Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18390
Revised: February 23, 2014
Accepted: June 20, 2014
Published online: December 28, 2014
Processing time: 421 Days and 14.8 Hours
AIM: To detect the expression of tumor necrosis factor-α (TNF-α) in colorectal cancer (CRC) cells among Saudi patients, and correlate its expression with clinical stages of cancer.
METHODS: Archival tissue specimens were collected from 30 patients with CRC who had undergone surgical intervention at King Khalid University Hospital. Patient demographic information, including age and gender, tumor sites, and histological type of CRC, was recorded. To measure TNF-α mRNA expression in CRC, total RNA was extracted from tumor formalin-fixed, paraffin-embedded, and adjacent normal tissues. Reverse transcription and reverse transcription polymerase chain reaction were performed. Colorectal tissue microarrays were constructed to investigate the protein expression of TNF-α by immunohistochemistry.
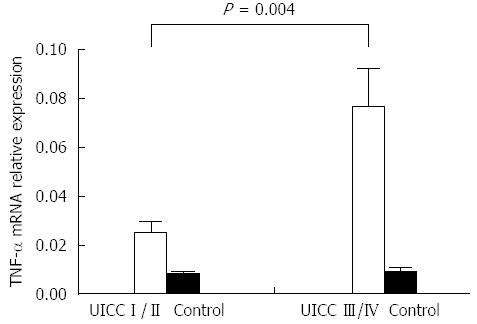
RESULTS: The relative expression of TNF-α mRNA in colorectal cancer was significantly higher than that seen in adjacent normal colorectal tissue. High TNF-α gene expression was associated with Stage III and IV neoplasms when compared with earlier tumor stages (P = 0.004). Eighty-three percent of patients (25/30) showed strong TNF-α positive staining, while only 10% (n = 3/30) of patients showed weak staining, and 7% (n = 2/30) were negative. We showed the presence of elevated TNF-α gene expression in cancer cells, which strongly correlated with advanced stages of tumor.
CONCLUSION: High levels of TNF-α expression could be an independent diagnostic indicator of colorectal cancer, and targeting TNF-α might be a promising prognostic tool by assessment of the clinical stages of CRC.
Core tip: The relative expression of tumor necrosis factor-α (TNF-α) mRNA in colorectal cancer tumor tissue was significantly higher than adjacent normal tissue. In addition, higher TNF-α gene expression was associated significantly with advanced tumor as compared to early tumor stages. Colorectal tumor tissue contained many TNF-α positive cells, whereas normal colorectal tissue contained very few positive cells. We showed elevated TNF-α gene expression by cancer cells, which correlated strongly with advanced tumor stages. High levels of TNF-α expression could be an independent prognostic indicator, and targeting TNF-α may be a promising tool, which can be used to follow-up patients with colorectal carcinoma.
- Citation: Obeed OAA, Alkhayal KA, Sheikh AA, Zubaidi AM, Vaali-Mohammed MA, Boushey R, Mckerrow JH, Abdulla MH. Increased expression of tumor necrosis factor-α is associated with advanced colorectal cancer stages. World J Gastroenterol 2014; 20(48): 18390-18396
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18390.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18390
Colorectal cancer (CRC) is the second most common malignancy in Saudi Arabia[1]. Worldwide, CRC is the third most common cancer in males and the second in females[2]. In addition, it is the third most common cause of cancer-related death. Between forty and fifty percent of patients die within five years of diagnosis despite the major advances in the diagnosis and management of CRC[3] in recent years. Surgery is the mainstay of treatment for all CRC cases, and aims at complete (R0) resection of the infiltrating neoplasm, and the removal of its lymphatic drainage. Nodal positive patients (UICC Stage III) are a well-recognized, high-risk population, and adjuvant treatment is usually administered in these patients.
The progressive growth of malignant neoplasm is accompanied by a decline in immune response through mechanisms that are poorly understood[4]. While many studies have improved our understanding of tumor initiation and progression, many questions regarding the phenomenon of an increasingly ineffective tumor immune response during tumor growth are in need of elucidation[5,6]. In fact, during cancer progression colorectal tumor cells acquire various characteristics that allow them to evade immunological surveillance[7-9]. Particularly, and with regard to therapeutic modulation of the immune system, it is important to understand tumor-specific immunological responses and mechanisms leading to induction or suppression of the immune system. In this respect, it has been hypothesized that tumor progression in immunocompetent individuals may reflect a failure of the immune system to recognize tumor antigen, or may result from subversion of anti-tumor responses[10,11]. Induction of inflammation by tumor-released cytokines has been proposed to play an important role in colorectal carcinogenesis[4,12]. Furthermore, tumor cells themselves can secrete pro-inflammatory cytokines, which contribute directly to malignant progression[13]. The complex interactions between the neoplastic and inflammatory cells, which are mediated by inflammatory cytokines, are essential features of the tumor microenvironment[14].
Tumor necrosis factor-α (TNF-α) is a proinflammatory cytokine predominantly produced by macrophages as well as tumor cells[15,16], and is a cytokine ligand of the TNF family that interacts with different receptors of the TNF receptor superfamily. The activity of this system may be one of the drivers of progression in CRC[17]. Its wide range of biological activities includes inflammation, apoptosis, cell proliferation and differentiation. In addition, it is a key molecule regulating inflammatory processes in tumor promotion. It has multiple effects on cell function by binding to specific high-affinity, cell-surface receptors. Apart from its apoptosis-inducing mechanisms, TNF-α may promote tumor growth at lower levels during cancer progression[15,16]. A mounting body of evidence has suggested that TNF-α mediates many critical processes of tumor progression, including oncogene activation, DNA damage, and tumor metastases[18,19]. High TNF-α expression is strongly associated with tumor recurrences in CRC patients and positive lymph node metastases[4].
TNF-α expression in cancer patients is detected by measuring the levels of the cytokine, indicating its potential role during tumor progression. Conversely, TNF-α has also been considered to be an anticancer agent[10,20]. There are some reports that TNF-α expression is increased in the serum of CRC patients[21], and may be useful as a marker for the early diagnosis of CRC[22]. For these reasons, we have focused on TNF-α expression in Saudi CRC patients. However, the clinical impact of TNF-α expression by cancer cells in patients with CRC remains unclear. Thus, we looked for the expression of this inflammatory cytokine in tumor tissues by reverse transcriptase-polymerase chain reaction (RT-PCR) analysis and immunohistochemistry. We also correlated protein expression with tumor stages in order to determine the role of TNF-α expression by the invading neoplastic cells in tumor progression.
Archival CRC paraffin blocks of patients with histologically proven CRC (n = 30), undergoing surgical resection between 2010 and 2011, were obtained from the archives of the Department of Pathology of King Saud University at King Khalid University Hospital. Patient demographic characteristics, including age and gender, tumor sites, and histological type of CRC, were recorded. The histological stage of the tumor was determined in accordance with the Union International Contre le Cancer (UICC)-TNM Staging System[23,24]. Tumor localization, UICC stages, and tumor differentiation (grading), in accordance with WHO classification[25] were documented in a database prospectus. Furthermore, and in order to minimize possible influences of radiotherapy and chemotherapy on TNF-α status and prognosis, all patients having undergone neo-adjuvant or adjuvant therapy in addition to surgical treatment were excluded from this cohort.
Preparation of total RNA: Total cellular RNA was extracted using the Ambion® RecoverAll™ Total Nucleic Acid Isolation Kit (Invitrogen, United States), for the extraction of total RNAs from formalin-fixed, paraffin-embedded (FFPE) tissues. Briefly, four FFPE sections (10 μm each) were obtained from all patient blocks, and adjacent normal tissue using a Leica semi-automatic microtome. Pure, concentrated RNA was eluted in elution buffer provided as a kit ingredient, and stored at -80 °C for further analysis. The purity of the total RNA was assessed by measuring the A260/280 ratio (1.8-2.0), using an ultraviolet spectrophotometer. To remove the contamination of genomic DNA, RNA samples were treated with RNAse-free DNA enzyme (Ambion, United States).
cDNA synthesis: Reverse transcription reaction was performed using a commercially available set of High Capacity cDNA Archive Kits (Applied Biosystems, United States). cDNA was prepared from 2 μg of total RNA, with random hexamer primers. According to the manufacturer’s instructions the mix was run on a PCR thermocycler gene as follow: 10 min at 25 °C, 2 h at 37 °C, 5 min at 85 °C, and kept at 4 °C thereafter on a PCR thermocycler Gene (Applied Biosystems, United States). cDNA was diluted to a final concentration of 5 ng/μL which constituted a matrix for further experiments.
Real time PCR: PCR samples were prepared using an iScript One-step RT-PCR Kit with SYBER Green (Bio-Rad, United States), and run on a Light Cycler (Roche) PCR machine. The 2-ΔΔCT method applied for relative quantification of PCR products. The following primers (BioNEER, United States) were used to detect the expression of TNF-α by RT-PCR: Forward 5’-CCTGCCCCAATCCCTTTATT -3’, Reverse 5’-CCCTAAGCCCCCAATTCTCT-3’), and GAPDH Forward 5’-TGCACCACCAACTGCTTAGC -3’, Reverse 5’-GGCATGGACTGTGGTCATGA. The result of TNF-α mRNA expression was measured relatively to GAPDH gene expression.
Colorectal tissue microarrays (TMA) were constructed, as previously described[26]. H and E stained sections of FFPE tumor samples were used to define representative areas of viable tumor tissue. From these areas, 1 mm diameter needle core biopsies were taken from corresponding areas on FFPE tumor blocks using a manual tissue arrayer (Arraymold Kit D IHCWORLD, United States). The cores were placed in recipient paraffin array blocks at defined coordinates. To ensure that representative parts of the tumors were examined, three cores of each tumor were taken. To account for tumor heterogeneity, cores were taken from central tumor arrays, as well as from the invasive border. The cores in the paraffin block were incubated for 30 min at 37 °C to improve adhesion between cores and paraffin of the recipient block. Paraffin TMA blocks were micro-dissected using a Leica semi-automatic microtome, and mounted on glass slides.
Immunohistochemistry staining was done on 5 μm sections of TMA blocks. The detection of TNF-α expression was performed using streptavidin-biotinylated horseradish peroxidase (S-ABC) kit (NovoLink Max Polymer Detection System, Novocastra, United Kingdom). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide in distilled H2O for 5 min, and then the slides were washed in Tris buffered saline (TBS) for 10 min. Nonspecific binding of antibodies was blocked by incubation with protein block (Novocastra, United Kingdom) for 5 min. Subsequently, the slides were incubated with human anti-TNF-α monoclonal mouse antibody (Abazyme, LLC, United States) primary antibody (1:100) for 1 hour at room temperature. Slides were washed in TBS 3x for 3 min, and then incubated with biotinylated anti-mouse IgG (Novocastra, United Kingdom) for 30 min. Peroxidase was detected using Diaminobenzedine (DAB) substrate (Novocastra, United Kingdom). Finally, slides were counterstained with Mayer’s hematoxylin (Novocastra, United Kingdom). As a negative control, the same procedure was conducted with the omission of the primary antibody. The expression of TNF-α in tumor and normal samples was analyzed using the eSlide capture device (ScanScope CS, Aperio Technologies Inc., Vista, CA United States).
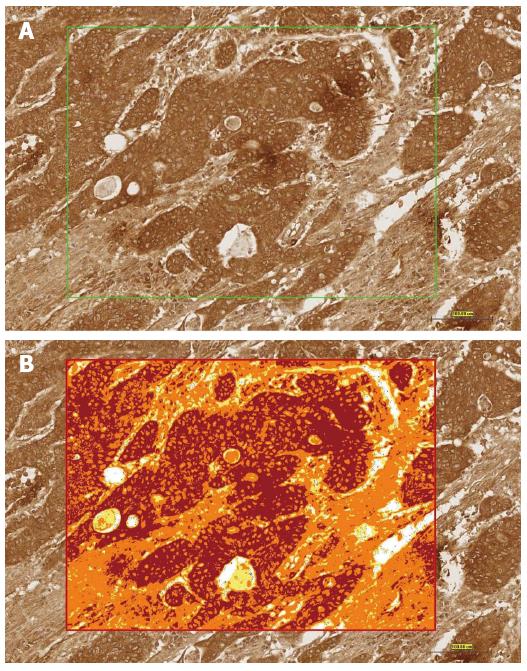
High-resolution, whole-slide digital scans of all TMA glass slides were created with a ScanScope slide scanner (Aperio Technologies, Inc.). The digital slide images were viewed by Aperio’s viewing software (ImageScope), and analyzed using Aperio’s image analysis algorithms. In each core, five square fields of a fixed area of 0.2645 μm2 were randomly selected (Figure 1A). The color deconvolution (color separation) algorithm (Aperio Technologies, Inc.) was then run on the selected area, and it generated an intensity ranges color markup image, segmenting and color-coding different parts of the image according to the intensity of positive staining. The intensity ranges showed four colors: Red indicated strong staining, Orange indicated moderate staining, Yellow indicated weak staining, and Blue indicated negative staining (Figure 1B). The area for each of these four intensity categories (expressed as a percent relative to the total analysis area), together with the average positive intensity and the average optical density, was also given as numerical output. The algorithm output also included a score (0-300) of TNF-α expression based on the percent of total area and the average optical density. The analysis output results were then exported to Excel sheets and subjected to statistical analysis, focusing mainly on the percentage of the total positive cells as the parameters to be statistically analyzed and compared.
Statistical analysis was performed using GraphPad Prism software (version 5.0). The means between the two groups were compared using Mann-Whitney test and paired t test to compare TNF-α mRNA levels in early and late tumor stage. P-value of ≤ 0.05 was considered significant.
The study complied with the requirements, and has been approved by the Ethics Committee of the King Saud University. Patient consent was obtained for this study.
The distribution of clinical pathological parameters among the patient cohort was covered by the TMA used in this study. As shown in Table 1, the majority of tumor samples approximately (16/30) were advanced at Stage III, whereas individual tumors were all at high grades (Grades II and III). Approximately (20/30) of the patients had already developed lymph node metastases.
| No. of cases (n = 30) | |
| Mean age (61 yr) | |
| < 61 | 18 (60) |
| ≥ 61 | 12 (40) |
| Gender | |
| Male | 17 (57) |
| Female | 13 (43) |
| Primary tumor | |
| Colon | 24 (80) |
| Rectum | 6 (20) |
| Tumor staging [Union internationale contre le cancer (UICC) 2010] | |
| pT2 | 10 (33.33) |
| pT3 | 19 (63.33) |
| pT4 | 1 (0.033) |
| Lymph node status (UICC 2010) | |
| pN0 | 10 (33.33) |
| pN1 | 12 (40) |
| pN1 | 8 (26.67) |
| Clinical staging (UICC 2010) | |
| Stage I | 8 (26.67) |
| Stage II | 2 (6.66) |
| Stage III | 16 (53.33) |
| Stage IV | 4 (13.33) |
| Histological grading (UICC 2010) | |
| G2 | 29 (96.67) |
| G3 | 1 (3.33) |
The relative levels of TNF-α mRNA in early CRC and tumor adjacent normal colorectal tissue were 0.02509 ± 0.02 and 0.00725 ± 0.01, respectively. The late stage of CRC compared to adjacent normal tissue was 0.07648 ± 0.1 and 0.009010 ± 0.01, respectively. Statistical analysis indicated that the expression level of TNF- mRNA was significantly higher in CRC compared to tumor adjacent normal CRC tissue (P < 0.05).
The relative expression of TNF-α mRNA in early- vs late-stage CRC was 0.02509 ± 0.02 and 0.07648 ± 0.1, respectively. The expression of TNF-mRNA was significantly higher in late CRC stage compared to early stage (P = 0.004) (Figure 2). Increased gene expression was seen in the advanced stages, with lymph node metastases compared with patients with no lymph node metastasis. The difference was not statistically significant (data not shown).
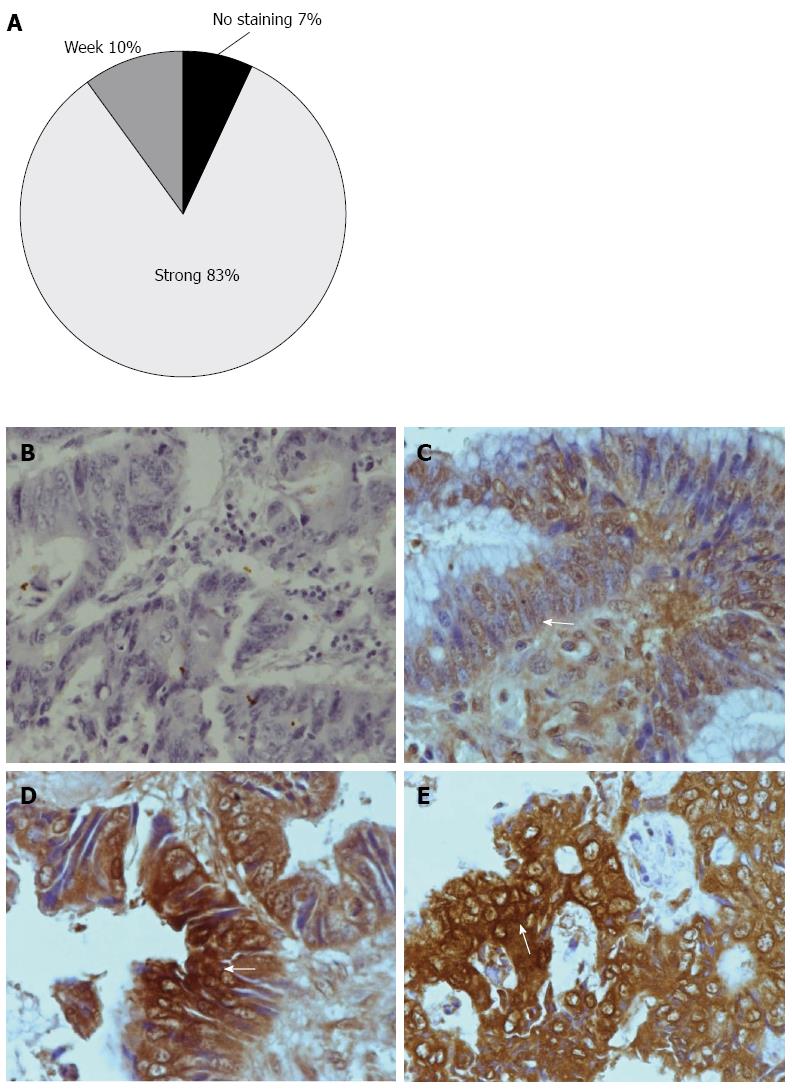
TNF-α positive cell displayed brown staining in the cytoplasm. Tumor tissues contained many TNF-α positive cells (Figure 3D and E), whereas normal colorectal tissues contained very few positive cells (Figure 3B and C). We conducted a semi-quantitative analysis based on the categorization of patient samples depending on the absence of staining, or the presence of a weak or strong staining intensity for TNF-α (Figure 1). 83% of the CRC samples showed strong positive staining, 10% showed weak staining, while 7% of samples showed negative staining for TNF-α (Figure 3A).
During cancer therapy, metastases induced by chronic inflammation is a major challenge. However, the underlying mechanisms are not completely elucidated. TNF-α has a wide range of biological activities, including apoptosis, inflammation, cell proliferation and differentiation[19]. Although TNF-α has been considered as an anti-cancer agent, it is currently recognized that chronically elevated TNF-α expression in CRC tissues may promote tumor growth, invasion and metastasis[20]. TNF-α expression is also correlated with tumor progression of colorectal adenocarcinoma. Increased TNF-α expression is correlated with tumor recurrence in CRC patients with metastases[4]. Also, TNF-α expression has been reported to increase in serum of CRC patients[21]. Recently, it has been reported that TNF-α induced epithelial mesenchymal transition (EMT), plays an indispensable role in CRC invasion and metastases, and thereby promotes CRC invasion and metastases[22].
We investigated the expression of TNF-α mRNA and protein levels in CRC patients. We showed that increased TNF-α gene transcription and protein expression levels in late stages of tumor progression are associated with advanced tumor stages. This is in accord with findings in other cancer entities that show high expression levels of TNF-α[27-29].
Immunohistochemistry results indicate that TNF-α is significantly expressed in later stages (Stage III, IV) of CRC compared to early stages (Stage I, II). TNF-α was expressed by CRC cells, but few positive cells were detected in stromal cells.
There is a link between inflammation and cancer[30-32], and TNF-α might play a significant role in this process[19]. It acts not only as a pro-inflammatory cytokine, but can also cause tumor development. Consequently, the use of TNF-α inhibitors as cancer therapeutics has been proposed[14].
Both immunohistochemistry and RT-PCR results were associated with tumor progression. These results could contribute to a further understanding of CRC pathogenesis in Saudi patients. Even if TNF-α is ultimately found not to contribute to the pathogenesis of CRC, it could still serve as an important indicator of disease progression.
We would like to thank Dr. Rehan Ahmad for his valuable scientific input with comments that greatly improved the manuscript.
Tumor necrosis factor-α (TNF-α) is a pro-inflammatory cytokine predominantly produced by macrophages as well as tumor cells. Elevated TNF-α expression in colorectal cancer (CRC) tissues may promote tumor growth, invasion and metastasis. CRC is a common type of tumor in Saudi Arabia, and has had a gradual increase in incidence.
In this study, archival tissue specimens were collected from 30 patients with CRC who had undergone surgical intervention. TNF-α mRNA and protein levels were detected by reverse transcriptase-polymerase chain reaction (RT-PCR) and immunohistochemistry, respectively.
The relative expression level of TNF-α mRNA in advanced CRC was significantly higher than in early stages. By immunohistochemistry, tissue tumor revealed a strongly positive TNF-α presence, with remarkable brown staining in the cytoplasm in 83% of patients, whereas normal colorectal tissue revealed very little evidence of TNF-α, as evidenced by no staining.
The authors found that high levels of TNF-α expression could be used as an independent diagnostic indicator of CRC, and targeting TNF-α could be a promising prognostic tool through its use in assessing the clinical stages of CRC.
CRC, RT-PCR, immunohistochemistry, tissue microarray.
The authors demonstrate the expression of TNF-α in CRC, and its correlation with advanced stages (III, IV) compared to early stages (I, II). The data is illustrative and promotes the need for further study.
P- Reviewer: Clemente A, Skoropad V, Wang F S- Editor: Zhai HH L- Editor: A E- Editor: Wang CH
| 1. | Al-Ahwal MS, Shafik YH, Al-Ahwal HM. First national survival data for colorectal cancer among Saudis between 1994 and 2004: what’s next? BMC Public Health. 2013;13:73. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Available from: http://www.wcrf.org/cancer_stastics/. |
| 3. | Compton CC, Fielding LP, Burgart LJ, Conley B, Cooper HS, Hamilton SR, Hammond ME, Henson DE, Hutter RV, Nagle RB. Prognostic factors in colorectal cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124:979-994. [PubMed] |
| 4. | Grimm M, Lazariotou M, Kircher S, Höfelmayr A, Germer CT, von Rahden BH, Waaga-Gasser AM, Gasser M. Tumor necrosis factor-α is associated with positive lymph node status in patients with recurrence of colorectal cancer-indications for anti-TNF-α agents in cancer treatment. Cell Oncol (Dordr). 2011;34:315-326. [PubMed] |
| 5. | Finke J, Ferrone S, Frey A, Mufson A, Ochoa A. Where have all the T cells gone? Mechanisms of immune evasion by tumors. Immunol Today. 1999;20:158-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 95] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Frey AB, Monu N. Signaling defects in anti-tumor T cells. Immunol Rev. 2008;222:192-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Lankiewicz S, Rother E, Zimmermann S, Hollmann C, Korangy F, Greten TF. Tumour-associated transcripts and EGFR deletion variants in colorectal cancer in primary tumour, metastases and circulating tumour cells. Cell Oncol. 2008;30:463-471. [PubMed] |
| 8. | Mesker WE, Liefers GJ, Junggeburt JM, van Pelt GW, Alberici P, Kuppen PJ, Miranda NF, van Leeuwen KA, Morreau H, Szuhai K. Presence of a high amount of stroma and downregulation of SMAD4 predict for worse survival for stage I-II colon cancer patients. Cell Oncol. 2009;31:169-178. [PubMed] |
| 9. | Veenendaal LM, Kranenburg O, Smakman N, Klomp A, Borel Rinkes IH, van Diest PJ. Differential Notch and TGFbeta signaling in primary colorectal tumors and their corresponding metastases. Cell Oncol. 2008;30:1-11. [PubMed] |
| 10. | Grimm M, Kim M, Rosenwald A, von Raden BH, Tsaur I, Meier E, Heemann U, Germer CT, Gasser M, Waaga-Gasser AM. Tumour-mediated TRAIL-Receptor expression indicates effective apoptotic depletion of infiltrating CD8+ immune cells in clinical colorectal cancer. Eur J Cancer. 2010;46:2314-2323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Jarnicki AG, Lysaght J, Todryk S, Mills KH. Suppression of antitumor immunity by IL-10 and TGF-beta-producing T cells infiltrating the growing tumor: influence of tumor environment on the induction of CD4+ and CD8+ regulatory T cells. J Immunol. 2006;177:896-904. [PubMed] |
| 12. | Balkwill F, Joffroy C. TNF: a tumor-suppressing factor or a tumor-promoting factor? Future Oncol. 2010;6:1833-1836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 350] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 13. | Burke F, Relf M, Negus R, Balkwill F. A cytokine profile of normal and malignant ovary. Cytokine. 1996;8:578-585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 96] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 14. | van Kempen LC, Ruiter DJ, van Muijen GN, Coussens LM. The tumor microenvironment: a critical determinant of neoplastic evolution. Eur J Cell Biol. 2003;82:539-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 174] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 15. | Zidi I, Mestiri S, Bartegi A, Amor NB. TNF-alpha and its inhibitors in cancer. Med Oncol. 2010;27:185-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 16. | Zins K, Abraham D, Sioud M, Aharinejad S. Colon cancer cell-derived tumor necrosis factor-alpha mediates the tumor growth-promoting response in macrophages by up-regulating the colony-stimulating factor-1 pathway. Cancer Res. 2007;67:1038-1045. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Reed JC. Apoptosis-targeted therapies for cancer. Cancer Cell. 2003;3:17-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 377] [Cited by in RCA: 360] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 18. | Balkwill F. Tumour necrosis factor and cancer. Nat Rev Cancer. 2009;9:361-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1478] [Cited by in RCA: 1407] [Article Influence: 87.9] [Reference Citation Analysis (0)] |
| 19. | Choo MK, Sakurai H, Koizumi K, Saiki I. Stimulation of cultured colon 26 cells with TNF-alpha promotes lung metastasis through the extracellular signal-regulated kinase pathway. Cancer Lett. 2005;230:47-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Szlosarek P, Charles KA, Balkwill FR. Tumour necrosis factor-alpha as a tumour promoter. Eur J Cancer. 2006;42:745-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 300] [Cited by in RCA: 291] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Csiszár A, Szentes T, Haraszti B, Balázs A, Petrányi GG, Pócsik E. The pattern of cytokine gene expression in human colorectal carcinoma. Pathol Oncol Res. 2004;10:109-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Wang H, Wang HS, Zhou BH, Li CL, Zhang F, Wang XF, Zhang G, Bu XZ, Cai SH, Du J. Epithelial-mesenchymal transition (EMT) induced by TNF-α requires AKT/GSK-3β-mediated stabilization of snail in colorectal cancer. PLoS One. 2013;8:e56664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 182] [Cited by in RCA: 224] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 23. | Greene FL. TNM staging for malignancies of the digestive tract: 2003 changes and beyond. Semin Surg Oncol. 2003;21:23-29. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 24. | Sobin LH, Christian W, UICC . TNM Classification of Malignant Tumors. 6th ed. New York: Willy-Liss Publisher 2002; . |
| 25. | Aaltonen L, Hamilton SR. Pathology and genetics of tumours of the digestive system. Tumours of small intestine. Lyon: IARC 2000; 69–91. |
| 26. | Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844-847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2991] [Cited by in RCA: 2974] [Article Influence: 110.1] [Reference Citation Analysis (0)] |
| 27. | Trompet S, de Craen AJ, Mooijaart S, Stott DJ, Ford I, Sattar N, Jukema W, Westendorp RG. High Innate Production Capacity of Proinflammatory Cytokines Increases Risk for Death from Cancer: Results of the PROSPER Study. Clin Cancer Res. 2009;15:7744-7748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 28. | Warzocha K, Ribeiro P, Renard N, Bienvenu J, Charlot C, Coiffier B, Salles G. Expression of genes coding for the tumor necrosis factor and lymphotoxin ligand-receptor system in non-Hodgkin’s lymphomas. Cancer Immunol Immunother. 2000;49:469-475. [PubMed] |
| 29. | Sati HI, Greaves M, Apperley JF, Russell RG, Croucher PI. Expression of interleukin-1beta and tumour necrosis factor-alpha in plasma cells from patients with multiple myeloma. Br J Haematol. 1999;104:350-357. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 71] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 30. | Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009;30:1073-1081. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 2081] [Article Influence: 130.1] [Reference Citation Analysis (1)] |
| 31. | Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5245] [Cited by in RCA: 5751] [Article Influence: 239.6] [Reference Citation Analysis (0)] |
| 32. | Balkwill F, Coussens LM. Cancer: an inflammatory link. Nature. 2004;431:405-406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 492] [Cited by in RCA: 495] [Article Influence: 23.6] [Reference Citation Analysis (0)] |