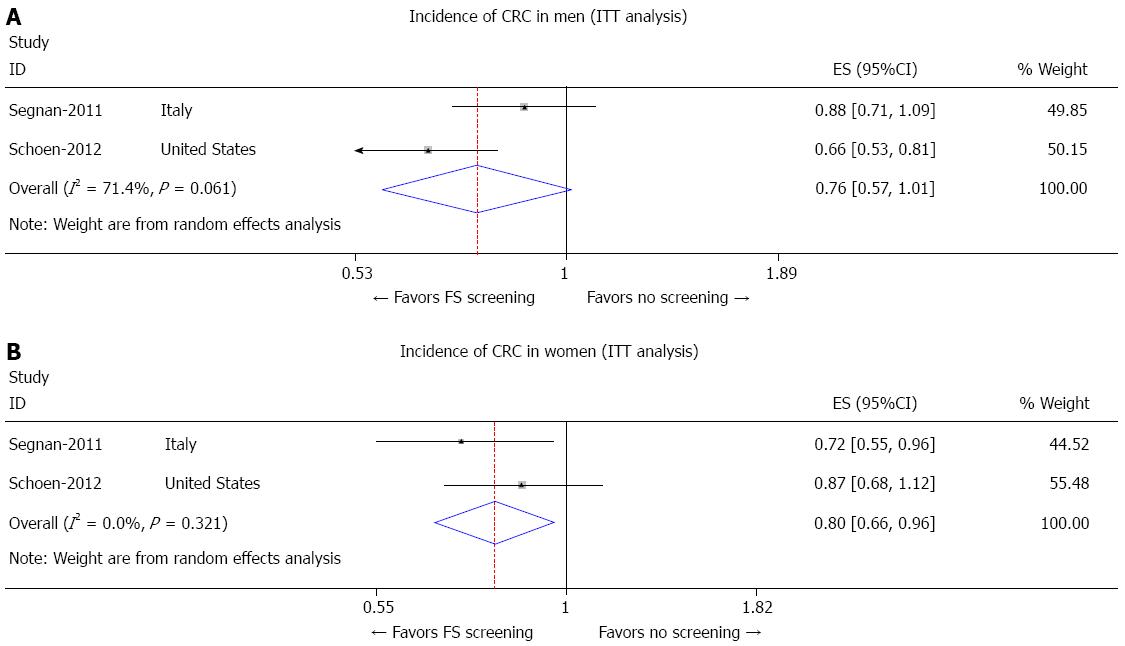
Two trials reported the overall incidence of CRC in men and women separately. Subgroup analysis showed 24% and 20% reduction in the overall incidence of CRC in men and women with FS screening, respectively. For men (Figure 4A), the pooled incidence of CRC was 0.76 (95%CI: 0.57-1.01). For women (Figure 4B), the pooled incidence of CRC was 0.80 (95%CI: 0.66-0.96).
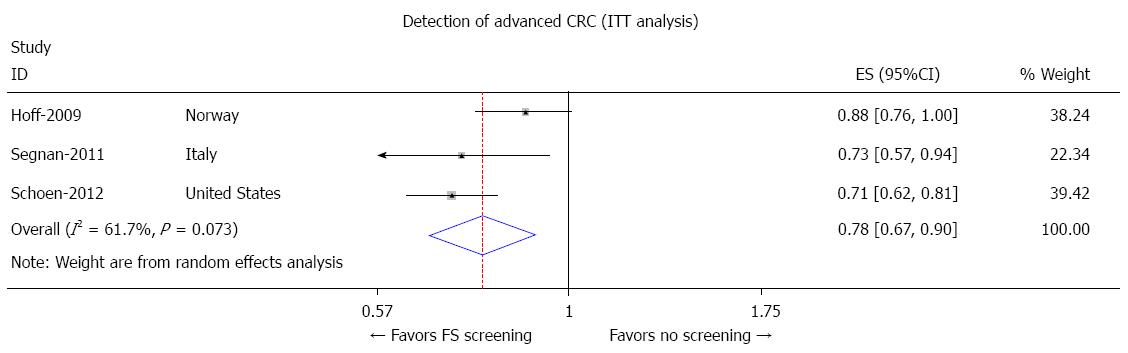
Patients undergoing FS screening were 22% less likely to be diagnosed with advanced CRC compared to the patients who did not NORCCAP screening. The pooled odds ratio for advanced CRC with FS screening was 0.78 (95%CI: 0.67-0.90) as shown in Figure 5.
FS screening reduced the overall mortality from CRC by 28%. The pooled RR for overall CRC mortality with FS screening was 0.72 (95%CI: 0.65-0.80) as shown in Figure 3A. FS screening reduced the mortality only from distal CRC by 43%. The pooled RR for distal CRC mortality by FS screening was 0.57 (95%CI: 0.45-0.72) as shown in Figure 3B. There was a statistically non-significant trend towards reduction in proximal CRC mortality with FS with pooled RR of 0.95 (95%CI: 0.77-1.17) as shown in Figure 3C.
The pooled RR for incidence and mortality from CRC for all studies combined comparing the effect of at least one screening FS against no assigned screening FS are summarized in Figures 2, 3 and 5.
No significant heterogeneity was observed between the studies (Figures 2, 3 and 5). Publication bias was assessed using Begg and Mazumdar rank correlation test, Egger’s test of the intercept and Duval and Tweedie’s trim and fill test. The test for publication bias was negative using both Begg and Mazumdar test P = 0.50 (Kendall’s tau b) and Egger’s test P = 0.49. Under the random effects model the incidence of CRC was 0.78 (95%CI: 0.74-0.83); using Trim and Fill these values were unchanged.
The current meta-analysis summarizes the results of 4 large population-based RCTs including 5865 cases of CRC. All four studies compared the incidence and mortality from CRC between an intervention group (those assigned to receive at least one screening FS) and control group (those not formally assigned to receive screening FS).
Our meta-analysis indicates that screening FS significantly reduces the incidence (RR = 0.78, 95%CI: 0.74-0.83) and mortality (RR = 0.72, 95%CI: 0.65-0.80) from CRC, with similar CRC incidence reduction in men and women. In addition, our meta-analysis shows that there is a significant decrease in the incidence of distal CRC (RR = 0.69, 95%CI: 0.63-0.75) and proximal CRC (RR = 0.91, 95%CI: 0.83-0.99) between the screening FS group and the control, as well as mortality from distal CRC (RR = 0.57, 95%CI: 0.45-0.72). However, there is no reduction in mortality from proximal CRC (RR = 0.95; 95%CI: 0.77-1.17). The current meta-analysis results suggest that the pooled benefits with FS are much more than that were suggested by the pooled estimates with guaiac FOBT in the Cochrane meta-analysis[3]. Newer versions of FOBT (FIT, Hemoccult II sensa) have been reported to have higher sensitivity in detection of CRC, but whether that leads to reduction in CRC mortality has never been evaluated in a RCT; if the increased sensitivity is associated with an increased detection of biologically less aggressive lesions the effect on CRC mortality may not be as marked as that on CRC detection. Colonoscopy has become the preferred first test for CRC screening in the United States[29,30]. However, it remains uncertain how much benefit visualization of the proximal part of the colon by colonoscopy can add to endoscopic screening for CRC; the estimates vary widely in the published cohort studies[31]. It has been shown that smaller neoplastic lesions (< 5 mm) and more flat, translucent lesions are more common in the proximal than distal colon which may make adenoma detection difficult in general[13,32,33]. In addition, visualization of the proximal colon mucosa is more likely to be hindered by suboptimal bowel preparation. There are documented significant differences in the biology of proximal and distal CRC[34]. It is extremely unfortunate no prior trial or an ongoing trial is directly compares FS to colonoscopy for CRC screening. Results of ongoing trials evaluating colonoscopy will be influenced by interim improvements in technology and quality of the procedures, which will thereby make it difficult to compare results of these colonoscopy trials to the older FS trials.
The pooled results from our meta-analysis are more conservative than observed in two other recently published meta-analyses[35,36], both of which included a study[21] that we considered to be a non-randomized trial showing a very large reduction in CRC incidence (80%) and CRC mortality (67%) with FS. This study[21] was excluded in our meta-analysis because it compared two cohorts selected from the source population, rather than identifying a study group and then randomizing the group into intervention and non-intervention arms and hence the authors of the original study reported it as a prospective controlled clinical trial, rather an RCT. In addition, our study is the only one which has provided pooled estimates of proximal and distal CRC incidence and mortality.
Meta-analysis is an important tool and sheds light on why trial results differ; raises research and editorial standards by calling attention to the strengths and weaknesses of the body of research in an area; and gives the practitioner an objective view of the research literature[37]. The current meta-analysis has some advantages. First, the number of total individuals undergoing FS and controls were substantial and that increased the statistical power for the analysis. Second, there was no evidence of publication bias or significant heterogeneity for most of the outcomes between the studies.
Our study also has several limitations that should be acknowledged. A meta-analysis is not able to solve problems related to confounding factors that could be inherent in the included studies. While criteria for “positive” FS were similar in all four studies, there were differences between them; a more liberal definition of “positive” screening FS led to higher number of colonoscopies in the PLCO which could have led to greater detection of CRC and premalignant lesions. In addition, in the PLCO trial, a large number of patients in the intervention group obtained two sigmoidoscopies during the study time frame which also likely increased the detection of CRC and premalignant lesions[17]. Variation in endoscopist’s ability/technique, proximal extent visualized with FS, as well as use of varied endoscope types and visual aids (i.e., narrow band imaging) likely impacted ability to detect CRC or premalignant lesions, but there are no analyses available on the effect of these factors on the CRC incidence and mortality from these trials. It is possible that these trials may be underestimating the efficacy of FS performed under the conditions which increase the detection rate of CRC and premalignant lesions. The PLCO trial authors have published results about CRC lesions that were missed in the screening FS group in the PLCO trial. Non-detected lesions were attributed to problems in patient compliance with initial and follow up endoscopy and bowel preparation (35.6%), limitation of the FS procedure with regard to reach of the FS and depth of insertion (43.9%), and limitation of endoscopists (20.5%)[38]. Increasing attention to these factors which are associated with better quality lower gastrointestinal endoscopy in the years after these trials were conducted could have led to improved outcomes after screening FS and the follow-up colonoscopy after “positive” FS.
Furthermore, the fact that participants had to indicate interest in obtaining a screening FS to be included in the ITT analysis makes the study population of the PLCO, United Kingdom, and SCORE trials different from the NORCCAP participants as those who would be willing to undergo screening may be more health-conscious as it is (and consequently have lower risk of developing CRC) and are more likely to actually obtain the screening test. Hence the results of these 3 trials are probably more directly applicable to those willing to undergo CRC screening than to the population at large. Long-term follow-up of the NORCAAP and carefully performed additional observational studies of screening FS in the general population will therefore be important. Lack of heterogeneity of results from NORCAAP with that from the other trials in the current meta-analysis is reassuring.
The four studies[17-20] had a range in the proportion of subjects in the intervention group who actually underwent FS which clearly could impact whether there was a significant change in the relative risk of incidence or mortality from CRC between the intervention and control groups. For example, in the NORCCAP trial, only 64.8% of the screening group participants actually underwent screening FS compared to 86% of screening group participants in the PLCO trial who obtained at least one screening FS[20]. It should be noted that in the NORCCAP trial, there was a significant reduction in mortality from CRC all-sites and distal CRC among those people who actually obtained a screening FS in the intervention group, but this reduction in mortality was not seen in the ITT analysis[20]. The researchers of the NORCCAP trial speculate that this could be explained also by self-selection, i.e., that the subjects who chose to attend were low-risk, healthier subjects who in general were more motivated to obtain a screening test[20]. This hypothesis should be tested in additional analysis adjusted for the characteristics of the participants and non-participants. Otherwise, as Sir Richard Peto noted in regards to the NORCCAP “although intention-to-treat analyses have their uses, and are in some circumstances essential, they may in other circumstances lead to false negative interpretations of important trial findings[39]”.
In addition to a varied proportion of participants in the screening group who actually obtained FS between the four studies, there was likely a significant difference between the PLCO trial and the European studies with regard to the number of screening endoscopies done in the control group. The PLCO trial authors note that there were a significant proportion of the subjects in the control group who obtained screening endoscopies given high rates of screening for CRC in the United States compared to European countries, and this may have marginalized the difference between the intervention and controls groups in the PLCO trial.
Another confounding variable could be follow-up time. For the PLCO, United Kingdom, and SCORE trials, the follow up time period was about the same which was around 11 years. The NORCCAP trial follow up time period was only 7 years and it is possible that this may not be enough time to account for the lag period in which a premalignant lesion can become CRC, thereby making it seem that there is no significant difference in mortality and incidence of CRC between the control and intervention group when it actually exists.
Although we did not find major publication bias, potential for publication bias cannot be completely excluded as small studies with null results tend not to be published (“file drawer problem”). Also analysis of publication bias was limited by the fact that only four studies were included in the analysis. No sub-group analyses were performed because of the limited number of studies included in the meta-analysis. Finally, we did not assess the adverse events with the intervention in the FS RCTs; however another recent meta-analysis[35] concluded that the reporting of adverse effects was incomplete in the FS RCTs and hence one would have to rely more on the adverse events reported in the cohort studies[8,14,16].
In conclusion, the pooled results from this meta-analysis confirm that screening FS reduces the overall incidence of and mortality from CRC. In addition, it reduces the incidence of and mortality from distal CRC, incidence of proximal CRC, and decreases the likelihood of subsequent diagnosis of advanced CRC. We believe, based on the proven benefits of FS, lower rates of complications with FS than with colonoscopy, and feasibility in clinical practice (demonstrated by use over many years in a very large Health Care Maintenance organization Kaiser Permanente[40]), FS should be offered as an option for CRC screening, particularly in the population-based CRC screening programs.
Colorectal cancer (CRC) is currently the second leading cancer killer in United States and fourth leading cause of death (694000 deaths) worldwide in 2012. Screening colonoscopies to detect adenomas and to diagnose early CRC is the mainstay intervention to mitigate this public health issue. However, screening rates have remained low with United States preventive task force reporting over 20 million United States adults who have not undergone the recommended colonoscopy.
In order to reduce incidence of CRC and cancer mortality it is imperative that we study and develop screening methods that can potentially increase accessibility, are inexpensive and are more acceptable by population.
Flexible sigmoidoscopy (FS) is a relatively inexpensive alternative to screening colonoscopy, and requires lesser or no conscious sedation as compared to colonoscopy. However reports regarding its efficacy as a screening tool to reduce CRC incidence and related mortality have been equivocal, largely due to flawed study designs, small sample sizes and variations in clinico-demographic features of the studies. The current study is a meta-analysis of all the randomized clinical trials comparing CRC incidence and related mortality outcomes in patients screened with FS with subjects not screened for CRC. The pooled data is derived from population based studies located in diverse geographical regions with varied clinicodemographic features. Thus our study results represent a more robust and generalizable estimation of effectiveness of colonoscopy in screening CRC and reducing associated mortality.
The results of the study suggest screening FS is efficacious in reducing CRC incidence and related mortality in population. FS based screening protocols can improve access to screening by providing an alternative to colonoscopy in resource limited settings.
FS is a minimally invasive examination of sigmoid colon using an endoscope based technique. Randomized clinical trial is a clinical trial which assigns treatments to patients randomly to compare effectiveness of two treatments. It is a gold standard of comparing treatments. Meta-analysis is a method of combining treatment effects from several studies in order to derive more conclusive and robust result on efficacy of treatments.
This meta-analysis provides an interesting insight about the effect of screening with FS on incidence and mortality of CRC. The manuscript is interesting and well written. The authors have gathered the results from all the large population-based randomized clinical trials which have evaluated the efficacy of FS on colorectal cancer incidence and mortality.
P- Reviewer: Chang WP, Giraldi G, Velasco I S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Available from: http://www.who.int/immunization/newsroom/factsheets/en/. |
| 2. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [PubMed] |
| 3. | Hewitson P, Glasziou P, Watson E, Towler B, Irwig L. Cochrane systematic review of colorectal cancer screening using the fecal occult blood test (hemoccult): an update. Am J Gastroenterol. 2008;103:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 719] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 4. | Kronborg O, Fenger C, Olsen J, Jørgensen OD, Søndergaard O. Randomised study of screening for colorectal cancer with faecal-occult-blood test. Lancet. 1996;348:1467-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1640] [Cited by in RCA: 1601] [Article Influence: 55.2] [Reference Citation Analysis (0)] |
| 5. | Mandel JS, Bond JH, Church TR, Snover DC, Bradley GM, Schuman LM, Ederer F. Reducing mortality from colorectal cancer by screening for fecal occult blood. Minnesota Colon Cancer Control Study. N Engl J Med. 1993;328:1365-1371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2174] [Article Influence: 67.9] [Reference Citation Analysis (1)] |
| 6. | Mandel JS, Church TR, Ederer F, Bond JH. Colorectal cancer mortality: effectiveness of biennial screening for fecal occult blood. J Natl Cancer Inst. 1999;91:434-437. [PubMed] |
| 7. | Jørgensen OD, Kronborg O, Fenger C. A randomised study of screening for colorectal cancer using faecal occult blood testing: results after 13 years and seven biennial screening rounds. Gut. 2002;50:29-32. [PubMed] |
| 8. | Whitlock EP, Lin JS, Liles E, Beil TL, Fu R. Screening for colorectal cancer: a targeted, updated systematic review for the U.S. Preventive Services Task Force. Ann Intern Med. 2008;149:638-658. [PubMed] |
| 9. | Hardcastle JD, Chamberlain JO, Robinson MH, Moss SM, Amar SS, Balfour TW, James PD, Mangham CM. Randomised controlled trial of faecal-occult-blood screening for colorectal cancer. Lancet. 1996;348:1472-1477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1858] [Cited by in RCA: 1830] [Article Influence: 63.1] [Reference Citation Analysis (0)] |
| 10. | Zauber AG, Winawer SJ, O’Brien MJ, Lansdorp-Vogelaar I, van Ballegooijen M, Hankey BF, Shi W, Bond JH, Schapiro M, Panish JF. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med. 2012;366:687-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1952] [Cited by in RCA: 2284] [Article Influence: 175.7] [Reference Citation Analysis (1)] |
| 11. | Baxter NN, Warren JL, Barrett MJ, Stukel TA, Doria-Rose VP. Association between colonoscopy and colorectal cancer mortality in a US cohort according to site of cancer and colonoscopist specialty. J Clin Oncol. 2012;30:2664-2669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 271] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 12. | Brenner H, Chang-Claude J, Seiler CM, Rickert A, Hoffmeister M. Protection from colorectal cancer after colonoscopy: a population-based, case-control study. Ann Intern Med. 2011;154:22-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 612] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 13. | Lakoff J, Paszat LF, Saskin R, Rabeneck L. Risk of developing proximal versus distal colorectal cancer after a negative colonoscopy: a population-based study. Clin Gastroenterol Hepatol. 2008;6:1117-1121; quiz 1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 188] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Imperiale TF, Wagner DR, Lin CY, Larkin GN, Rogge JD, Ransohoff DF. Risk of advanced proximal neoplasms in asymptomatic adults according to the distal colorectal findings. N Engl J Med. 2000;343:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 668] [Article Influence: 26.7] [Reference Citation Analysis (0)] |
| 15. | Anderson JC, Alpern Z, Messina CR, Lane B, Hubbard P, Grimson R, Ells PF, Brand DL. Predictors of proximal neoplasia in patients without distal adenomatous pathology. Am J Gastroenterol. 2004;99:472-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Church J. Complications of colonoscopy. Gastroenterol Clin North Am. 2013;42:639-657. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 17. | Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, Bresalier R, Andriole GL, Buys SS, Crawford ED. Colorectal-cancer incidence and mortality with screening flexible sigmoidoscopy. N Engl J Med. 2012;366:2345-2357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 730] [Cited by in RCA: 756] [Article Influence: 58.2] [Reference Citation Analysis (1)] |
| 18. | Atkin WS, Edwards R, Kralj-Hans I, Wooldrage K, Hart AR, Northover JM, Parkin DM, Wardle J, Duffy SW, Cuzick J. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375:1624-1633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1242] [Cited by in RCA: 1136] [Article Influence: 75.7] [Reference Citation Analysis (0)] |
| 19. | Segnan N, Armaroli P, Bonelli L, Risio M, Sciallero S, Zappa M, Andreoni B, Arrigoni A, Bisanti L, Casella C. Once-only sigmoidoscopy in colorectal cancer screening: follow-up findings of the Italian Randomized Controlled Trial--SCORE. J Natl Cancer Inst. 2011;103:1310-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 428] [Cited by in RCA: 451] [Article Influence: 32.2] [Reference Citation Analysis (0)] |
| 20. | Hoff G, Grotmol T, Skovlund E, Bretthauer M. Risk of colorectal cancer seven years after flexible sigmoidoscopy screening: randomised controlled trial. BMJ. 2009;338:b1846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 225] [Cited by in RCA: 215] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 21. | Thiis-Evensen E, Hoff GS, Sauar J, Langmark F, Majak BM, Vatn MH. Population-based surveillance by colonoscopy: effect on the incidence of colorectal cancer. Telemark Polyp Study I. Scand J Gastroenterol. 1999;34:414-420. [PubMed] |
| 22. | Higgins JP, Green S. Cochrane Handbook for Systematic Reviews of Interventions v5.1.0. Available from: http://www.cochrane.org/training/cochrane-handbook. |
| 23. | DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177-188. [PubMed] |
| 24. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25779] [Article Influence: 1120.8] [Reference Citation Analysis (0)] |
| 25. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46470] [Article Influence: 2112.3] [Reference Citation Analysis (3)] |
| 26. | Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634. [PubMed] |
| 27. | Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455-463. [PubMed] |
| 28. | Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54:1046-1055. [PubMed] |
| 29. | Klabunde CN, Lanier D, Nadel MR, McLeod C, Yuan G, Vernon SW. Colorectal cancer screening by primary care physicians: recommendations and practices, 2006-2007. Am J Prev Med. 2009;37:8-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 165] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 30. | Meissner HI, Breen N, Klabunde CN, Vernon SW. Patterns of colorectal cancer screening uptake among men and women in the United States. Cancer Epidemiol Biomarkers Prev. 2006;15:389-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 443] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 31. | Thosani N, Guha S, Singh H. Colonoscopy and colorectal cancer incidence and mortality. Gastroenterol Clin North Am. 2013;42:619-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 32. | Hurlstone DP, Cross SS, Adam I, Shorthouse AJ, Brown S, Sanders DS, Lobo AJ. A prospective clinicopathological and endoscopic evaluation of flat and depressed colorectal lesions in the United Kingdom. Am J Gastroenterol. 2003;98:2543-2549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 113] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Konishi K, Fujii T, Boku N, Kato S, Koba I, Ohtsu A, Tajiri H, Ochiai A, Yoshida S. Clinicopathological differences between colonic and rectal carcinomas: are they based on the same mechanism of carcinogenesis? Gut. 1999;45:818-821. [PubMed] |
| 34. | Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 597] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 35. | Holme Ø, Bretthauer M, Fretheim A, Odgaard-Jensen J, Hoff G. Flexible sigmoidoscopy versus faecal occult blood testing for colorectal cancer screening in asymptomatic individuals. Cochrane Database Syst Rev. 2013;9:CD009259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 104] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 36. | Elmunzer BJ, Hayward RA, Schoenfeld PS, Saini SD, Deshpande A, Waljee AK. Effect of flexible sigmoidoscopy-based screening on incidence and mortality of colorectal cancer: a systematic review and meta-analysis of randomized controlled trials. PLoS Med. 2012;9:e1001352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 153] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 37. | Goodman SN. Have you ever meta-analysis you didn’t like? Ann Intern Med. 1991;114:244-246. [PubMed] |
| 38. | Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Church T, Laiyemo AO, Bresalier R, Hickey T, Riley T, Prorok PC. Colorectal cancers not detected by screening flexible sigmoidoscopy in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial. Gastrointest Endosc. 2012;75:612-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 39. | Peto R. Colorectal cancer. Limitations of trial. BMJ. 2009;338:b2531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 40. | Doria-Rose VP, Levin TR, Selby JV, Newcomb PA, Richert-Boe KE, Weiss NS. The incidence of colorectal cancer following a negative screening sigmoidoscopy: implications for screening interval. Gastroenterology. 2004;127:714-722. [PubMed] |