Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18427
Revised: August 1, 2014
Accepted: September 12, 2014
Published online: December 28, 2014
Processing time: 207 Days and 16.7 Hours
AIM: To determine the effect of different Roux-en-Y gastric bypass procedures in gastric carcinoma patients with type 2 diabetes mellitus.
METHODS: A retrospective analysis of the clinical data of 54 patients with gastric cancer and type 2 diabetes mellitus treated in the Department of General Surgery from January 2006 to June 2013 was conducted. The patients underwent gastrectomy using different Roux-en-Y gastric bypass procedures (traditional, n = 26; modified, n = 28). Fasting plasma glucose (FPG), two hour postprandial blood glucose (2 h PBG) and hemoglobin A1c (HbA1c) were analyzed before surgery (0 mo) and 1, 3 and 6 mo after surgery.
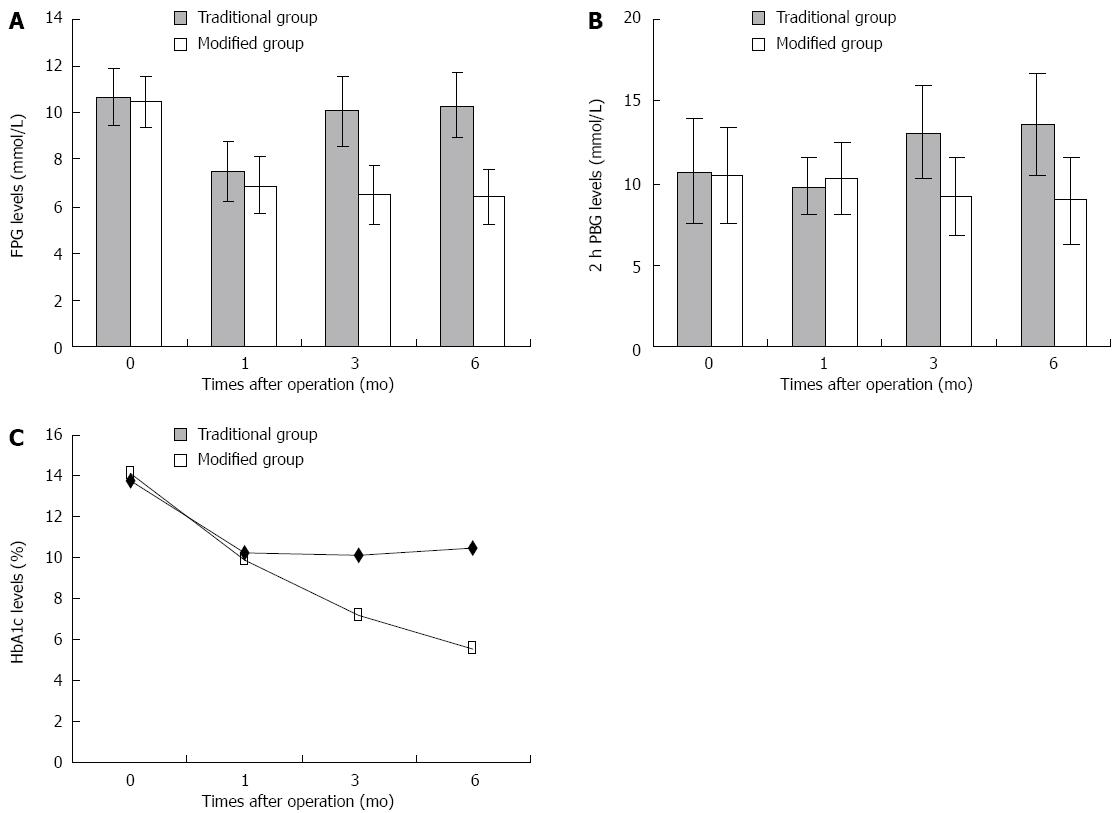
RESULTS: FPG and 2 h PBG levels were significantly decreased 1 mo after surgery in the traditional Roux-en-Y gastric bypass group (FPG 7.5 ± 1.3 vs 10.7 ± 1.2, P < 0.05) (2 h PBG 10.2 ± 1.8 vs 13.8 ± 3.2, P < 0.05). FPG and 2 h PBG levels were significantly decreased after surgery in the modified Roux-en-Y gastric bypass group (FPG 6.9 ± 1.2 vs 10.5 ± 1.1, 6.5 ± 1.3 vs 10.5 ± 1.1, 6.4 ± 1.2 vs 10.5 ± 1.1, P < 0.05) (2 h PBG 9.9 ± 2.2 vs 14.1 ± 2.9, 9.2 ± 2.4 vs 14.1 ± 2.9, 8.9 ± 2.6 vs 14.1 ± 2.9, P < 0.05). Compared with the levels before surgery, HbA1c levels were significantly decreased 3 and 6 mo after surgery (7.2 ± 1.1 vs 10.5 ± 1.1, 5.5 ± 1.1 vs 10.5 ± 1.1, P < 0.05). Significant differences between the two groups regarding FPG, 2 h PBG and HbA1c concentration were observed 3 and 6 mo after surgery (FPG 10.1 ± 1.5 vs 6.5 ± 1.3, 10.3 ± 1.4 vs 6.4 ± 1.2, P < 0.05) (2 h PBG 13.1 ± 2.8 vs 9.2 ± 2.4, 13.6 ± 3.1 vs 8.9 ± 2.6, P < 0.05) (HbA1c 10.1 ± 1.4 vs 7.2 ± 1.1, 10.5 ± 1.3 vs 5.5 ± 1.1, P < 0.05).
CONCLUSION: Modified Roux-en-Y gastric bypass can improve glucose metabolism in type 2 diabetic patients with gastric cancer.
Core tip: Type 2 diabetes mellitus (T2DM) is a common chronic disease and is a threat to public health. Modified Roux-en-Y gastric bypass surgery, which results in weight loss and a hypoglycemic effect, has been used in the treatment of patients with T2DM and obesity. However, the therapeutic mechanism involved is unclear. The aim of this study was to evaluate the efficacy of two Roux-en-Y gastric bypass procedures (traditional and modified) on blood glucose control in patients with T2DM and gastric cancer. It is suggested that modified Roux-en-Y gastric bypass may improve glucose metabolism in T2DM patients more effectively than the traditional procedure.
- Citation: Xiong SW, Zhang DY, Liu XM, Liu Z, Zhang FT. Comparison of different gastric bypass procedures in gastric carcinoma patients with type 2 diabetes mellitus. World J Gastroenterol 2014; 20(48): 18427-18431
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18427.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18427
Type 2 diabetes mellitus (T2DM) is the most common form of diabetes[1]. It is frequently seen in the patients who undergo surgery. Modified gastric bypass surgery, based on traditional Roux-en-Y digestive tract reconstruction was initiated to treat obese patients in the 1990s[2]. It was later found that this procedure was also effective in patients with T2DM[3], and the efficiency of this procedure reached 83%[4-11], although the therapeutic mechanism involved is still unclear. Traditional Roux-en-Y digestive tract reconstruction is a technique commonly used in gastric cancer surgery, and it is unclear whether it has a hypoglycemic effect in patients with T2DM as there is a lack of research in this field. In this study, we retrospectively analyzed blood glucose control in patients with T2DM and gastric cancer who underwent gastrectomy of stomach neoplasms using either modified or traditional Roux-en-Y gastric bypass surgery.
A retrospective analysis of the clinical data of 54 patients with gastric cancer and T2DM in the Department of General Surgery from January 2006 to June 2013 was conducted. The patients underwent gastrectomy of stomach neoplasms using two Roux-en-Y gastric bypass procedures (traditional, n = 26 or modified, n = 28). Follow-up data were complete. The patients in this study were diagnosed with T2DM in accordance with the diagnostic criteria of the American Diabetes Association (2003). Specific parameters measured were as follows: symptoms of diabetes mellitus, random blood glucose ≥ 11.1 mmol/L, fasting plasma glucose ≥ 7.0 mmol/L and an oral glucose tolerance test at 2 h ≥ 11.1 mmol/L. If the patients met one of the above conditions, and a retest the following day conformed to the standards of the diagnosis of diabetes, the patient was then diagnosed with diabetes mellitus. Islet cell antibodies, antibody to insulin, glutamic acid decarboxylase antibody, glycosylated hemoglobin A1c (HbA1c) and C peptide level were examined to define T2DM. All patients were diagnosed with gastric cancer by gastroscopic biopsy and a pathology report prior to surgery. The gender, age, duration of diabetes and tumor TNM stage of patients in the two surgical groups was comparable (P > 0.05). Statistical analysis data of Traditional group compared with modified group as follows: gender 19/7 vs 18/10; age 53.1 ± 5.0 years vs 51.3 ± 4.5 years; duration of diabetes 8.6 ± 4.1 vs 9.0 ± 3.8; Tumor TNM stage (n) 2/9/14/1 vs 3/7/15/3.
According to the location of cancer in the gastric cavity, the patients underwent radical total gastrectomy or distal gastrectomy, stomach perigastric lymph node dissection, and then Roux-en-Y reconstruction of the digestive tract.
In the traditional surgery group, the jejunum was cut 10-15 cm from the Treitz ligament, and the distal end of the jejunum was connected to the residual stomach or esophagus by an end-to-end or end-to-side anastomosis. The distal jejunum at 40-45 cm was connected to the proximal jejunum by an end-to-side anastomosis. In the modified surgery group, the jejunum was cut 75-100 cm from the Treitz ligament, and then the distal end of the jejunum was connected to the residual stomach or esophagus by an end-to-end or end-to-side anastomosis. The distal jejunum at 75-100 cm was connected to the proximal jejunum by an end-to-side anastomosis.
Fasting plasma glucose, two hour postprandial blood glucose (2 h PBG), and HbA1c levels in the two groups were determined before surgery (0 mo) and 1, 3, and 6 mo after surgery, respectively. These values were compared with those at other time points within the group and at the same time points between groups.
The measurement data were expressed as the mean ± SD. Statistical analyses were performed by the T test using SPSS 17.0 (SPSS Inc., Chicago, IL, United States). The count data were determined by the χ2 test. P-values of < 0.05 were considered statistically significant.
Compared with the preoperative FPG and 2 h PBG levels, in the traditional surgery group these levels decreased 1 mo after surgery (P < 0.05), however, 3 and 6 mo after surgery these values were gradually restored to preoperative levels. The HbA1c level after 1, 3 and 6 mo was not significantly reduced (P > 0.05). In the modified surgery group, the FPG and 2 h PBG levels at 1, 3 and 6 mo after surgery and the HbA1c levels at 3 and 6 mo decreased significantly (P < 0.05). When the FPG, 2 h PBG and HbA1c levels at the same time point were compared between the two groups, the differences were not statistically significant at 1 mo after surgery, however, the differences at 3 and 6 mo after surgery were statistically significant (P < 0.05) (Table 1, Figure 1).
| n | 0 mo | 1 mo | 3 mo | 6 mo | |||||||||
| FPG mmol/L | 2 h PBG mmol/L | HbA1c | FPG mmol/L | 2 h PBG mmol/L | HbA1c | FPG mmol/L | 2 h PBG mmol/L | HbA1c | FPG mmol/L | 2 h PBG mmol/L | HbA1c | ||
| Traditional group | 26 | 10.7 ± 1.2 | 13.8 ± 3.2 | 10.7% ± 1.2% | 7.5 ± 1.3a | 10.2 ± 1.8a | 9.8% ± 1.1% | 10.1 ± 1.5c | 13.1 ± 2.8c | 10.1% ± 1.4%c | 10.3 ± 1.4c | 13.6 ± 3.1c | 10.5% ± 1.3%c |
| Modified group | 28 | 10.5 ± 1.1 | 14.1 ± 2.9 | 10.5% ± 1.1% | 6.9 ± 1.2a | 9.9 ± 2.2a | 10.2% ± 1.3% | 6.5 ± 1.3a | 9.2 ± 2.4a | 7.2% ± 1.1%a | 6.4 ± 1.2a | 8.9 ± 2.6a | 5.5% ± 1.1%a |
According to statistics, 90%-95% of patients with DM have T2DM. At present, DM is the third most common chronic non-communicable disease in humans after tumors and cardiovascular disease, and the incidence rate is rising each year. Patients for surgical operation are frequently complicated with T2DM. DM has various etiological factors and complicated mechanisms; therefore, curing DM has become an urgent global medical goal[12].
During the 1990’s, modified Roux-en-Y reconstruction of the digestive tract also known as gastric bypass surgery (GBP) for the treatment of severe obesity resulted in a good outcome. Thus, GBP has now become the standard surgical technique for obesity. Surprisingly, GBP has a good hypoglycemic effect in obese patients with T2DM. Previous research showed that 83%-86% of T2DM patients maintained normal levels of blood glucose following GBP[13,14]. In a follow-up study, Patriti et al[15] showed that this surgical procedure was also efficacious in T2DM patients without obesity. Therefore, GBP surgery for T2DM was widely used in the hope of curing patients with T2DM.
Gastric bypass surgery has improved based on Roux-en-Y digestive tract reconstruction of the traditional Roux-en-Y procedure; however, the mechanism of the improvement in hypoglycemia following Roux-en-Y reconstruction is unclear. Whether the surgical technique has a hypoglycemic effect requires further investigation.
Traditional Roux-en-Y reconstruction of the digestive tract is a common technique in gastric cancer, and some patients with gastric cancer also have T2DM, therefore, research on the hypoglycemic effect of traditional digestive tract reconstruction is important and may provide a theoretical basis for patients undergoing Roux-en-Y digestive tract reconstruction.
In the present study, we compared the hypoglycemic effect of two types of Roux-en-Y digestive tract reconstruction. We found that in the traditional surgery group, FPG and 2 h PBG levels decreased 1 mo after surgery compared with preoperative levels, however, FPG and 2 h PBG levels were gradually restored to the level before surgery after 3 and 6 mo. The HbA1c level after 1, 3 and 6 mo was not significantly decreased. In the modified surgery group, the FPG, 2 h PBG levels at 1, 3 and 6 mo after surgery and the HbA1c levels at 3 and 6 mo after surgery decreased significantly. A comparison of the FPG, 2 h PBG and HbA1c levels at the same time point between the two groups, showed no statistically significant differences at 1 mo after surgery, however, statistically significant differences at 3 and 6 mo after surgery were observed. This indicated that traditional digestive tract reconstruction had a hypoglycemic effect in the short-term[16], but did not last, thus this hypoglycemic effect was not ideal. The modified digestive tract reconstruction procedure had a better hypoglycemic effect and lasted longer. Many researchers have investigated this hypoglycemic mechanism. At present, there are many hypotheses related to this mechanism, including gastric inhibitory peptide (GIP)[17] which is the core of the “foregut hypothesis” and glucagon-like peptide-1 (GLP-1)[18-20] which is the core of the “hindgut hypothesis” which have received significant attention[21]. The “foregut hypothesis” considers that food in the duodenum and proximal jejunum stimulate the production of GIP, thus improving insulin resistance[22]. The “hindgut hypothesis” considers that chyme in the terminal ileum and colon promotes the secretion of GLP-1, and stimulates the secretion of glucose-dependent insulin, which improves the insulin effect and the proliferation of islet B cells[23-25].
Following the modified digestive tract reconstruction procedure, food no longer flows through the distal stomach, duodenum and proximal jejunum, and directly enters the distal jejunum then the ileum, thereby reducing the secretion of GIP and stimulating GLP-1, which alters peripheral endocrine factors and the role of islet cells and related target organs. Thus resulting in better regulation of blood glucose[26].
Type 2 diabetes mellitus (T2DM) is one of the most serious diseases that threaten public health. Modified Roux-en-Y gastric bypass surgery, which has better weight loss and hypoglycemic effect, has been applied to the treatment of T2DM patients with obesity. However, the therapeutic mechanism to this function is still unclear due to the lack of research in this field.
Modified gastric bypass surgery, based on traditional Roux-en-Y digestive tract reconstruction was initiated to treat obese patients in the 1990s. It was later found that this procedure was also effective in patients with T2DM, and the efficiency of this procedure reached 83%, although the therapeutic mechanism involved is still unclear.
The aim of this study was to test the efficacy of traditional and modified Roux-en-Y gastric bypass surgeries on blood glucose control of the patients with type 2 diabetes mellitus and gastric cancer. It is suggested that modified Roux-en-Y gastric bypass may improve glucose metabolism of T2DM patients more effectively than the traditional operation mode may do.
Modified Roux-en-Y gastric bypass could provide a better hypoglycemic support to type 2 diabetic patients with gastric cancer. This gastric bypass procedure may provide a potential alternative for patients.
Authors reported that in this study, patients with gastric cancer and type 2 diabetes mellitus underwent gastrectomy using different Roux-en-Y gastric bypass procedures. The results indicated that modified Roux-en-Y gastric bypass may improve glucose metabolism in T2DM patients more effectively than the traditional procedure. This is very important to know for the clinical setting and educators in the world.
P- Reviewer: Izawa KP S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Rubino F. Is type 2 diabetes an operable intestinal disease? A provocative yet reasonable hypothesis. Diabetes Care. 2008;31 Suppl 2:S290-S296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 165] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 2. | Wittgrove AC, Clark GW, Tremblay LJ. Laparoscopic Gastric Bypass, Roux-en-Y: Preliminary Report of Five Cases. Obes Surg. 1994;4:353-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 568] [Cited by in RCA: 458] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 3. | Buchwald H, Estok R, Fahrbach K, Banel D, Jensen MD, Pories WJ, Bantle JP, Sledge I. Weight and type 2 diabetes after bariatric surgery: systematic review and meta-analysis. Am J Med. 2009;122:248-256.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1816] [Cited by in RCA: 1733] [Article Influence: 108.3] [Reference Citation Analysis (0)] |
| 4. | Yip K, Heinberg L, Giegerich V, Schauer PR, Kashyap SR. Equivalent weight loss with marked metabolic benefit observed in a matched cohort with and without type 2 diabetes 12 months following gastric bypass surgery. Obes Surg. 2012;22:1723-1729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Mingrone G, Panunzi S, De Gaetano A, Guidone C, Iaconelli A, Leccesi L, Nanni G, Pomp A, Castagneto M, Ghirlanda G. Bariatric surgery versus conventional medical therapy for type 2 diabetes. N Engl J Med. 2012;366:1577-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1340] [Cited by in RCA: 1276] [Article Influence: 98.2] [Reference Citation Analysis (0)] |
| 6. | Lee WJ, Hur KY, Lakadawala M, Kasama K, Wong SK, Lee YC. Gastrointestinal metabolic surgery for the treatment of diabetic patients: a multi-institutional international study. J Gastrointest Surg. 2012;16:45-51; discussion 51-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Raj PP, Kumaravel R, Chandramaliteeswaran C, Vaithiswaran V, Palanivelu C. Laparoscopic duodenojejunal bypass with sleeve gastrectomy: preliminary results of a prospective series from India. Surg Endosc. 2012;26:688-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Reoch J, Mottillo S, Shimony A, Filion KB, Christou NV, Joseph L, Poirier P, Eisenberg MJ. Safety of laparoscopic vs open bariatric surgery: a systematic review and meta-analysis. Arch Surg. 2011;146:1314-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 97] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 9. | Hirao M, Takiguchi S, Imamura H, Yamamoto K, Kurokawa Y, Fujita J, Kobayashi K, Kimura Y, Mori M, Doki Y. Comparison of Billroth I and Roux-en-Y reconstruction after distal gastrectomy for gastric cancer: one-year postoperative effects assessed by a multi-institutional RCT. Ann Surg Oncol. 2013;20:1591-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 219] [Cited by in RCA: 254] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 11. | Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1140] [Cited by in RCA: 1306] [Article Influence: 87.1] [Reference Citation Analysis (1)] |
| 12. | Spanou M, Tziomalos K. Bariatric surgery as a treatment option in patients with type 2 diabetes mellitus. World J Diabetes. 2013;4:14-18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 7] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Schauer PR, Kashyap SR, Wolski K, Brethauer SA, Kirwan JP, Pothier CE, Thomas S, Abood B, Nissen SE, Bhatt DL. Bariatric surgery versus intensive medical therapy in obese patients with diabetes. N Engl J Med. 2012;366:1567-1576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1639] [Cited by in RCA: 1590] [Article Influence: 122.3] [Reference Citation Analysis (0)] |
| 14. | Lee WJ, Chong K, Ser KH, Lee YC, Chen SC, Chen JC, Tsai MH, Chuang LM. Gastric bypass vs sleeve gastrectomy for type 2 diabetes mellitus: a randomized controlled trial. Arch Surg. 2011;146:143-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 327] [Cited by in RCA: 331] [Article Influence: 23.6] [Reference Citation Analysis (1)] |
| 15. | American Diabetes Association. Standards of medical care in diabetes--2013. Diabetes Care. 2013;36 Suppl 1:S11-S66. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2371] [Cited by in RCA: 2481] [Article Influence: 206.8] [Reference Citation Analysis (0)] |
| 16. | An JY, Kim YM, Yun MA, Jeon BH, Noh SH. Improvement of type 2 diabetes mellitus after gastric cancer surgery: short-term outcome analysis after gastrectomy. World J Gastroenterol. 2013;19:9410-9417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 17. | Rao RS, Kini S. GIP and bariatric surgery. Obes Surg. 2011;21:244-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 18. | Bose M, Oliván B, Teixeira J, Pi-Sunyer FX, Laferrère B. Do Incretins play a role in the remission of type 2 diabetes after gastric bypass surgery: What are the evidence? Obes Surg. 2009;19:217-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 19. | Laferrère B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J, Hart AB, Olivan B. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care. 2007;30:1709-1716. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 429] [Cited by in RCA: 393] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 20. | Vetter ML, Cardillo S, Rickels MR, Iqbal N. Narrative review: effect of bariatric surgery on type 2 diabetes mellitus. Ann Intern Med. 2009;150:94-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 125] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 21. | Chen MC, Lee YC, Lee WJ, Liu HL, Ser KH. Diet behavior and low hemoglobin level after laparoscopic mini-gastric bypass surgery. Hepatogastroenterology. 2012;59:2530-2532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 22. | Abdelmalak B, Ibrahim M, Yared JP, Modic MB, Nasr C. Perioperative glycemic management in insulin pump patients undergoing noncardiac surgery. Curr Pharm Des. 2012;18:6204-6214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Aroda VR, Henry RR, Han J, Huang W, DeYoung MB, Darsow T, Hoogwerf BJ. Efficacy of GLP-1 receptor agonists and DPP-4 inhibitors: meta-analysis and systematic review. Clin Ther. 2012;34:1247-1258.e22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 189] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 24. | Park YJ, Ao Z, Kieffer TJ, Chen H, Safikhan N, Thompson DM, Meloche M, Warnock GL, Marzban L. The glucagon-like peptide-1 receptor agonist exenatide restores impaired pro-islet amyloid polypeptide processing in cultured human islets: implications in type 2 diabetes and islet transplantation. Diabetologia. 2013;56:508-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 25. | Guo N, Sun J, Chen H, Zhang H, Zhang Z, Cai D. Liraglutide prevents diabetes progression in prediabetic OLETF rats. Endocr J. 2013;60:15-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 26. | Hamilton R, Thai XC, Ameri D, Pai MP. Oral bioavailability of linezolid before and after Roux-en-Y gastric bypass surgery: is dose modification necessary in obese subjects? J Antimicrob Chemother. 2013;68:666-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |