Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18420
Revised: October 23, 2014
Accepted: December 1, 2014
Published online: December 28, 2014
Processing time: 120 Days and 11.1 Hours
AIM: To investigate perioperative outcomes in patients undergoing modified laparoscopic splenectomy and azygoportal disconnection (MLSD) with intraoperative autologous cell salvage.
METHODS: We retrospectively evaluated outcomes in 79 patients admitted to the Clinical Medical College of Yangzhou University with cirrhosis, portal hypertensive bleeding and secondary hypersplenism who underwent MLSD without (n = 46) or with intraoperative cell salvage and autologous blood transfusion, including splenic blood and operative hemorrhage (n = 33), between February 2012 and January 2014. Their intraoperative and postoperative variables were compared. These variables mainly included: operation time; estimated intraoperative blood loss; volume of allogeneic blood transfused; visual analog scale for pain on the first postoperative day; time to first oral intake; initial passage of flatus and off-bed activity; perioperative hemoglobin (Hb) concentration; and red blood cell concentration.
RESULTS: There were no significant differences between the groups in terms of duration of surgery, estimated intraoperative blood loss and overall perioperative complication rate. In those receiving salvaged autologous blood, Hb concentration increased by an average of 11.2 ± 4.8 g/L (P < 0.05) from preoperative levels by the first postoperative day, but it had fallen by 9.8 ± 6.45 g/L (P < 0.05) in the group in which cell salvage was not used. Preoperative Hb was similar in the two groups (P > 0.05), but Hb on the first postoperative day was significantly higher in the autologous blood transfusion group (118.5 ± 15.8 g/L vs 102.7 ± 15.6 g/L, P < 0.05). The autologous blood transfusion group experienced significantly fewer postoperative days of temperature > 38.0 °C (P < 0.05).
CONCLUSION: Intraoperative cell salvage during MLSD is feasible and safe and may become the gold standard for liver cirrhosis with portal hypertensive bleeding and hypersplenism.
Core tip: Because of the impairment of hepatic synthetic function and intractable coagulopathy, one of the most serious complications during laparoscopic splenectomy and azygoportal disconnection (LSD) is rapid loss of large volumes of blood. Intraoperative cell salvage and autologous blood transfusion of splenic blood and operative hemorrhage during LSD can increase hemoglobin concentration and reduce or obviate the need for intraoperative allogeneic transfusion. An autologous blood salvage device can minimize intraoperative blood loss and make full use of the large red cell pool sequestered in an enlarged spleen. At the same time, it will encourage more surgeons to perform LSD.
- Citation: Jiang GQ, Bai DS, Chen P, Qian JJ, Jin SJ, Yao J, Wang XD. Modified laparoscopic splenectomy and azygoportal disconnection combined with cell salvage is feasible and might reduce the need for blood transfusion. World J Gastroenterol 2014; 20(48): 18420-18426
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18420.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18420
Surgery for patients with liver cirrhosis complicated by portal hypertensive variceal bleeding has evolved substantially in terms of techniques and indications. The two basic strategies are to create a shunt or to devascularize; shunting is presently the preferred surgical treatment for recurrent variceal hemorrhage in western countries[1,2], while devascularization is used more commonly in China[3,4]. For patients with liver cirrhosis, portal hypertensive bleeding and hypersplenism, open splenectomy and azygoportal disconnection is generally accepted as the most effective approach. With the recent significant development in laparoscopic skills and technical advances in surgical instruments, laparoscopic splenectomy and azygoportal disconnection (LSD) has proved to be a feasible, effective and safe procedure and is gradually gaining acceptance as the invasive treatment of choice for this challenging group of patients[5-11]. We were the first to report a modified LSD technique (MLSD) that further reduces complications and is associated with a more rapid recovery[12,13].
One of the most serious perioperative complications during LSD is rapid loss of large volumes of blood, not least because of the impairment of hepatic synthetic function and intractable coagulopathy. The aim of the current study was to use intraoperative cell salvage and autologous blood transfusion during MLSD to minimize intraoperative blood loss, exploit the large volume of blood sequestered in the enlarged spleen, and decrease the need for allogeneic blood transfusion.
We retrospectively reviewed the medical records of all patients diagnosed with liver cirrhosis, portal hypertensive bleeding and secondary hypersplenism who underwent MLSD at the Clinical Medical College of Yangzhou University, China, between February 2012 and January 2014. We identified a series of 79 consecutive patients; of these, 46 underwent MLSD without intraoperative cell salvage and autologous blood transfusion and 33 were treated with the cell salvage technique.
As part of the consent procedure before surgery, all patients were informed that MLSD was at an early stage of development and evaluation compared with the open technique and a written informed choice was made as to which procedure to undergo. Between February 2012 and January 2014, 82 patients underwent surgery and, excluding three who selected open surgery, the remaining 79 patients underwent MLSD. Cell salvage and autologous blood transfusion were not used in the first 46 consecutive procedures, but were used in all of the subsequent 33 procedures. The same surgical team performed all the operations. The Ethics Committee of the Clinical Medical College of Yangzhou University, China gave approval for the conduct of the study.
The following demographic and clinical characteristics were collected retrospectively: age; sex; etiology of cirrhosis; Child-Pugh classification; longitudinal diameter of the spleen; spleen volume index; and preoperative hemoglobin (Hb) and red blood cell (RBC) concentrations. Intraoperative data collected included: operation time; estimated intraoperative blood loss; and volume of allogeneic blood transfused. Postoperative data included: visual analog scale (VAS) for pain on the first postoperative day; time to first oral intake, initial passage of flatus and off-bed activity; duration of postoperative hospital stay; number of days with temperature > 38 °C; proportion of patients who were not pyrexial; and perioperative complications. Blood analysis included: Hb concentration; white blood cell (WBC) count; platelet count; and concentrations of alanine aminotransferase (ALT) and creatinine determined preoperatively and 1, 3 and 7 d after surgery.
Spleen volume index was calculated from the product of the greatest superoinferior, anteroposterior and mediolateral lengths measured at the level of the splenic hilum from images acquired by computed tomography or magnetic resonance imaging[14].
The VAS for pain was recorded using a questionnaire that rated pain intensity on a scale of 0-10[15], with 0 representing no pain and 10 representing very severe pain.
After induction of general anesthesia, patients were placed in the supine position with their legs apart. Pneumoperitoneum was maintained at 13 mmHg using CO2. A five-port method was chosen, including one 5 mm, three 10 mm and one 12 mm port. The splenic artery was ligated using a Hem-o-lok. A LigaSure vessel-sealing device (Covidien, Boulder, CO, United States) was used to dissociate the ligaments surrounding the spleen. The splenic artery and vein were transected en bloc using a linear laparoscopic vascular stapler [ECHELON 60 ENDOPATH Stapler (Ethicon Endo-Surgery, Cincinnati, United States)] through the 12 mm port. During the laparoscopic azygoportal disconnection procedure, all paraesophageal venous collaterals were divided using the LigaSure vessel-sealing device from posterior to anterior, from superior to inferior, and from left to right.
The spleen was removed from the abdominal cavity through the 12 mm port using an electromechanical morcellator (TSCS, Hangzhou, China) consisting of a motor-driven cutting tube and a large claw forceps. After the spleen had been grasped by the forceps, a cylindrical spleen tissue sample was gradually cut by a motor-driven cutting tube and removed through its lumen. These steps were repeated until the entire spleen had been removed.
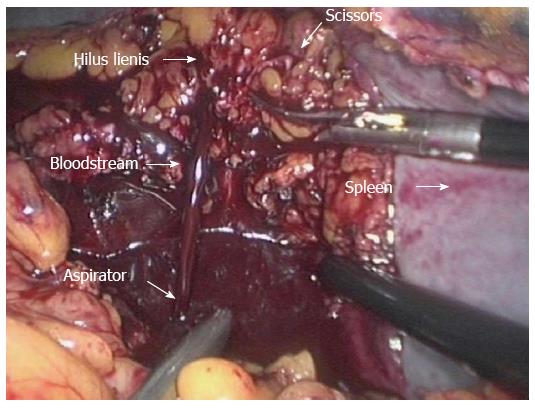
Cell salvage was used throughout the whole procedure. Intraoperative blood loss and the blood sequestered within the enlarged spleen were collected and processed in heparinized saline by a cell saver (Autologous Blood Recovery System, Beijing Jingjing Medical Equipment Co. Ltd., China). Sequestered splenic blood was collected at two different times: once the spleen had been completely detached, blood was drained through many incisions in the hilus lienis vessels and collected from the peritoneum (Figure 1); and a small quantity of blood was also extracted from the spleen after its morcellation. The washed red blood cells were transfused immediately after the anticoagulated blood was processed with a standard procedure by means of the Autologous Blood Recovery System.
Data are presented as the mean ± SD, or proportions (%). Group means were compared using Student’s t test and proportions using χ2 test, as appropriate. All statistical analyses were performed using SPSS version 13.0 software (SPSS, Chicago, IL, United States). P < 0.05 was considered statistically significant.
Of the 79 patients undergoing MLSD, 45 (57.0%) were men. The operation was performed successfully in all patients and none required conversion to an open procedure. Patients were divided into one of two groups on the basis of the intraoperative transfusion technique used: Group A comprised the first consecutive 46 patients operated on between February 2012 and May 2013 who were eligible to receive allogeneic blood if needed; and Group B comprised the latter consecutive 33 patients operated on between May 2013 and January 2014 who underwent cell salvage and autologous transfusion.
Group A comprised 29 men and 17 women, aged 33-75 years (mean 54.7 ± 10.5 years), who had been admitted to hospital as a consequence of recurrent portal hypertensive variceal bleeding. The liver cirrhosis stage was Child-Pugh A in 30 patients and Child-Pugh B in 16. The etiology of liver disease is described in Table 1. All 46 patients had splenomegaly, the longitudinal diameter of the spleen ranged from 11.0 to 26.5 cm, and spleen volume index from 648.0 to 3776.2 cm3.
| Variable | Group A (n = 46) | Group B (n = 33) | P value |
| Sex | |||
| Male | 29 | 16 | 0.197 |
| Female | 17 | 17 | |
| Age (yr) | 54.67 ± 10.52 | 52.55 ± 9.76 | 0.364 |
| Etiology | |||
| HBV cirrhosis | 23 | 21 | 0.229 |
| HCV cirrhosis | 3 | 3 | 1.000 |
| Schistosoma cirrhosis | 6 | 1 | 0.253 |
| Alcoholic cirrhosis | 3 | 0 | 0.369 |
| Autoimmunity liver cirrhosis | 9 | 5 | 0.612 |
| Cryptogenic | 2 | 3 | 0.700 |
| Child-Pugh classification | 0.479 | ||
| A | 30 | 24 | |
| B | 16 | 9 | |
| Longitudinal diameter of spleen (cm) | 18.03 ± 2.97 | 19.16 ± 2.95 | 0.101 |
| Spleen volume index (cm3) | 1599.88 ± 791.84 | 1763.89 ± 715.56 | 0.348 |
Group B comprised 33 patients (16 men and 17 women) who underwent MLSD with intraoperative cell salvage and autologous blood transfusion. Their age ranged from 30 to 77 years (mean 52.6 ± 9.8 years) and they were also hospitalized owing to repeated variceal bleeding. The liver cirrhosis stage was Child-Pugh A in 24 patients and Child-Pugh B in nine. The etiology of liver disease is shown in Table 1. Spleen diameters ranged from 13.9 to 24.5 cm and spleen volume index ranged from 760.1 to 3049.8 cm3.
Patients’ baseline demographic and clinical characteristics are shown in Table 1. There were no significant differences between the groups in terms of age, sex, etiology of cirrhosis, Child-Pugh classification, longitudinal spleen diameter or spleen volume index. All patients were discharged in good health after surgery.
The intraoperative and postoperative details of the two groups are shown in Table 2. There were no significant differences between the groups in terms of operation time (221.0 ± 56.9 min in Group A vs 208.8 ± 54.2 min in Group B, P > 0.05); intraoperative estimated blood loss (179 ± 158 mL in Group A vs 158 ± 220 mL in Group B, P > 0.05); proportion requiring intraoperative allogeneic blood transfusion (4.3% in Group A vs none in Group B, P > 0.05); VAS for pain on the postoperative day 1 (2.5 ± 0.8 in Group A vs 2.7 ± 0.9 in Group B, P > 0.05); time to first oral intake (1.5 ± 0.6 d in Group A vs 1.3 ± 0.6 d in Group B, P > 0.05); time to first passage of flatus (2.4 ± 0.9 d in Group A vs 2.6 ± 0.9 d in Group B, P > 0.05); time to off-bed activity (2.6 ± 0.7 d in Group A vs 2.4 ± 0.7 d in Group B, P > 0.05); postoperative hospital stay (10.7 ± 2.6 d in Group A vs 11.3 ± 2.3 d in Group B, P > 0.05); and overall perioperative complication rate (13.0% in Group A vs 12.1% in Group B, P > 0.05).
| Variable | Group A (n = 46) | Group B (n = 33) | P value |
| Operative time (min) | 220.98 ± 56.92 | 208.79 ± 54.24 | 0.341 |
| Estimated blood loss (mL) | 179.35 ± 157.96 | 158.18 ± 220.35 | 0.620 |
| Volume of blood transfusion (n) | 2 | 0 | 0.626 |
| VAS score of the first day | 2.50 ± 0.84 | 2.73 ± 0.91 | 0.255 |
| First oral intake time after operation (d) | 1.46 ± 0.62 | 1.33 ± 0.60 | 0.380 |
| Initial passage of flatus time (d) | 2.43 ± 0.91 | 2.58 ± 0.90 | 0.498 |
| Postoperative off-bed activity time (d) | 2.57 ± 0.69 | 2.39 ± 0.66 | 0.270 |
| Postoperative hospital stay (d) | 10.72 ± 2.62 | 11.30 ± 2.32 | 0.308 |
| Perioperative complications | 6 | 4 | 1.000 |
| Asymptomatic portal vein thrombosis | 5 | 3 | 1.000 |
| Pancreatic fistula | 1 | 1 | 1.000 |
| Incision complications | 0 | 0 | |
| Pneumonia | 0 | 0 | |
| Abdominal infection | 0 | 0 | |
| Emergency operation for bleeding | 0 | 0 |
There was no significant difference in preoperative Hb between the groups (112.5 ± 15.2 g/L in Group A vs 107.0 ± 15.4 g/L in Group B, P > 0.05). However, Hb on postoperative day 1 was significantly higher in Group B (118.5 ± 15.8 g/L vs 102.7 ± 15.6 g/L in Group A, P < 0.05). By postoperative day 1, Hb had fallen by 9.8 ± 6.4 g/L from its preoperative value in Group A, which was significantly lower than that before surgery (102.7 ± 15.61 g/L compared with 112.5 ± 15.2 g/L, P < 0.05). In contrast, Hb concentration rose by 11.2 ± 4.8 g/L perioperatively in Group B, which was significantly higher than the preoperative concentration (118.5 ± 15.8 g/L compared with a 107.0 ± 15.4 g/L, P < 0.05).
Similarly, there was no significant difference in preoperative RBC count between the groups (3.880 × 109/L ± 0.632 × 109/L in Group A vs 3.727 × 109/L ± 0.550 × 109/L in Group B, P > 0.05). However, RBC count on postoperative day 1 was significantly higher in Group A (4.212 × 109/L ± 0.530 × 109/L vs 3.558 ± 0.647 × 109/L in Group B, P < 0.05). By postoperative day 1, the RBC count had fallen by 0.339 × 109/L ± 0.244 × 109/L from its preoperative value in Group A, which was significantly lower than that before surgery (3.558 × 109/L ± 0.647 × 109/L compared with a preoperative value of 3.880 × 109/L ± 0.632 × 109/L, P < 0.05). In contrast, RBC count rose by 0.486 × 109/L ± 0.293 × 109/L perioperatively in Group B, which was significantly higher than the preoperative concentration (4.212 × 109/L ± 0.530 × 109/L compared with 3.727 × 109/L ± 0.550 × 109/L, P < 0.05).
Body temperature and WBC count data are shown in Table 3. None of the patients in either group had fever before surgery. Following surgery, the mean number of days when body temperature exceeded 38.0 °C was significantly greater in Group A (3.8 ± 2.9 d vs 2.3 ± 2.9 d in Group B, P < 0.05). Seven patients in Group A (15.2%) and 11 in Group B (33.3%) did not develop postoperative pyrexia, but this difference was not significant. There was no significant difference in pre- or postoperative WBC count between the groups.
| Variable | Group A (n = 46) | Group B (n = 33) | P value |
| Postoperative subfebrile (T > 38.0 °C) days | 3.67 ± 2.92 | 2.33 ± 2.91 | 0.047 |
| No fever, n | 7 | 11 | 0.058 |
| WBC day 0, 109/L | 3.03 ± 1.85 | 2.35 ± 1.39 | 0.082 |
| WBC day 1, 109/L | 11.33 ± 3.50 | 10.97 ± 3.61 | 0.654 |
| WBC day 3, 109/L | 11.25 ± 3.61 | 11.89 ± 4.09 | 0.464 |
| WBC day 7, 109/L | 8.73 ± 2.56 | 9.33 ± 3.83 | 0.400 |
The preoperative and postoperative serum ALT concentrations were not significantly different between the groups (Table 4). Renal function was also not significantly different: the preoperative and postoperative serum creatinine concentrations were broadly comparable between the groups (Table 4).
| Variable | Group A (n = 46) | Group B (n = 33) | P value |
| ALT day 0, U/L | 30.98 ± 23.33 | 29.64 ± 15.21 | 0.773 |
| ALT day 1, U/L | 37.52 ± 18.47 | 33.27 ± 15.80 | 0.228 |
| ALT day 3, U/L | 29.78 ± 23.27 | 31.97 ± 40.12 | 0.761 |
| ALT day 7, U/L | 20.67 ± 11.73 | 21.52 ± 13.59 | 0.769 |
| Creatinine day 0, umol/L | 72.35 ± 17.63 | 71.33 ± 16.83 | 0.797 |
| Creatinine day 1, umol/L | 75.58 ± 16.37 | 73.18 ± 23.00 | 0.589 |
| Creatinine day 3, umol/L | 60.15 ± 16.25 | 60.97 ± 20.53 | 0.843 |
| Creatinine day 7, umol/L | 61.19 ± 14.54 | 60.52 ± 16.03 | 0.846 |
LSD is a challenging procedure in cirrhotic patients with portal hypertensive bleeding and secondary hypersplenism. Low platelet count, development of collateral vessels and intractable coagulopathy mean that there is a substantial risk of torrential and potentially unmanageable intraoperative hemorrhage.
To date, only one study has examined the use of intraoperative splenic blood salvage during LSD and reported that cell salvage significantly increased postoperative Hb and minimized the risks and complications of perioperative allogeneic transfusion[6].
We believe we are the first to report the MLSD technique[12,13], in which the spleen is removed through the existing trocar incision using an electromechanical morcellator without the need for an enlarged incision. It is a feasible, effective and safe surgical procedure, embodies excellent minimally invasive surgery and may become the gold standard surgical procedure for liver cirrhotic patients with bleeding portal hypertension and hypersplenism.
We have used cell salvage and autologous blood transfusion during every MLSD procedure since May 2013 in a different cohort of patients from that reported in this study. There are important differences between the above-mentioned and our studies. In the study of Wang et al[6], intraoperative blood was not collected and the blood sequestered in the enlarged spleen was not salvaged until the end of the operation. In our study, both intraoperative blood and the blood sequestered in the enlarged spleen were collected and splenic blood was salvaged on two different occasions. Sequestered blood released from the spleen through many incisions in the hilus lienis vessels was collected from the left subphrenic space where it had collected after the patient had been placed in the Trendelenburg position (rather than it being allowed to disperse throughout the peritoneal cavity). This method allows sequestered blood to be collected earlier and the volume maximized. If sequestered blood is only collected at the end of surgery then the volume and quality of blood may be reduced by clot formation within the spleen. At the end of the operation, during laparoscopic extraction of the spleen using the morcellator, a small quantity of blood released from splenic tissue was also collected. The time spent collecting blood was usually < 10 min.
In the study of Wang et al[6], in order to salvage the blood trapped in the enlarged spleen, they needed to manipulate the large spleen into a bag in a small working space, which may have been difficult and tedious for the surgeon. Then, the blood was aspirated through the suction apparatus that was pierced into the splenic pulp from various points. There may be a risk with this method in that the blood can be lost in the peritoneal cavity if the bag is punctured unexpectedly.
We found that postoperative hepatic and renal function was not affected by intraoperative autologous blood transfusion. Preoperative liver and renal function reflected by serum ALT and creatinine concentrations was comparable between the groups and there were no significant differences on postoperative days 1, 3 or 7. Patients with leukocytopenia due to hypersplenism frequently have WBC counts below the lower limit of normal, but there was also no significant difference in perioperative WBC counts between the groups.
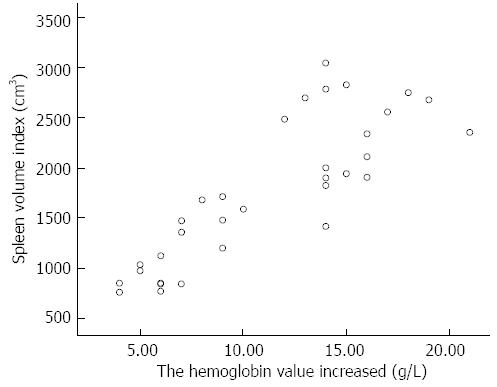
We also found that Hb significantly declined in the group in which autologous blood transfusion was not used, but that Hb significantly rose in the group in which it was. These findings concur with those reported by Wang et al[6]. In the group in which cell salvage was not used, mean intraoperative blood loss was 179 mL, resulting in a mean decrease in Hb of 9.8 g/L. The conservation of intraoperative blood loss using the cell saver and use of sequestered splenic blood resulted in a perioperative increase in Hb of 11.2 g/L. The extent of increase in perioperative Hb positively correlated with the longitudinal diameter of the spleen (Pearson correlation = 0.733, P < 0.05) and spleen volume index (Pearson correlation = 0.844, P < 0.05) (Figure 2). The strength of the correlation was greater for spleen volume index, suggesting that it more accurately reflects the volume of sequestered blood.
Scarcity of blood products can make the clinical management of torrential bleeding challenging, particularly if Rhesus-negative products are needed. Cell salvage and autologous blood transfusion addresses these organizational issues as well as the physiological complications of massive or exchange transfusion. In this series, two patients undergoing MLSD without cell salvage required allogeneic blood transfusion. None of the patients in whom cell salvage was used required allogeneic blood, even one patient in whom perioperative blood loss was measured at 1.3 L. In that case, the patient suffered no complications and his Hb rose from 97 g/L preoperatively to 113 g/L on postoperative day 1. Another patient’s blood type was A Rhesus-negative; compatible blood was not available in the local transfusion center and without cell salvage this patient would not have been able to undergo surgery.
We also found that patients who underwent perioperative cell salvage had significantly fewer postoperative days when their temperature exceeded 38.0 °C. Of the 33 patients in Group B, 11 (33.3%) did not exhibit postoperative subfebrile temperature, compared with seven of the 46 patients (15.2%) in Group A. This difference was not significant (P = 0.058), probably owing to the small sample size. There are three potential mechanisms that may explain a diminished inflammatory response in patients who undergo cell salvage: a normal or increased rather than depleted Hb; avoidance of the complications of allogeneic blood transfusion; and presence of anti-inflammatory immune factors such as tuftsin in the blood harvested from the spleen. In cells of monocytic origin, such as macrophages, microglia and neutrophils, tuftsin promotes phagocytic activity[16-18].
In conclusion, our findings show that MLSD with intraoperative cell salvage and autologous blood transfusion is feasible and safe. It increases Hb and reduces the need for allogeneic blood transfusion, relieving pressure on transfusion services and surgical staff. Intraoperative cell salvage and autologous blood transfusion may become the gold standard for cirrhotic patients with portal hypertensive bleeding and secondary hypersplenism undergoing MLSD.
The authors thank Yao Tang (a biostatistician of Clinical Medical College of Yangzhou University) for her assistance with statistical analysis.
Whether intraoperative cell salvage and autologous blood transfusion during modified laparoscopic splenectomy and azygoportal disconnection (MLSD) can increase hemoglobin (Hb) concentration and reduce or obviate the need for intraoperative allogeneic transfusion has not been investigated thoroughly.
So far, only one study has examined the use of intraoperative splenic blood salvage during LSD and reported that cell salvage significantly increased postoperative Hb concentration and minimized the risks and complications of perioperative allogeneic transfusion.
In this study, the authors examined the influence of intraoperative cell salvage and autologous blood transfusion of intraoperative hemorrhage and sequestered splenic blood on postoperative outcomes after MLSD. As well as standard use of cell salvage to collect and process blood lost during surgery, the authors salvaged blood sequestered in the enlarged spleen. This was achieved in two ways: (1) by making many incisions in the hilus lienis vessels after the spleen had been dissected en bloc. Blood drained from the spleen in this way was salvaged from the left subphrenic space with the patient in the Trendelenburg position; and (2) by collecting and salvaging a small quantity of blood released from splenic tissue after extraction of the spleen through a laparoscopic port site using an electromechanical morcellator.
Intraoperative cell salvage and autologous blood transfusion during MLSD is necessary and safe. Cell salvage substantially increases Hb concentration and reduces the need for allogeneic blood transfusion, relieving pressure on transfusion services and surgical staff. It may become the gold standard for cirrhotic patients with portal hypertensive bleeding and secondary hypersplenism undergoing MLSD.
A cell saver, the Autologous Blood Recovery System suctions, washes and filters blood so it can be given back to the patient instead of being thrown away. One advantage of this is that the patient receives his/her own blood instead of donor blood so there is no risk of contracting outside diseases. Because the blood is recirculated, there is no limit to the amount of blood that can be given back to the patient.
The study is interesting and relevant. The manuscript has several flaws and should be revised; the discussion especially needs major revision.
P- Reviewer: Boettcher M, Noguera J, Piccolo G S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Zhang DN
| 1. | Henderson JM, Boyer TD, Kutner MH, Galloway JR, Rikkers LF, Jeffers LJ, Abu-Elmagd K, Connor J. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology. 2006;130:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 2. | Rosemurgy AS, Bloomston M, Clark WC, Thometz DP, Zervos EE. H-graft portacaval shunts versus TIPS: ten-year follow-up of a randomized trial with comparison to predicted survivals. Ann Surg. 2005;241:238-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 3. | Xu XB, Cai JX, Leng XS, Dong JH, Zhu JY, He ZP, Wang FS, Peng JR, Han BL, Du RY. Clinical analysis of surgical treatment of portal hypertension. World J Gastroenterol. 2005;11:4552-4559. [PubMed] |
| 4. | Shi B, Yang Z, Wang X, Xu J, Lu X, Liang F, Mu Q, Wu TH. Selective periesophagogastric devascularization in portal hypertension: results of 56 patients. Hepatogastroenterology. 2009;56:492-497. [PubMed] |
| 5. | Jiang XZ, Zhao SY, Luo H, Huang B, Wang CS, Chen L, Tao YJ. Laparoscopic and open splenectomy and azygoportal disconnection for portal hypertension. World J Gastroenterol. 2009;15:3421-3425. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 35] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 6. | Wang Y, Ji Y, Zhu Y, Xie Z, Zhan X. Laparoscopic splenectomy and azygoportal disconnection with intraoperative splenic blood salvage. Surg Endosc. 2012;26:2195-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Yamamoto J, Nagai M, Smith B, Tamaki S, Kubota T, Sasaki K, Ohmori T, Maeda K. Hand-assisted laparoscopic splenectomy and devascularization of the upper stomach in the management of gastric varices. World J Surg. 2006;30:1520-1525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 8. | Wang YD, Ye H, Ye ZY, Zhu YW, Xie ZJ, Zhu JH, Liu JM, Zhao T. Laparoscopic splenectomy and azygoportal disconnection for bleeding varices with hypersplenism. J Laparoendosc Adv Surg Tech A. 2008;18:37-41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | Zheng X, Liu Q, Yao Y. Laparoscopic splenectomy and esophagogastric devascularization is a safe, effective, minimally invasive alternative for the treatment of portal hypertension with refractory variceal bleeding. Surg Innov. 2013;20:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Xin Z, Qingguang L, Yingmin Y. Total laparoscopic versus open splenectomy and esophagogastric devascularization in the management of portal hypertension: a comparative study. Dig Surg. 2009;26:499-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 30] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Li SL, Li YC, Xu WL, Shi BJ. Laparoscopic splenectomy and periesophagogastric devascularization with endoligature for portal hypertension in children. J Laparoendosc Adv Surg Tech A. 2009;19:545-550. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Jiang G, Qian J, Yao J, Wang X, Jin S, Bai D. A New Technique for Laparoscopic Splenectomy and Azygoportal Disconnection. Surg Innov. 2013;21:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Bai DS, Qian JJ, Chen P, Yao J, Wang XD, Jin SJ, Jiang GQ. Modified laparoscopic and open splenectomy and azygoportal disconnection for portal hypertension. Surg Endosc. 2014;28:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 14. | Saygili OB, Tarhan NC, Yildirim T, Serin E, Ozer B, Agildere AM. Value of computed tomography and magnetic resonance imaging for assessing severity of liver cirrhosis secondary to viral hepatitis. Eur J Radiol. 2005;54:400-407. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22:1-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 760] [Cited by in RCA: 745] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 16. | Wardowska A, Dzierzbicka K, Szaryńska M, Dabrowska-Szponar M, Wiśniewska K, Myśliwski A, Trzonkowski P. Analogues of muramyl dipeptide (MDP) and tuftsin limit infection and inflammation in murine model of sepsis. Vaccine. 2009;27:369-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 17. | Bhasin M, Wu M, Tsirka SE. Modulation of microglial/macrophage activation by macrophage inhibitory factor (TKP) or tuftsin (TKPR) attenuates the disease course of experimental autoimmune encephalomyelitis. BMC Immunol. 2007;8:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 18. | Wu M, Nissen JC, Chen EI, Tsirka SE. Tuftsin promotes an anti-inflammatory switch and attenuates symptoms in experimental autoimmune encephalomyelitis. PLoS One. 2012;7:e34933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |