Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18260
Revised: May 12, 2014
Accepted: May 19, 2014
Published online: December 28, 2014
Processing time: 286 Days and 18.1 Hours
AIM: To investigate the role of pre-B-cell leukemia homeobox (PBX)3 in migration and invasion of colorectal cancer (CRC) cells.
METHODS: We detected PBX3 expression in five cell lines and surgical specimens from 111 patients with CRC using real-time reverse transcription-polymerase chain reaction. We forced expression of PBX3 in low metastatic HT-29 and SW480 cells and knocked down expression of PBX3 in highly metastatic LOVO and HCT-8 cells. Wound healing and Boyden chamber assays were used to detect cell migration and invasion after altered expression of PBX3. Western blot was performed to detect the change of signaling molecule ERK1/2 following PBX3 overexpression.
RESULTS: High level of PBX3 expression was correlated with the invasive potential of CRC cells, and significantly associated with lymph node invasion (P = 0.02), distant metastasis (P = 0.04), advanced TNM stage (P = 0.03) and poor overall survival of patients (P < 0.05). Ectopic expression of PBX3 in low metastatic cells was shown to promote migration and invasion, while inhibited PBX3 expression in highly metastatic cells suppressed migration and invasion. Furthermore, upregulation of phosphorylated extracellular signal-regulated kinase (ERK)1/2 was found to be one of the targeted molecules responsible for PBX3-induced CRC cell migration and invasion.
CONCLUSION: PBX3 induces invasion and metastasis of CRC cells partially through activation of the MAPK/ERK signaling pathway.
Core tip: Pre-B-cell leukemia homeobox (PBX)3 is a poor prognostic factor for colorectal cancer by promoting cell migration and invasion. We investigated the role of PBX3 in migration and invasion of colorectal cancer cells, and found that PBX3 could induce the invasion and metastasis of colorectal cancer cells partially through activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase signaling pathway.
-
Citation: Han HB, Gu J, Ji DB, Li ZW, Zhang Y, Zhao W, Wang LM, Zhang ZQ. PBX3 promotes migration and invasion of colorectal cancer cells
via activation of MAPK/ERK signaling pathway. World J Gastroenterol 2014; 20(48): 18260-18270 - URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18260.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18260
Colorectal cancer (CRC) is the third most common type of cancer with > 1.2 million new cases each year worldwide. Up to 50% of all patients eventually develop metastases and 90% of them cannot undergo radical resection, leading to the majority of cancer deaths[1]. Although much progress has been made in the identification and characterization of the major changes involved in CRC carcinogenesis and metastasis, searching for molecules that could serve as prognostic markers and/or therapeutic targets remains the highest-priority task in the fight against CRC.
Pre-B-cell leukemia homeobox (PBX), a family of transcription factors, has been reported to play an important role in tumor growth. PBX belongs to the TALE (three amino acid loop extension) family with a highly conserved homologous domain. It usually binds to specific DNA sequences by interacting with other homologous proteins (e.g., Meis and HOX), resulting in transcription activation or suppression of the target genes[2]. Increased expression of PBX is closely correlated with tumor growth and progression in malignancies including ovarian cancer[3], melanoma and prostate cancer[4,5]. Interference of interaction between PBX/HOX can induce apoptosis, leading to tumor growth inhibition of ovarian cancer[6], kidney cancer[7], non-small cell lung cancer[8] and breast cancer[9]. Therefore, the PBX family is likely to be closely associated with malignant behavior of the tumor cells and could be a target molecule for cancer treatment.
In recent years, PBX3, a member of the human PBX family, has been continuously reported to be associated with tumor growth and progression. In 2011, it was reported that expression level of PBX3 is significantly increased in malignant prostate cancer tissues[10]. In 2012, it was reported that forced expression of PBX3 could reverse the inhibitory effect arising from miRNA (miR)-181 on proliferation of leukemia cells[11]. In 2013, PBX3 was found to be an important cofactor of HOXA9 in leukemogenesis[12]. Direct targeting of HOXA/PBX3 impairs leukemia growth and sensitizes cells to standard chemotherapy[13]. Recently, PBX3 was also reported to be upregulated in gastric cancer and to regulate cell proliferation[14]. Together, the data suggest that PBX3, as an oncogene in some leukemias and solid cancers, is an important factor for regulating malignant biological characters of cancer cells.
We have previously reported that PBX3, as a downstream target gene of let-7c, could overcome the inhibitory effects of microRNA let-7c on cell growth and metastasis in CRC[15]. In the present study, we detected expression of PBX3 in tissues from patients with CRC and clarified its role in cancer cell invasion. Our data provide evidence that PBX3 gene expression signatures may have specific prognostic or therapeutic value for CRC.
Human CRC cell lines (HT-29, RKO, LOVO, SW480 and HCT8) were all originated from the American Type Culture Collection and cultured in RPMI 1640 medium with 10% fetal bovine serum (FBS) in a humidified atmosphere of 5% CO2 at 37 °C. The CRC specimens were collected, with informed consent, from 111 patients underwent surgery at Beijing Cancer Hospital, following the protocols approved by the Ethics Committee of Peking University Hospital. These CRC patients were all confirmed by definitive histological diagnosis after surgery and received no preoperative chemotherapy or radiotherapy. The matched normal tissues were defined as the farthest tissue at least more than 5 cm from the same tumor tissue. TNM classification was staged according to the American Joint Committee on Colorectal Cancer[16]. Those patients with synchronous liver metastases defined as M1 stage underwent both liver metastatic lesion and primary tumor surgery.
Total RNA was extracted from cells and tissues using the RNeasy Mini Kit (Qiagen, Valencia, CA, United States) according to the manufacturer’s instruction. A total of 2 μg RNA was reverse transcribed using oligo-d(T)15 primers and Moloney murine leukemia virus (MMLV) reverse transcriptase. Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed using rTaq DNA Polymerase (Takara Bio, Otsu, Shiga, Japan) on a Bio-Rad Thermal Cycler. Real-time quantitative PCR (Q-PCR) was performed using SYBR Green PCR Master Mix (Toyobo, Osaka, Japan) on ABI 7500 Fast including three independent experiments in triplicate. The primer sequences were the same as those described previously[15]. Relative gene expression normalized to GAPDH was calculated by 2-ΔCt, where ΔCt = Ct (Target) - Ct (Reference). The 2-ΔΔCt method was used for fold change calculation.
pELNS-PBX3 plasmid was constructed as described previously[15] and the PBX3 gene was subcloned into lentivirus plenti6-TR vector (Invitrogen, Carlsbad, CA, United States). For depletion of endogenous PBX3 expression, short hairpin RNA (shRNA) targeting 592-613 bp or 928-949 bp was inserted into a lentivirus vector downstream of the U6 promoter. Lentiviral particles were prepared by co-transfection with shuttle vector constructs and the ViraPower Packaging Mix (Invitrogen) in HEK-293FT cells, according to the manufacturer’s protocol. Cells infected with lentiviruses were screened with blasticidin (5 μg/mL) to achieve stable overexpression or silencing in cell lines.
About 4 × 105 cells were seeded into six-well culture plates and an incision was made in the central area of the confluent cells 24 h later. Cell migration near the wound area in confluent monolayers was monitored under a microscope (Leica, Wetzlar, Germany) at the time of scratching and after 72 h. The experiment was performed in triplicate with three independent repeats.
To evaluate the effect of PBX3 on cell migration and invasion further, 2.5 × 104 cells in 100 μL RPMI-1640 with 1% FBS were plated into the upper chamber of the Transwell chamber (8-μm pore size; Corning Incorporated, Corning, NY, United States). A total of 500 μL of RPMI-1640 with 10% FBS was loaded into the lower chamber to serve as a chemoattractant for the cells. After 24 h, cells migrated to or invaded the other side of the membrane and were counted and imaged under a microscope (Leica), after fixing with 2% methanol and staining with 1% crystal violet solution. The experiments were repeated three times in triplicate.
Cell proteins were extracted using RIPA buffer containing complete protease and phosphatase inhibitor cocktail (Roche, Mannheim, Germany). Extracted protein (20 μg) was separated by 10% SDS-PAGE and blotted onto PVDF membranes (Millipore, Billerica, CA, United States). Rabbit anti-PBX3 (1:3000 dilution; Epitomics, Burlingame, CA, United States), rabbit anti-extracellular signal-regulated kinase (ERK) and anti-phospho-ERK (1:3000 dilution; Cell Signaling Technology, Danvers, MA, United States), and mouse anti-β-actin (1:50 000 dilution; Roche) were used as primary antibodies. Horseradish-peroxidase-conjugated goat anti-rabbit or anti-mouse IgG (cwBiotech, Beijing, China) was used as secondary antibodies. Signals were detected with a chemiluminescence (ECL) kit (Millipore).
Continuous variables with normal distribution are presented as mean ± SD of three independent experiments run in triplicate, or otherwise as the median. Categorical variables are presented as frequency or percentage. Differences between two groups were assessed by Student’s t test or Mann-Whitney test unless specified otherwise. A P value < 0.05 was considered statistically significant.
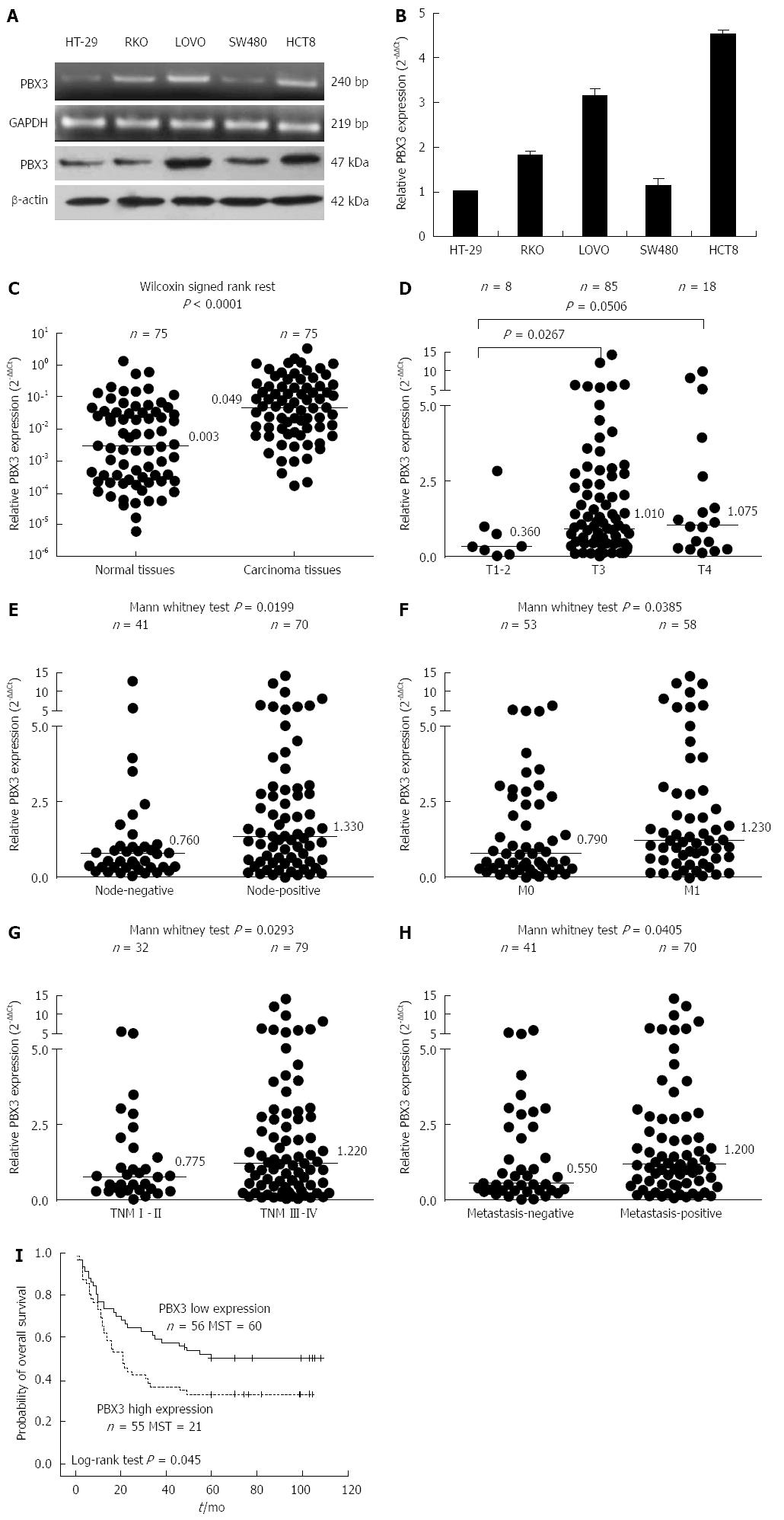
To determine the potential role of PBX3 in cell invasion, the expression levels of PBX3 were detected in CRC cell lines. As shown in Figure 1A and B, the relative expression of PBX3 at both the mRNA (Figure 1A by RT-PCR, Figure 1B by real-time Q-PCR) and protein levels (Figure 1A by Western blot) were higher in cells with relatively high invasive ability (LOVO and HCT8) than in those with relatively low or no invasive potential (HT-29 and SW480). The results suggested that a high level of PBX3 expression is associated with invasion and metastasis of CRC cells.
To determine the relationship between the expression level of PBX3 and clinical pathological variables, we examined PBX3 expression in 75 human CRC tissues and matched normal tissues. As shown in Figure 1C, the PBX3 expression was upregulated about 16-fold in cancer tissues compared with normal tissues (median: 0.049 vs 0.003; Wilcoxon signed rank test P < 0.0001, n = 75 for each group). We focused on the role of PBX3 in tumor invasion and metastasis, thus, we further detected its expression in 111 carcinoma tissues from patients with detailed follow-up information. As shown in Table 1 and Figure 1D-H, high levels of PBX3 expression were significantly associated with local depth of invasion (Figure 1D, T3 vs T1-2, P = 0.0267), lymph node metastases (Figure 1E, P = 0.0199), synchronous liver metastases (Figure 1F, P = 0.0385), advanced TNM stage (Figue 1G, P = 0.0293), and metastasis (including synchronous and metachronous metastasis, Figure 1H, P = 0.0405). There was no significant difference in PBX3 expression with regard to sex, age, venous invasion, histological type, and degree of differentiation. The results indicated that high level of PBX3 expression was related to malignant invasion and metastasis of CRC cells.
| Variable | Case | PBX3 expression1 Median (range) | P value2 |
| Sex | |||
| Male | 63 (56.8) | 0.590 (0.00-14.16) | 0.527 |
| Female | 48 (43.2) | 0.690 (0.04-12.84) | |
| Age | |||
| ≤ 60 | 51(45.9) | 1.010 (0.02-14.16) | 0.425 |
| > 60 | 60 (54.1) | 0.945 (0.00-12.18) | |
| Venous invasion | |||
| Absent | 69 (62.2) | 0.870 (0.00-14.16) | 0.557 |
| Present | 42 (37.8) | 1.095 (0.07-9.83) | |
| Histological type | |||
| Adenocarcinoma | 108 (97.3) | 0.995 (0.00-14.16) | 0.874 |
| Mucinous adenocarcinoma | 3 (2.7) | 1.620 (0.20-0.67) | |
| Differentiation3 | |||
| Poor | 16 (14.8) | 0.865 (0.12-12.84) | 0.714 |
| Moderate | 75 (69.4) | 0.990 (0.02-14.16) | |
| Well | 17 (15.7) | 1.080 (0.00-8.19) | |
| Tumor stage | |||
| 1-2 | 8 (7.2) | 0.360 (0.02-2.84) | 0.211 |
| 3 | 85 (76.6) | 1.010 (0.00-14.16) | |
| 4 | 18 (16.2) | 1.075 (0.10-9.83) | |
| Lymph node metastasis | |||
| Negative | 41 (36.9) | 0.7600 (0.02-12.84) | 0.0199a |
| Positive | 70 (63.1) | 1.3300 (0.0-14.16) | |
| Metastasis stage | |||
| M0 | 53 (47.7) | 0.790 (0.02-6.44) | 0.0385a |
| M1 | 58 (52.3) | 1.230 (0.00-14.16) | |
| TNM stage | |||
| I-II | 32 (28.8) | 0.775 (0.02-5.50) | 0.0293a |
| III-IV | 79 (71.2) | 1.220 (0.00-14.16) | |
| Metastasis | |||
| Absent | 41 (36.9) | 0.550 (0.02-5.90) | 0.0405a |
| Present (SM and MM) | 70 (63.1) | 1.200 (0.00-14.16) |
Kaplan-Meier curve analysis revealed that high expression of PBX3, grouped by a cut-off value of median PBX3 level in cancer tissues, predicted poor patient survival. Figure 1I shows that the overall survival time for patients with high PBX3 expression (median: 21 mo; n = 55) was significantly shorter than that for patients with low PBX3 expression (median: 60 mo; n = 56). However, Cox proportional hazard regression analysis failed to reveal that the expression of PBX3 was an independent prognostic factor for survival of patients with CRC (data not shown). These data indicated that increased PBX3 expression predicted poor prognosis.
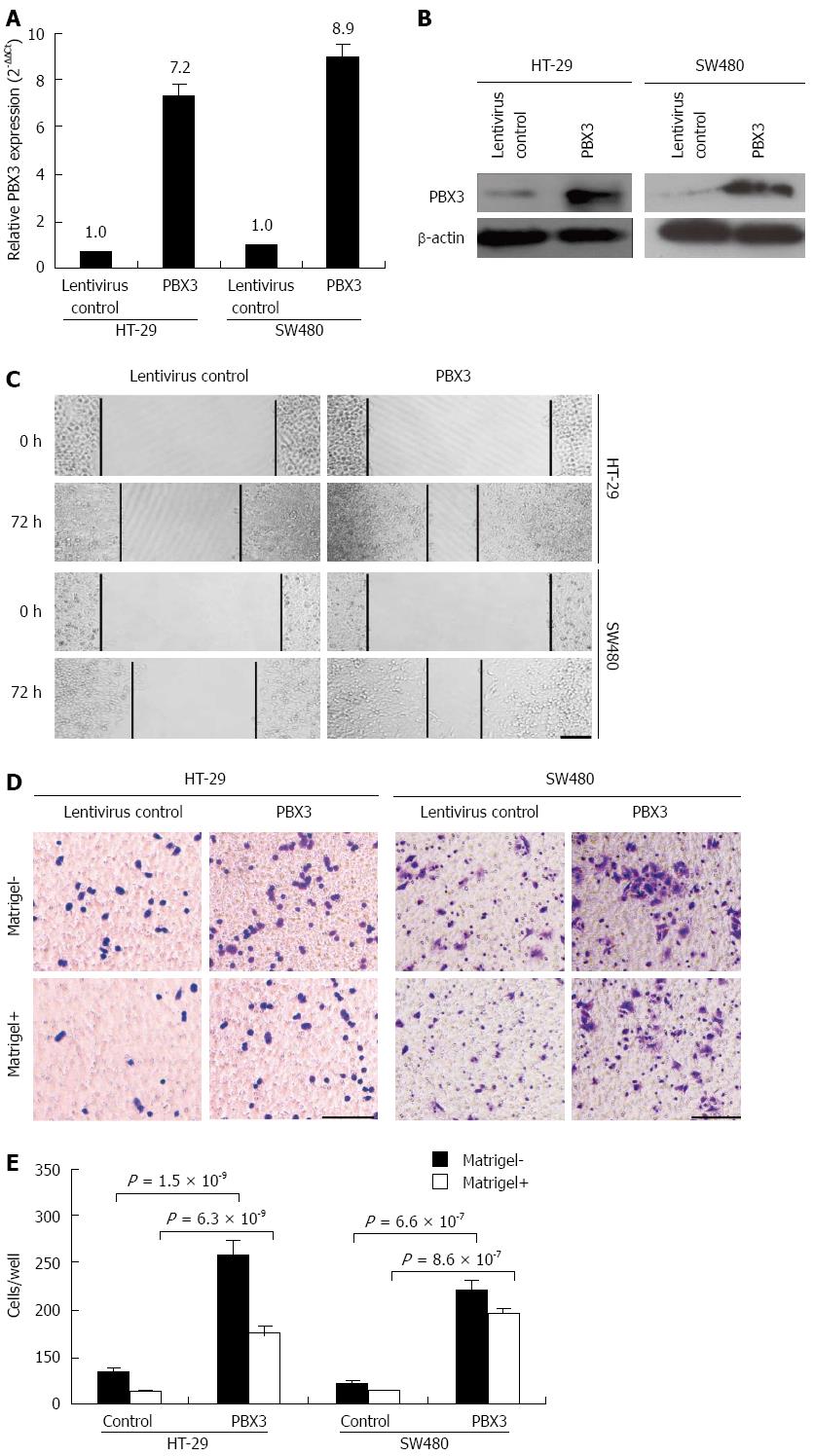
The relationship between increased expression of PBX3 and cell invasion of CRC drove us to explore the possible biological functions of PBX3 in cancer cells, especially the effect on cell migration and invasion. We overexpressed PBX3 in the low metastatic HT-29 and SW480 cells. As shown in Figure 2A, the relative expression of PBX3 was increased about 7.2- and 8.9-fold for HT-29 and SW480 cells, respectively, after infection with PBX3-overexpressing lentiviruses. PBX3 protein expression was also increased markedly (Figure 2B). Both HT-29 and SW480 cells with ectopic expression of PBX3 showed a significant increase in cell spreading, by wound healing assay (Figure 2C), cell migration and invasion by Transwell assay (Figure 2D), as compared with control cells. Figure 2E shows that the number of cells that migrated through the Transwell chamber was increased about 4.7- and 5.3-fold in HT-29 and SW480 cells, respectively, after overexpression of PBX3, compared with control cells. At the same time, the number of invasive cells was also increased about 5.9- and 6.3-fold in HT-29 and SW480 cells, respectively. Collectively, overexpression of PBX3 promoted cell spreading, migration and invasion.
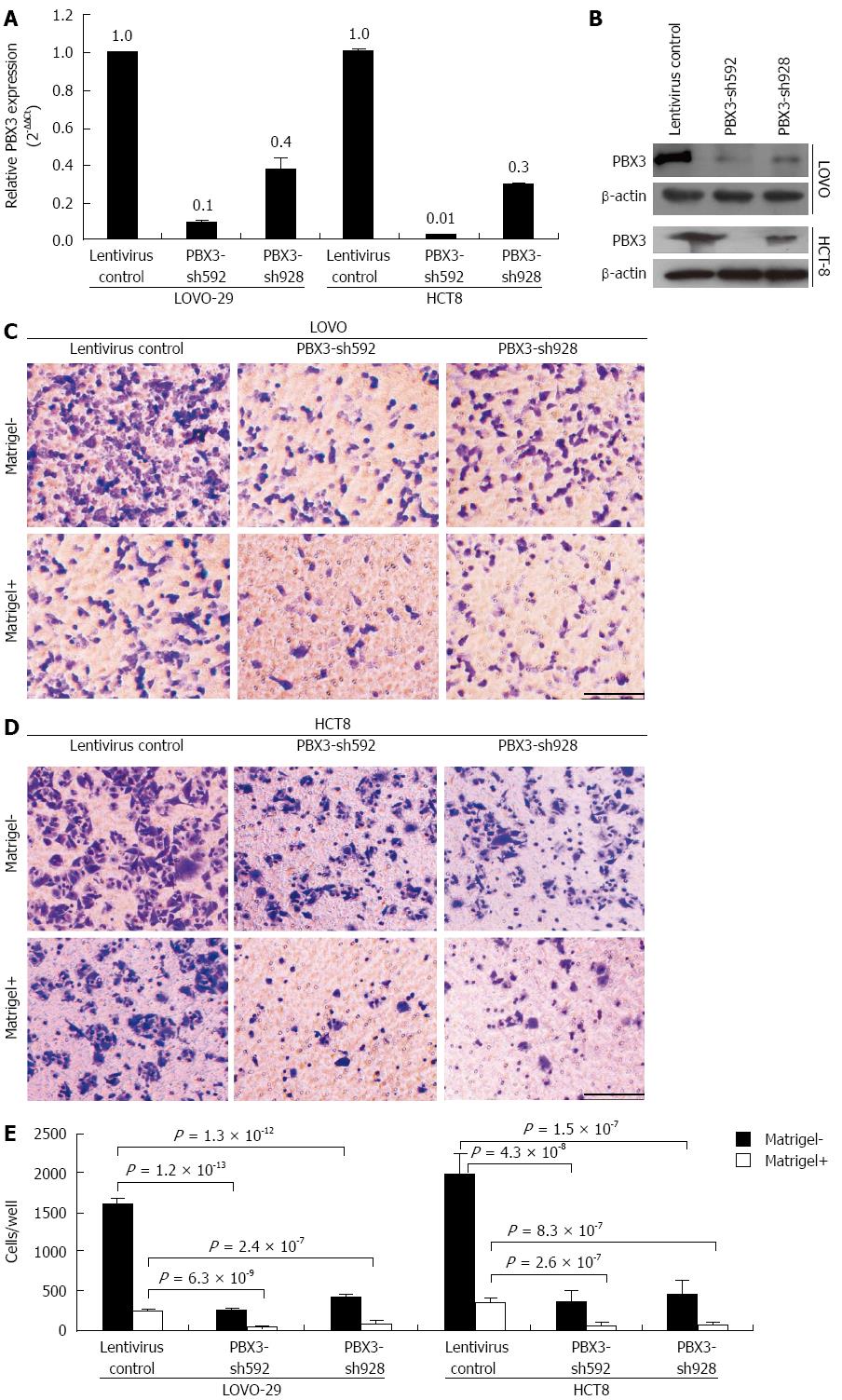
To validate if PBX3 is indeed necessary for CRC cell migration and invasion, we inhibited the expression of PBX3 using lentiviral shRNA constructs in highly metastatic cell lines, LOVO and HCT8. As shown in Figure 3A, the levels of PBX3 expression were decreased about 90% and 61% after infection with PBX3-shRNA592 and shRNA928 in LOVO cells, and about 97% and 70% for PBX3-shRNA592 and shRNA928 in HCT8 cells (Figure 3A), respectively. PBX3 expression was also suppressed at protein level (Figure 3B). Consistent with the results with overexpression of PBX3 in HT-29 and SW480 cells, knockdown of PBX3 expression in LOVO and HCT8 cells inhibited cell motility and invasion (Figure 3C-E). These data support that inhibition of PBX3 expression suppresses cell motility and invasion in vitro.
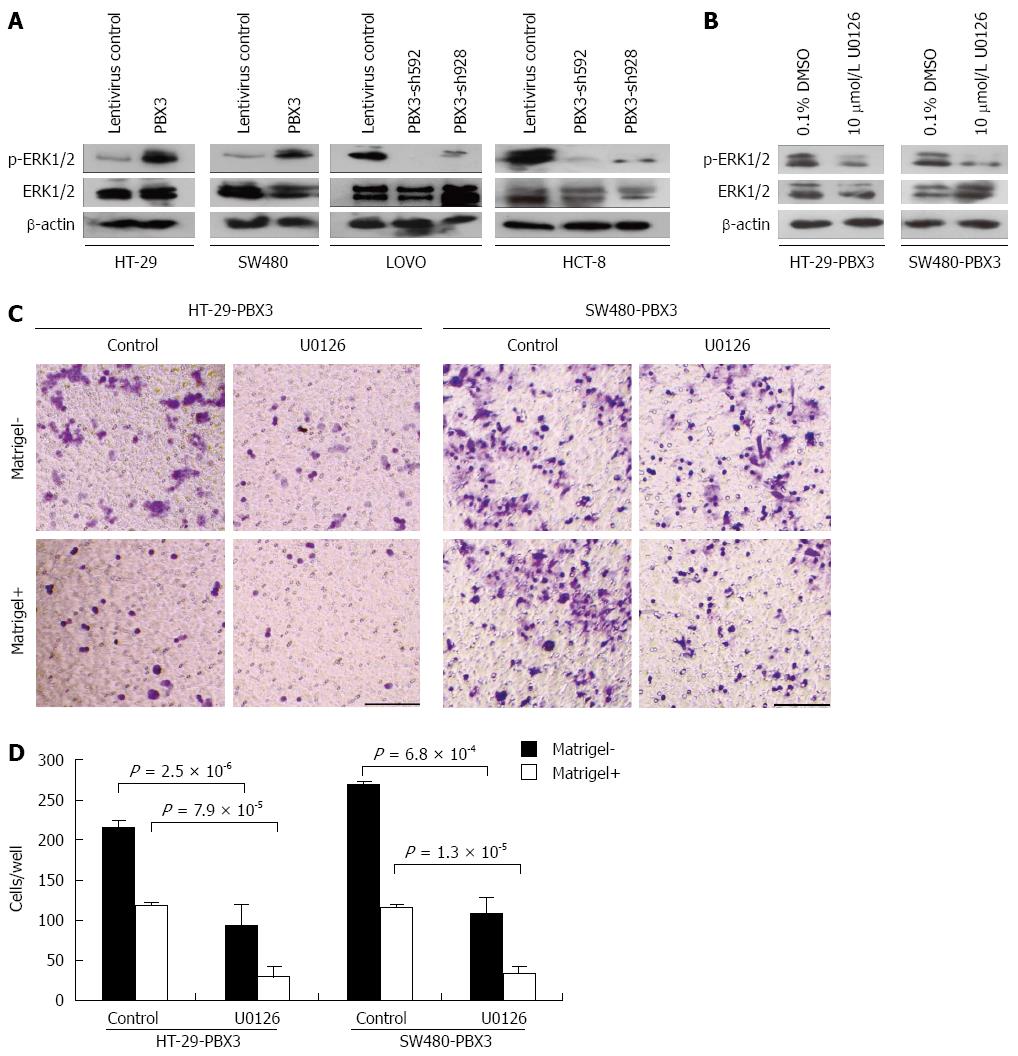
Our preliminary data with RNA-seq analysis of differentially regulated genes indicate that MAPK/ERK signaling pathway was significantly enriched after ectopic expression of PBX3 (data not shown). The MAPK/ERK signaling pathway, which was reported to be associated with CRC migration and invasion, was therefore chosen for further study. We determined the changes in expression of phospho-ERK1/2 and ERK1/2 in cells with altered expression of PBX3. As expected, phospho-ERK1/2 expression was increased after overexpression of PBX3 and decreased after silencing PBX3 (Figure 4A). These data clearly demonstrated that PBX3 expression resulted in an activation of MAPK/ERK signaling pathway, at least partially.
To confirm whether the effect of PBX3 overexpression on cell motility and invasion was through the MAPK/ERK pathway, cells ectopically expressing PBX3 were treated with ERK1/2 inhibitor U0126. Figure 4B shows that the upregulation of phospho-ERK1/2 by PBX3 overexpression in HT-29 and SW480 cells was greatly reduced by incubation with 10 μmol/L U0126 for 48 h. In the meantime, the promotive effects of PBX3 on cell migration and invasion were also markedly reversed (Figure 4C and D). These data suggest that ERK1/2 inhibition reverses the effects of PBX3 overexpression on cell motility and that the MAPK/ERK pathway is essential for the function of PBX3.
PBX proteins are central to multiple gene regulatory networks of interactions between PBX proteins and other transcription factors[17]. They bind to specific DNA sequences and have been implicated in crucial developmental processes, either as co-factors to members of the HOX family or by HOX-independent functions[2]. In addition to the regular DNA binding sequences for PBX proteins, PBX3, in the form of monomer or homologous dimer, also shows high affinity binding to the “TGATTGATTTGAT” sequence, suggesting its different regulation of downstream target genes from other members. Here, based on our previous observation that let-7c functions as a metastasis suppressor by targeting PBX3, we verified the effect of PBX3 on CRC invasion and metastasis. Our data demonstrate that PBX3 is a potential marker of poor prognosis in CRC. First, malignant cancer cells are always accompanied by a higher level of PBX3 expression. Similar findings are also seen in malignant prostate tissue stained with anti-human PBX3 antibody by immunohistochemistry[10,15]. Second, high levels of PBX3 expression are associated with depth of invasion and clinical stage, which is consistent with the results reported in gastric cancer[14]. In addition, our study has revealed, for the first time, that patients with higher PBX3 expression are more likely to develop metastasis and have poor overall survival after surgery.
MAPK/ERK signaling plays a key role in many CRC-related biological processes, including cell proliferation[18], tumor invasiveness[19], stem-like phenotypes[20], as well as resistance to chemotherapy[21]. It can be activated by many tumor-related proteins and involved in their function as a network master[21,22]. In this study, we identified activation of ERK1/2 as a key downstream step in PBX3 function in CRC invasion and metastasis. However, the molecule(s) that directly responsible for the MAPK pathway activation remains to be revealed is still to be confirmed by further studies. It has been reported that PBX proteins can control the vertebrate developmental processes via transforming growth factor β signaling[23], Wnt-β-catenin signaling[24], and protein kinase A signaling[25]. Further studies are needed to verify whether these pathways are also involved in the PBX3-mediated invasion and metastasis of CRC.
We thank Drs. Hua-Mei Dong and Arthur Berg, Associate Professor of Biostatistics and Bioinformatics at Penn State College of Medicine, for revising the manuscript.
Tumor metastasis is the major cause of death in patients with colorectal cancer (CRC), so it is important to understand the underlying mechanisms of metastases in order to find molecules that can serve as prognostic markers or therapeutic targets. Pre-B-cell leukemia homeobox (PBX) is a family of transcription factors, which is reported to play an important role in tumor growth and progression. However, the role of PBX3 in CRC is still unclear.
PBX3 is a member of the human PBX family, which has frequently been reported to be associated with tumor growth and progression in recent years. In the area of oncology, the research hotspot focuses on the role of PBX3 in tumorigenesis and malignant characters of cancer cells as a target of miR-181, let-7d and let-7c, or as a co-factor of other transcription factors including HOXA9. The research hotspot also focuses on direct targeting of HOXA/PBX3 for therapeutic purposes.
In the previous study, the authors reported that PBX3, as a downstream target gene of let-7c, overcame the inhibitory effect on cell growth and metastasis initiated by let-7c in CRC. In this study, the authors detected the expression of PBX3 in CRC tissues and verified the effect of PBX3 on CRC cell invasion. By detecting the expression of PBX3 by real-time polymerase chain reaction, this study showed that the high level of PBX3 expression in CRC was correlated with the invasive potential of CRC cells, and significantly associated with lymph node invasion, distant metastasis, advanced TNM stage, and poor overall survival. By gain- and loss-of-function in CRC cells with different metastatic potentials, it was confirmed that PBX3 could promote migration and invasion of CRC cells. In this study, the authors also reported that activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase signaling pathway might be partially involved in PBX3-mediated cell invasion and metastasis of CRC cells.
The results suggest that PBX3 gene expression signatures have specific prognostic value, and PBX3 as a direct molecular target could have therapeutic value in CRC.
CRC (also known as colon cancer, rectal cancer, bowel cancer or colorectal adenocarcinoma) is a cancer with uncontrolled cell growth in the colon or rectum, or in the appendix. TALE (three-amino acid extension loop) homeobox proteins are highly conserved transcription regulators, including MEIS and PBX families. They are best characterized as co-factors for HOX proteins. PBX refers to a family of transcription factors, belonging to TALE homeobox proteins.
This was a good study in which the authors analyzed the expression profile of PBX3 in five CRC cell lines and clinical specimens from patients with CRC. In this study, the authors also analyzed the role and potential mechanism of PBX3 in CRC migration and invasion. The results are interesting and suggest that PBX3 is a potential prognostic and/or therapeutic target that could be used in CRC.
P- Reviewer: Kuramitsu Y, Zhang GJ S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Ricci-Vitiani L, Fabrizi E, Palio E, De Maria R. Colon cancer stem cells. J Mol Med (Berl). 2009;87:1097-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 2. | Laurent A, Bihan R, Omilli F, Deschamps S, Pellerin I. PBX proteins: much more than Hox cofactors. Int J Dev Biol. 2008;52:9-20. [PubMed] |
| 3. | Crijns AP, de Graeff P, Geerts D, Ten Hoor KA, Hollema H, van der Sluis T, Hofstra RM, de Bock GH, de Jong S, van der Zee AG. MEIS and PBX homeobox proteins in ovarian cancer. Eur J Cancer. 2007;43:2495-2505. [PubMed] |
| 4. | Shiraishi K, Yamasaki K, Nanba D, Inoue H, Hanakawa Y, Shirakata Y, Hashimoto K, Higashiyama S. Pre-B-cell leukemia transcription factor 1 is a major target of promyelocytic leukemia zinc-finger-mediated melanoma cell growth suppression. Oncogene. 2007;26:339-348. [PubMed] |
| 5. | Kikugawa T, Kinugasa Y, Shiraishi K, Nanba D, Nakashiro K, Tanji N, Yokoyama M, Higashiyama S. PLZF regulates Pbx1 transcription and Pbx1-HoxC8 complex leads to androgen-independent prostate cancer proliferation. Prostate. 2006;66:1092-1099. [PubMed] |
| 6. | Morgan R, Plowright L, Harrington KJ, Michael A, Pandha HS. Targeting HOX and PBX transcription factors in ovarian cancer. BMC Cancer. 2010;10:89. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 78] [Cited by in RCA: 85] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 7. | Shears L, Plowright L, Harrington K, Pandha HS, Morgan R. Disrupting the interaction between HOX and PBX causes necrotic and apoptotic cell death in the renal cancer lines CaKi-2 and 769-P. J Urol. 2008;180:2196-2201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Plowright L, Harrington KJ, Pandha HS, Morgan R. HOX transcription factors are potential therapeutic targets in non-small-cell lung cancer (targeting HOX genes in lung cancer). Br J Cancer. 2009;100:470-475. [PubMed] |
| 9. | Morgan R, Boxall A, Harrington KJ, Simpson GR, Gillett C, Michael A, Pandha HS. Targeting the HOX/PBX dimer in breast cancer. Breast Cancer Res Treat. 2012;136:389-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Ramberg H, Alshbib A, Berge V, Svindland A, Taskén KA. Regulation of PBX3 expression by androgen and Let-7d in prostate cancer. Mol Cancer. 2011;10:50. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Li Z, Huang H, Li Y, Jiang X, Chen P, Arnovitz S, Radmacher MD, Maharry K, Elkahloun A, Yang X. Up-regulation of a HOXA-PBX3 homeobox-gene signature following down-regulation of miR-181 is associated with adverse prognosis in patients with cytogenetically abnormal AML. Blood. 2012;119:2314-2324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 139] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 12. | Li Z, Zhang Z, Li Y, Arnovitz S, Chen P, Huang H, Jiang X, Hong GM, Kunjamma RB, Ren H. PBX3 is an important cofactor of HOXA9 in leukemogenesis. Blood. 2013;121:1422-1431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 114] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 13. | Dickson GJ, Liberante FG, Kettyle LM, O’Hagan KA, Finnegan DP, Bullinger L, Geerts D, McMullin MF, Lappin TR, Mills KI. HOXA/PBX3 knockdown impairs growth and sensitizes cytogenetically normal acute myeloid leukemia cells to chemotherapy. Haematologica. 2013;98:1216-1225. [PubMed] |
| 14. | Li Y, Sun Z, Zhu Z, Zhang J, Sun X, Xu H. PBX3 is overexpressed in gastric cancer and regulates cell proliferation. Tumour Biol. 2014;35:4363-4368. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 15. | Han HB, Gu J, Zuo HJ, Chen ZG, Zhao W, Li M, Ji DB, Lu YY, Zhang ZQ. Let-7c functions as a metastasis suppressor by targeting MMP11 and PBX3 in colorectal cancer. J Pathol. 2012;226:544-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 121] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Ratto C, Ricci R. Potential pitfalls concerning colorectal cancer classification in the seventh edition of the AJCC Cancer Staging Manual. Dis Colon Rectum. 2011;54:e232. [PubMed] |
| 17. | Capellini TD, Zappavigna V, Selleri L. Pbx homeodomain proteins: TALEnted regulators of limb patterning and outgrowth. Dev Dyn. 2011;240:1063-1086. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 18. | Qing H, Gong W, Che Y, Wang X, Peng L, Liang Y, Wang W, Deng Q, Zhang H, Jiang B. PAK1-dependent MAPK pathway activation is required for colorectal cancer cell proliferation. Tumour Biol. 2012;33:985-994. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 19. | Levidou G, Saetta AA, Gigelou F, Karlou M, Papanastasiou P, Stamatelli A, Kavantzas N, Michalopoulos NV, Agrogiannis G, Patsouris E. ERK/pERK expression and B-raf mutations in colon adenocarcinomas: correlation with clinicopathological characteristics. World J Surg Oncol. 2012;10:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Horst D, Chen J, Morikawa T, Ogino S, Kirchner T, Shivdasani RA. Differential WNT activity in colorectal cancer confers limited tumorigenic potential and is regulated by MAPK signaling. Cancer Res. 2012;72:1547-1556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 21. | Su N, Peng L, Xia B, Zhao Y, Xu A, Wang J, Wang X, Jiang B. Lyn is involved in CD24-induced ERK1/2 activation in colorectal cancer. Mol Cancer. 2012;11:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 22. | Zhao SL, Hong J, Xie ZQ, Tang JT, Su WY, Du W, Chen YX, Lu R, Sun DF, Fang JY. TRAPPC4-ERK2 interaction activates ERK1/2, modulates its nuclear localization and regulates proliferation and apoptosis of colorectal cancer cells. PLoS One. 2011;6:e23262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Bailey JS, Rave-Harel N, McGillivray SM, Coss D, Mellon PL. Activin regulation of the follicle-stimulating hormone beta-subunit gene involves Smads and the TALE homeodomain proteins Pbx1 and Prep1. Mol Endocrinol. 2004;18:1158-1170. [PubMed] |
| 24. | Ferretti E, Li B, Zewdu R, Wells V, Hebert JM, Karner C, Anderson MJ, Williams T, Dixon J, Dixon MJ. A conserved Pbx-Wnt-p63-Irf6 regulatory module controls face morphogenesis by promoting epithelial apoptosis. Dev Cell. 2011;21:627-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 25. | Kilstrup-Nielsen C, Alessio M, Zappavigna V. PBX1 nuclear export is regulated independently of PBX-MEINOX interaction by PKA phosphorylation of the PBC-B domain. EMBO J. 2003;22:89-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |