Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18228
Revised: June 28, 2014
Accepted: August 13, 2014
Published online: December 28, 2014
Processing time: 264 Days and 4.9 Hours
AIM: To investigate the therapeutic effects of mesenchymal stem cells (MSCs) transplanted intraperitoneally and intravenously in a murine model of colitis.
METHODS: MSCs were isolated from C57BL/6 mouse adipose tissue. MSC cultures were analyzed according to morphology, cellular differentiation potential, and surface molecular markers. Experimental acute colitis was induced in C57BL/6 mice by oral administration of 2% dextran sulfate sodium (DSS) in drinking water ad libitum from days 0 to 7. Colitis mice were treated with 1 × 106 MSCs via intraperitoneal or intravenous injection on days 2 and 5. The disease activity index was determined daily based on the following parameters: weight loss, stool consistency and presence of blood in the feces and anus. To compare morphological and functional differences in tissue regeneration between different MSC injection modalities, mice were euthanized on day 8, and their colons were examined for length, weight, and histopathological changes. Inflammatory responses were determined by measuring the levels of different serum cytokines using a CBA Th1/Th2/Th17 kit. Apoptotic rates were evaluated by terminal deoxynucleotidyl transferase-mediated dUDP-biotin nick end labeling assay.
RESULTS: Intravenous infusion of MSCs was more effective than intraperitoneal treatment (P < 0.001) in reducing the clinical and histopathologic severity of colitis, which includes weight loss, diarrhea and inflammation. An histological evaluation demonstrated decreased colonic inflammation based on reduced crypt loss and reduced infiltration of inflammatory cells. This therapeutic effect was most likely mediated by the down-regulation of pro-inflammatory cytokines [interleukin (IL)-6 and tumor necrosis factor (TNF)]; and by the up-regulation of anti-inflammatory cytokines (IL-10 and IL-4). Intravenous transplantation also induced high levels of IFN that lead to activation of the immunosuppressive activity of the MSCs, which did not occur with intraperitoneal transplantation (P = 0.006). An increase in apoptotic T cells was observed after intravenous, but not intraperitoneal, MSC infusion, suggesting that MSCs can induce apoptosis in resistant T cells in colonic inflammation (P = 0.027).
CONCLUSION: Our results demonstrate that intravenous treatment is a superior method for reducing colon inflammation compared with intraperitoneal therapy.
Core tip: After receiving appropriate biological signals during injury or tissue inflammation, mesenchymal stem cells (MSCs) can migrate to the affected site and suppress effector T cells to modulate inflammatory responses and tissue regeneration. Currently, little is known regarding the optimal delivery strategy for MSCs for the treatment of ulcerative colitis. To our knowledge, no studies have shown which method of cell transplantation is best for the treatment of colon inflammation. The present study demonstrates that intravenous treatment resulted in reduced colon inflammation and is the best route for cell therapy in ulcerative colitis.
-
Citation: Gonçalves FDC, Schneider N, Pinto FO, Meyer FS, Visioli F, Pfaffenseller B, Lopez PLDC, Passos EP, Cirne-Lima EO, Meurer L, Paz AH. Intravenous
vs intraperitoneal mesenchymal stem cells administration: What is the best route for treating experimental colitis? World J Gastroenterol 2014; 20(48): 18228-18239 - URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18228.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18228
Inflammatory bowel disease (IBD), which includes ulcerative colitis (UC) and Crohn’s disease (CD), is characterized by chronic inflammation, abdominal pain, visceral hypersensitivity, and diarrhea[1,2]. UC presents mucosal T cell dysfunction, inflammatory cell infiltration, and abnormal cytokine production. This ultimately leads to a defective immune response to enteric antigens, which causes chronic intestinal inflammation[3-5]. The resistance of T cells to apoptosis contributes to inappropriate T cell accumulation in colitis and, consequently, to the perpetuation of chronic mucosal inflammation[6].
Colitis therapy involves immunosuppressive drugs that induce remission of intestinal inflammation and associated symptoms. Although medical treatment is effective for inducing and maintaining remission, no therapeutic option exists that can definitively reverse colon inflammation[7]. Therefore, novel therapeutic strategies, such as stem cell therapy, are needed for non-responsive patients and to reduce the side effects associated with current therapy.
Mesenchymal stem cells (MSCs), which are present in adipose tissue and in several other tissues, exhibit great plasticity[8]. In appropriate culture conditions, MSCs are able to differentiate into cartilage, bone, muscle, and tendon/ligament[9,10]. In addition, they also have low immunogenicity and somewhat display immunosuppressive proprieties[11]. MSCs can trigger the release of several soluble factors, including anti-inflammatory cytokines which act on the immune system to modulate immune response[12]. Moreover, the capacity to suppress T cell activities and induce apoptosis provides a rationale for applying these cells in IBD therapy[13,14].
Several studies demonstrate the ability of MSCs to preferentially migrate to sites of injury when infused in animal models. After receiving appropriate signals during tissue inflammation, MSCs can migrate to affected sites where they assist in recovery, displaying high therapeutic potential with regards to tissue repair and/or the control of local inflammation[15]. The expression of growth factors, cytokines and extracellular matrix receptors by MSCs may drive this process[16,17]. These cells have great therapeutic potential in regenerative medicine due to their capacity for differentiation in vitro as well as their secretion of many bioactive molecules[18]. Still, little is known regarding the optimal delivery strategy for MSCs to treat IBD. The present study compared intravenous and intraperitoneal routes of administration for MSC in the treatment of UC to clarify the best cell therapeutic methodology to enhance the success of UC treatment.
Male C57BL/6 mice, 8-12 wk old, were purchased from Unidade de Experimentação Animal (UEA) of Hospital de Clínicas de Porto Alegre (HCPA) - Universidade Federal do Rio Grande do Sul (UFRGS). Mice were maintained at the house facilities, at a controlled humidity (50%) and temperature (20-22 °C), a 12 h light-dark cycle, and were fed standard diet and drinking water ad libitum. All procedures were performed in accordance to UFRGS guidelines for animal experimentation and the Brazilian Federal Law 11.794/08, which establishes procedures for the scientific use of animals and regulates the registration of experimentation centers. This study was approved by the Institutional Research Ethics Committee CEUA-HCPA and is registered under the number 11-0244.
Epididymal fat from male C57BL/6 mice was aseptically removed, dissected from visible blood vessels, and enzymatically digested for 30 min at 37 °C in Dulbecco’s Modified Eagle’s Medium (DMEM; Carlsbad, Gibco, CA, United States) low glucose supplemented with 1 mg/mL of collagenase type I (Sigma, St. Louis, MO, United States). Cell suspensions were centrifuged and the pellet was resuspended in DMEM with 200 mL/L fetal bovine serum (FBS; Carlsbad, Gibco, CA, United States) and antibiotics. Cells were then plated in 6-well culture dishes and incubated at 37 °C in a humidified atmosphere containing 50 mL/L CO2. Non-adherent cells were removed after 72 h in culture. Adherent cells achieving 80% confluence were passaged using 2.5 mL/L Trypsin-EDTA solution (Gibco, Carlsbad, CA, United States) and maintained in DMEM supplemented with 200 mL/L FBS, 100 units/mL penicillin and 100 mg/mL streptomycin (Gibco, Carlsbad, CA, United States). MSCs were used between passages 3-6.
To characterize MSCs in accordance with The International Society for Cellular Therapy Statement[19], three different experimental procedures were employed according to Gonçalves et al[20]. Adipogenic differentiation was induced by culturing MSCs in DMEM 100 mL/L FBS, 15 mmol/L Hepes (Sigma, St. Louis, MO, United States), supplemented with 10-8 mol/L dexamethasone (Sigma, St. Louis, MO, United States), 5 μg/mL insulin and 50 μg/mL indomethacin (Sigma, St. Louis, MO, United States). Adipocytes were easily discerned from the undifferentiated cells by phase-contrast microscopy. To further confirm their identity, cells were fixed with 40 g/L paraformaldehyde and stained with Oil Red (Sigma, St. Louis, MO, United States) after 21 d of adipogenic differentiation. Secondly, to induce osteogenic differentiation, MSCs were cultured in DMEM 100 mL/L FBS, 15 mmol/L Hepes, supplemented with 10-8 mol/L dexamethasone, 5 μg/mL ascorbic acid 2-phosphate (Sigma, St. Louis, MO, United States) and 10 mmol/L β-glycerolphosphate (Sigma, St. Louis, MO, United States). To observe calcium deposition, cultures were fixed and stained with Alizarin Red stain (Sigma, MO, United States) after 21 d of osteogenic differentiation. Finally, chondrogenic differentiation was induced by culturing MSCs in DMEM, 15 mmol/L Hepes, supplemented with 6.25 μg/mL insulin, 5 μg/mL ascorbic acid 2-phosphate and 10 ng/mL TGF-β (Sigma, St. Louis, MO, United States). To verify the presence of proteoglycans, cells were fixed and stained with Alcian Blue (Vetec, Duque de Caxias, RJ, BRA) after 21 d of chondrogenic differentiation.
To characterize the cell population according to surface molecular markers, immunophenotyping was performed. Approximately 1 × 106 MSCs were placed in sterile tubes and washed twice by centrifugation at 2000 r/min for 5 min at room temperature (RT). MSCs were then resuspended in phosphate-buffered saline (PBS) and incubated for 30 min at RT with phycoerythrin (PE) conjugated antibodies against mouse CD34, CD11bc, CD44, and CD90 (Becton-Dickinson, Franklin Lakes, NJ, United States). All assays were conducted using antibody concentrations recommended by the manufacturers. Cells were collected and washed with PBS by centrifugation at 1500 r/min for 10 min at RT, and fluorescence analysis was carried out with the BD FACS-Calibur flow cytometer (Becton-Dickinson, Franklin Lakes, NJ, United States). Data were analyzed using Cellquest and PAINTA-GATE software.
Acute colitis was induced by oral administration of 2% dextran sulfate sodium (DSS; MP Biomedicals, Solon, OH, United States) from day 0 to day 7 in drinking water ad libitum. On days 2 and 5 of the protocol, MSCs (1 × 106 cells/120 μL PBS) were delivered via intraperitoneal (DSS-MSC IP) or intravenous (DSS-MSC IV) tail vein (n = 5/group) injection. The saline group (DSS-Saline) received PBS (120 μL) injected according to the same protocol (n = 5). Mice receiving pure water instead of DSS were used as controls (Naive). The disease activity index (DAI) score was determined by an investigator blinded to the protocol. Animals were observed daily for weight loss, stool consistency and presence of blood in the feces and anus. A score from 0 to 4 was assigned for each parameter, resulting in the total DAI score ranging from 0 (unaffected) to 12 (severe colitis) as per Gonçalves et al[21].
After 8 d of DSS administration, mice were euthanized by cervical dislocation of spine and colons were removed from the cecum to the anus. Samples were measured and weighed as an indirect assessment of inflammation.
Colons were fixed in 40 g/L paraformaldehyde, processed and embedded in paraffin to obtain longitudinal medial cuts. Colon sections (4 μm) were stained with hematoxylin-eosin (HE) and analyzed using a halogen light microscope. Histological score was blindly determined as per Dieleman et al[22]. Each parameter of the histological score, such as severity of inflammation (0-3), depth of inflammation (0-3), regeneration (0-4) and crypt damage (0-4), was multiplied by the percentage of compromised tissue (1 point for 25%, 2 points for 26%-50%, 3 points for 51%-75%, and 4 points for 76%-100%). Therefore, inflammation and extent have a range from 0 to 12, and regeneration and crypt damage have a range from 0 to 16.
Following isoflurane-induced anesthesia, blood samples were collected by retro-orbital puncture for serum separation. Samples were collected in blood collection tube containing coagulant and centrifuged at 7000 r/min for 20 min. After separation, serum was stored at -80 °C until cytokine determination. Cytokine levels in the serum were determined using a CBA Th1/Th2/Th17 kit from BD Pharmingen according to the manufacturer’s recommendations.
To determine apoptosis, fragmented DNA was stained by the terminal deoxynucleotidyl transferase (TdT)-mediated dUDP-biotin nick end labeling (TUNEL) assay using an in situ Cell Death Detection Kit (Roche, San Francisco, CA, United States). After deparaffinization, sections were incubated with 20 μg/mL proteinase K solution for 30 min at 37 °C. After rinsing, slides were incubated with a labeling reaction mix containing TdT enzyme for 1 h at 37 °C in a humidified atmosphere in the dark. Samples were analyzed with a fluorescence microscope using an excitation wavelength in the range of 450-500 nm and detection in the range of 515-565 nm (green). Nine microscopic fields were quantified in pixels at 200 × magnification. ImageJ software (National Institute of Health, Bethesda, MA, United States) was used for apoptosis analysis. Apoptotic T cells were detected by immunohistochemical staining using an anti-CD3 antibody (Cell Marque, CA, United States).
Results were shown as the mean ± SE for each group. Statistical analysis was performed using SPSS (Version 18.0) statistical software. Generalized Estimated Equations (GEE) was used for DAI and weight loss analysis. For multiple comparisons (colon weight and length, histological analysis, cytokine and apoptosis quantification), non parametric Kruskal-Wallis test was used. In cases displaying significant differences, post hoc analysis was performed with Bonferroni test. P < 0.05 was considered to be statistically significant.
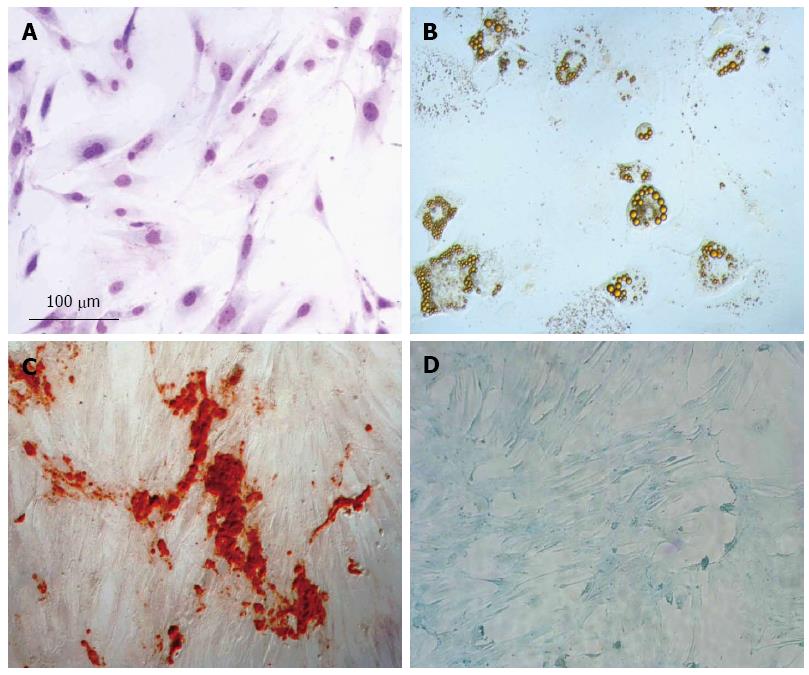
MSCs were obtained by plating out an adipose cell suspension in tissue culture dishes and propagating the resulting adherent cells. Between 5 to 7 d after initial plating, the isolated cells developed into visible systematic colonies of adherent fibroblast-like cells and, with further time in culture, became morphologically homogeneous due to the depletion of other stromal cells (Figure 1A). As demonstrated in Figure 1B, a clear potential for adipogenic differentiation was detected by Oil Red, which stains lipid vacuoles. Figure 1C shows osteogenic differentiation as detected by Alizarin Red, which stains calcium deposits. Chondrogenic differentiation was confirmed by Alcian Blue, which stains proteoglycans (Figure 1D). Using flow cytometry, we determined that the majority of cells preserved their characteristic CD44+, CD90+, CD11bc- and CD34- phenotypes and also confirmed that they retained their differentiation potential.
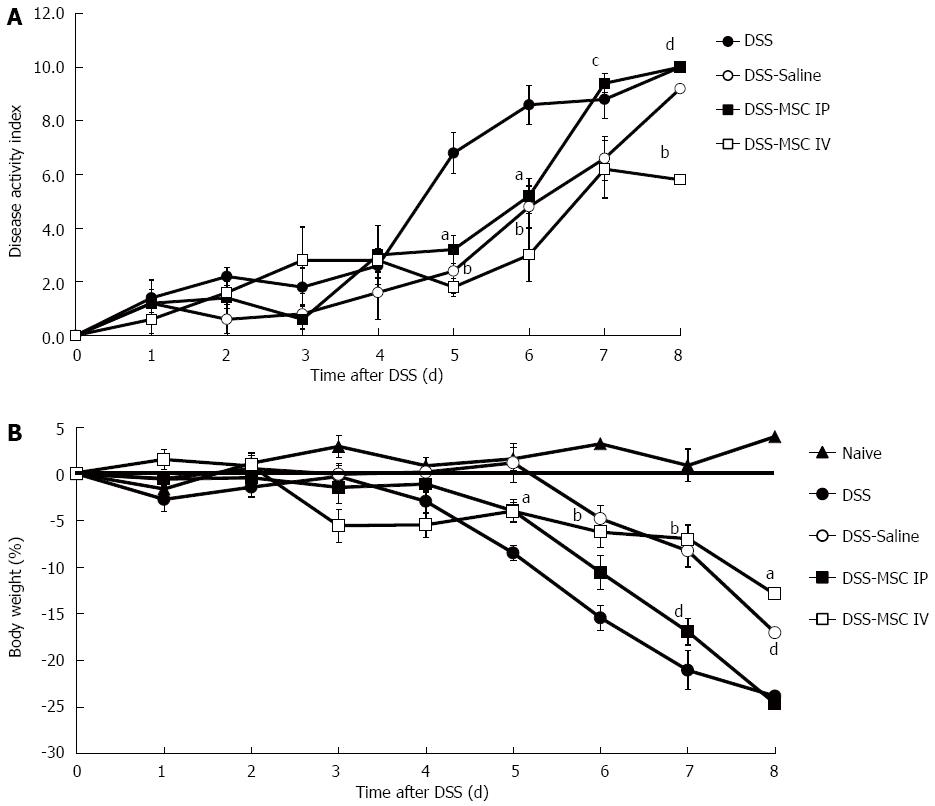
Mice exposed to oral administration of 2% DSS over 7 d presented a significant increase in disease activity index (DAI), which was characterized by acute colitis, bloody diarrhea and sustained weight loss. Transplantation of MSCs significantly reduced DAI scores on days 5 (1.80 ± 0.33 DSS-MSC IV and 3.20 ± 0.52 DSS-MSC IP vs 6.80 ± 0.76 DSS, P < 0.001 and P < 0.05, respectively) and 6 (3.00 ± 0.98 DSS-MSC IV and 5.20 ± 0.65 DSS-MSC IP vs 8.60 ± 0.72 DSS, P < 0.001 and P < 0.05, respectively). However, starting on day 7, the DSS-MSC IP group demonstrated a higher DAI value than the DSS-MSC IV group (9.40 ± 0.35 vs 6.20 ± 1.70, P < 0.05). On day 8, we observed clinical improvement in mice treated with MSCs via intravenous injection compared with both the group treated with MSCs via intraperitoneal injection (10.00 ± 0.00 vs 5.80 0.43, P < 0.001) and the untreated group (10.00 ± 0.00 vs 5.80 0.43, P < 0.001) (Figure 2A).
In the MSC-treated groups, weight loss was significantly decreased at day 5 compared to DSS group (-4.07 ± 1.03 g DSS-MSC IV and -3.98 ± 1.21 g DSS-MSC IP vs -8.4 ± 0.82 g DSS, P < 0.05). However, starting from day 6, only the DSS-MSC IV group was able to avoid excessive weight loss (-3.00 ± 0.98 g vs -8.60 ± 0.72 g, P < 0.001). From day 7 onward, a significant difference in weight loss was observed between the intraperitoneal and intravenous treatment groups (-9.40 ± 0.35 g vs -6.20 ± 1.07 g, P < 0.001) (Figure 2B).
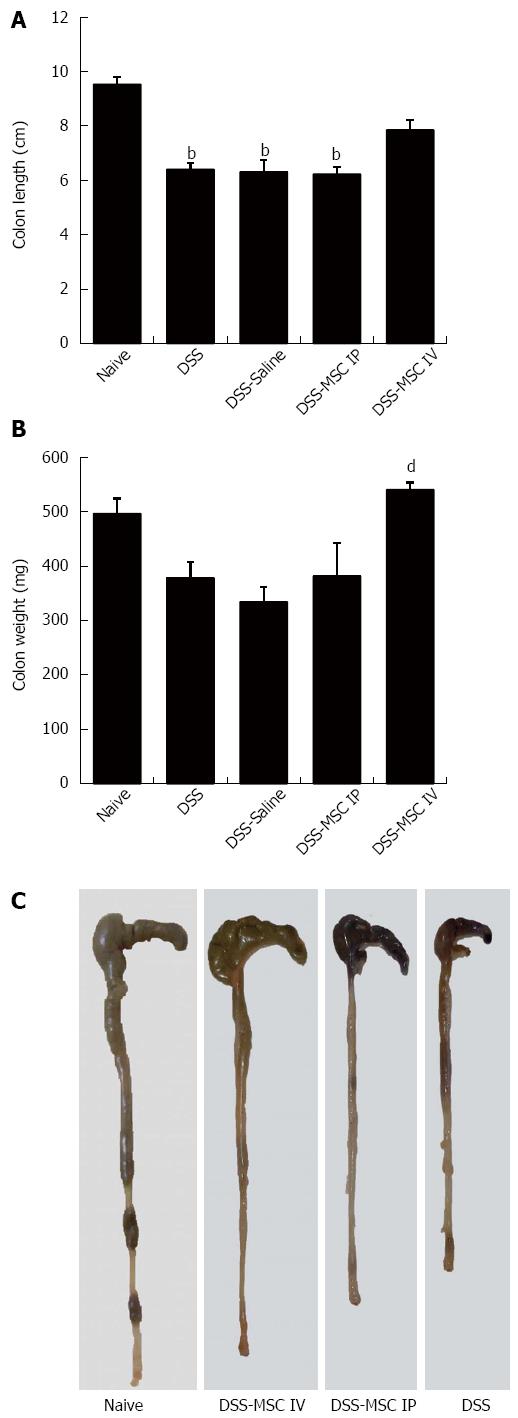
On day 8, macroscopic findings in colons from the DSS group included severe shortening of the colon and bloody stools compared to the Naive group (6.4 ± 0.54 cm vs 9.5 ± 0.66 cm, P < 0.001), symptoms that are indicative of UC. A significant difference in colon length was also observed between the DSS-MSC IP group and Naive group (6.22 ± 0.63 cm vs 9.5 ± 0.66 cm, P < 0.001) (Figure 3A and C). Mice from DSS-MSC IV group did not display significant macroscopic changes compared to the Naive group. However, significant differences in colon weight were noted when these mice were compared to untreated DSS mice (532 ± 32 mg vs 377 ± 67 mg, P = 0.009) (Figure 3B).
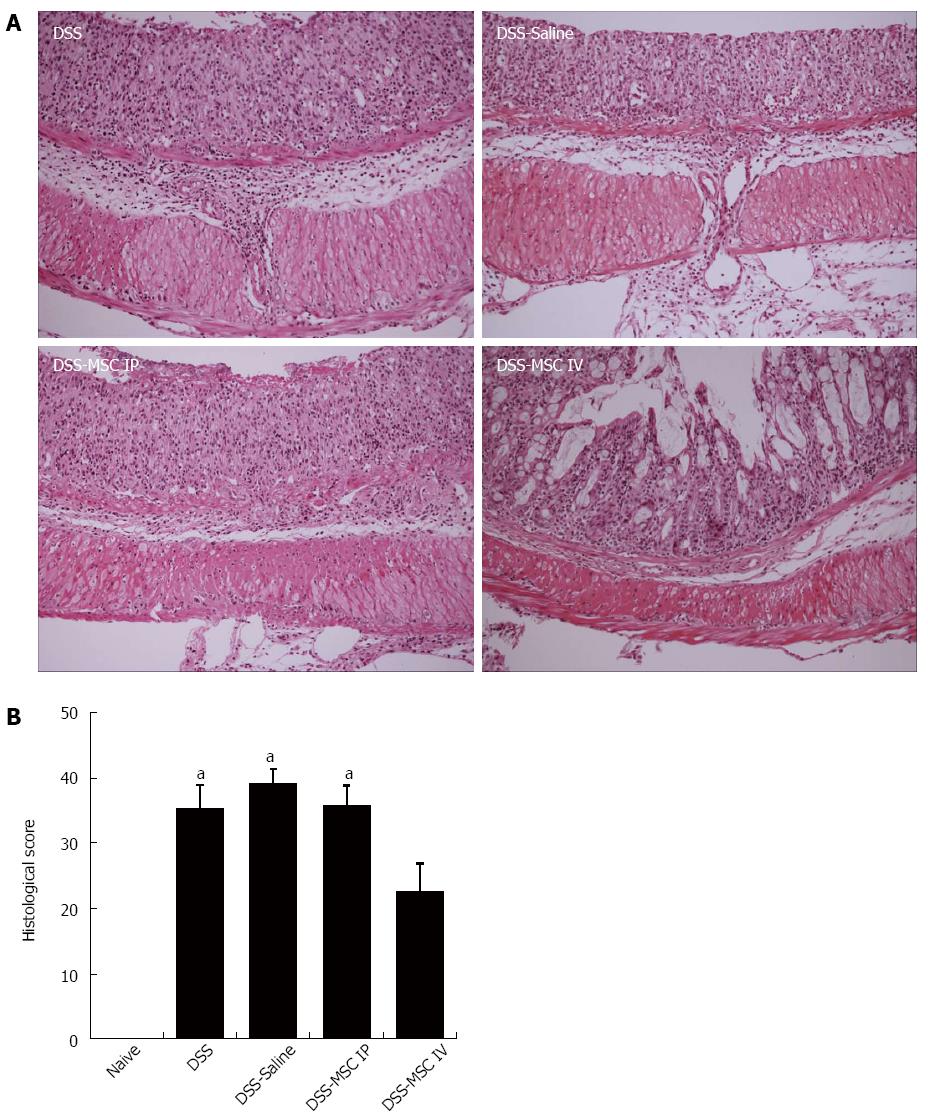
Intense colonic inflammation was observed in mucosa and submucosa stratum, with loss of goblet cells, crypt damage, and extensive mucosal ulceration. Moreover, areas of edema in the submucosa were observed in the untreated DSS, DSS-Saline and DSS-MSC IP groups when compared to the Naive group (P < 0.05). Inflammatory cell infiltration, including neutrophils and mononuclear cells, was also observed.
In contrast, the DSS-MSC IV group presented lower levels of inflammation, which was confined to the mucosal layer, and minimal ulceration when compared to the DSS-MSC IP group, demonstrating a strong tendency toward histological recovery (P = 0.051) that was consistent with the clinical score (Figure 4).
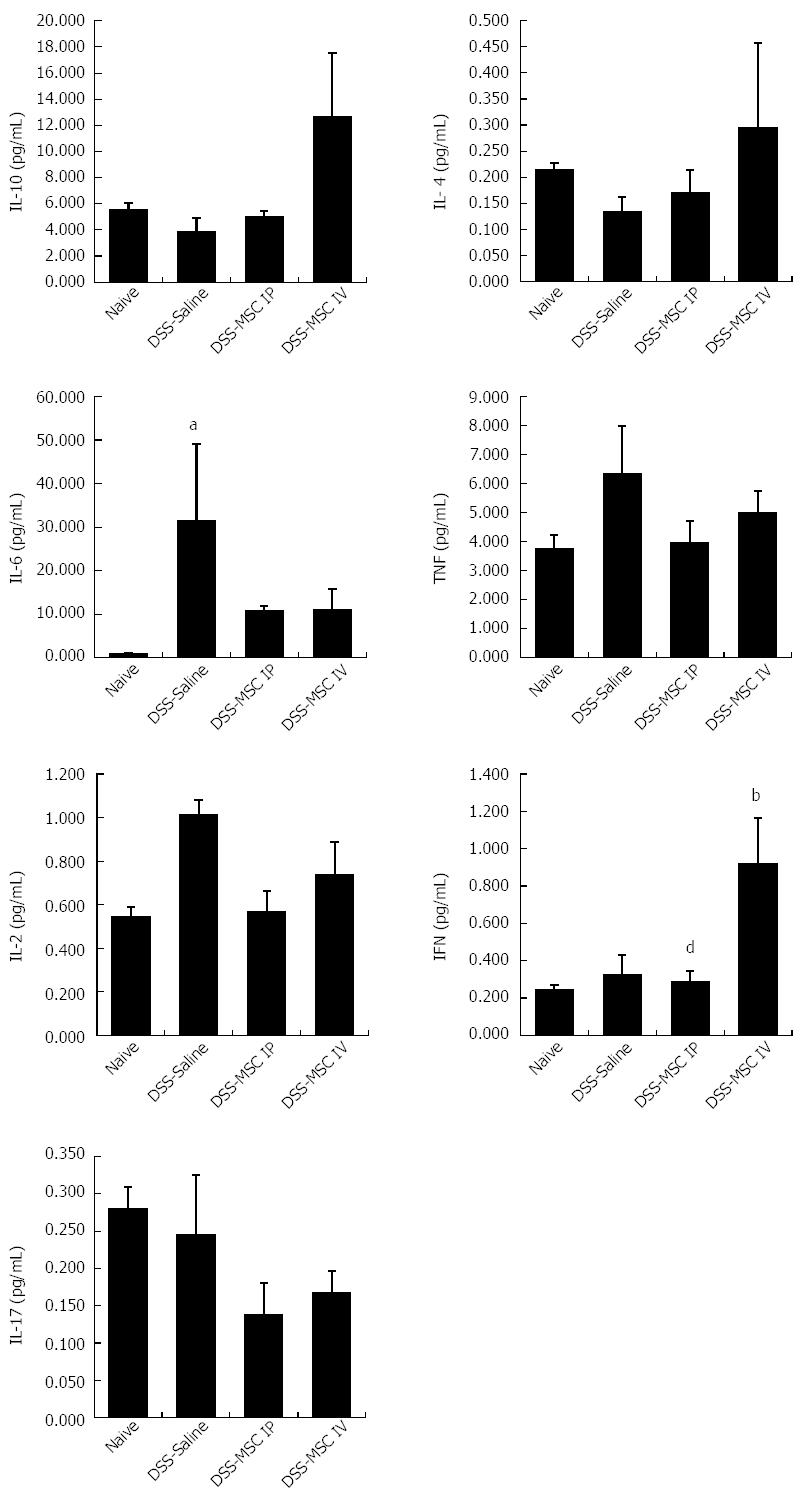
To analyze the effect of MSCs on the production of inflammatory mediators mechanistically involved in acute colitis, serum cytokine profiling was performed. In mice administered DSS, serum levels of the anti-inflammatory cytokines IL-10 and IL-4 did not differ statistically from the Naive group, even though the DSS-MSC IV group demonstrated higher levels of these cytokines (P > 0.05). The pro-inflammatory cytokines IL-6 and TNF play key roles in mediating acute inflammatory reactions. Acute DSS colitis associated with significantly elevated levels of IL-6 compared to the Naive group (P = 0.006). The MSC-treated group showed lower levels of IL-6 than the untreated DSS group, while TNF levels were higher in DSS group, although these changes were not significantly different (P > 0.05). The pro-inflammatory cytokines IL-2 and IFN are related to chronic inflammation. Therefore, as expected in a model of acute colitis, the levels of these cytokines were not altered when compared to the Naive group (P > 0.05) Nonetheless, IFN levels in the DSS-MSC IV group were significantly higher than in the Naive group, demonstrating that the presence of MSCs influences the levels of IFN (P = 0.006). Interestingly, there were differences between the DSS-MSC IP and DSS-MSC IV groups (P = 0.006), suggesting that the delivery route may have an effect IFN production. Levels of IL-17A, a pro-inflammatory cytokine, were similar between the untreated DSS group and the Naive group (P > 0.05), suggesting that colonic Th cells do not exhibit a Th17 profile in this acute DSS-colitis model (Figure 5).
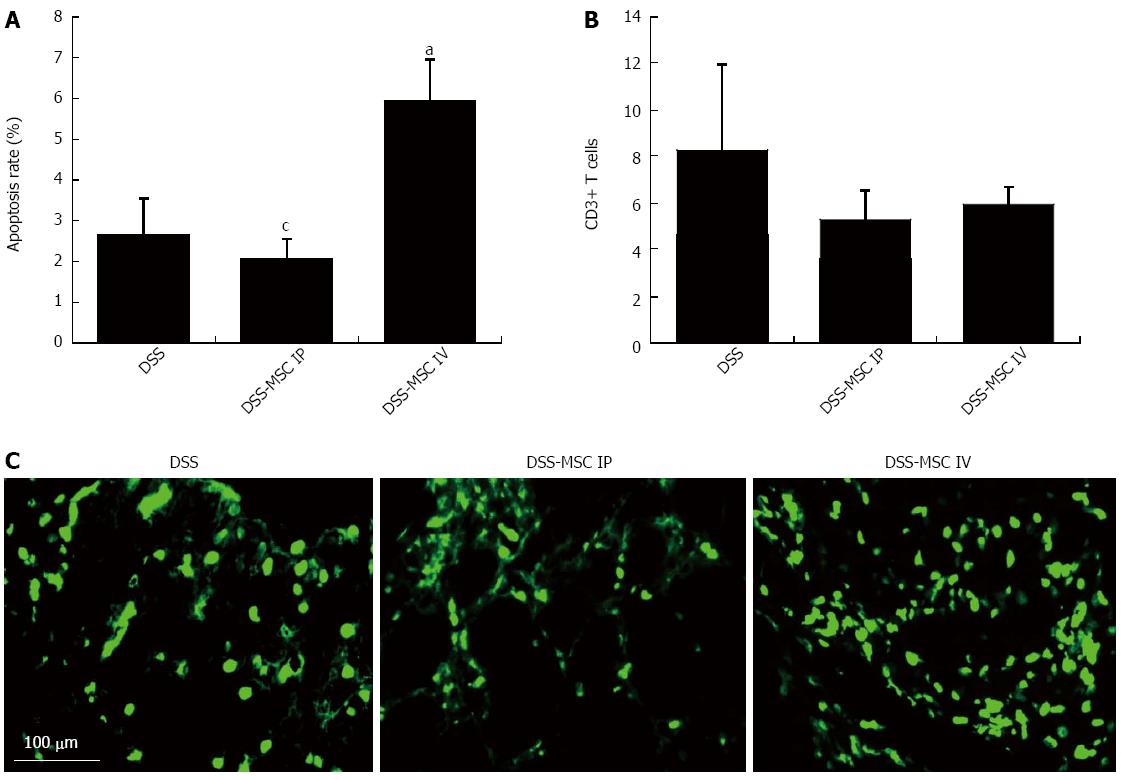
It is known that the resistance of T cells to apoptosis contributes to inappropriate T cell accumulation in UC and the perpetuation of chronic mucosal inflammation[6]. In our study, the frequency of TUNEL-positive mononuclear cells was significantly higher in mice from the DSS-MSC IV group compared to the untreated DSS group (5.92% ± 1.00% vs 2.66% ± 0.89%, P = 0.027). This significant difference was also observed between the DSS-MSC IP and DSS-MSC IV groups (2.08% ± 0.48% vs 5.92% ± 1.00%, P = 0.027) (Figure 6A). There were few apoptotic cells present in DSS and DSS-MSC IP groups. In the DSS-MSC IV group, these apoptotic cells were more prominent in the epithelium and lamina propria (Figure 6C). The presence of T cells was confirmed by CD3 staining, as demonstrated in Figure 6B. The MSCs-treated groups showed a lower number of CD3+ T cells, although this difference was not significant.
Previous studies have focused on the attractive regenerative properties of MSCs, and there is evidence indicating that MSCs can promote regeneration of injured tissue. After receiving appropriate biological signals during injury or tissue inflammation, MSCs can migrate to affected sites and assist in their recovery, having a high therapeutic potential in tissue repair and controlling in situ inflammation[15]. Mechanisms directing in vivo homing and engraftment of MSCs are not well described and depend on complex interactions between many signaling events. Thus, it is important to elucidate the best route of delivery for providing effective cell therapy to an inflamed colon. Our study tested two different routes of MSCs transplantation in a murine model of UC to evaluate their functionality and potential beneficial effects.
Several preclinical studies have transplanted MSCs by intravenous injection[7,23-27], while others have used an intraperitoneal injection route[3,5] as a treatment for DSS-induced colitis. Nevertheless, none of these studies have presented a comparative analysis of the best route of cell transplantation for the treatment of UC. Our results demonstrated that intravenous MSC treatment reduced diarrhea with blood, and improved stool consistency, body weight loss, and the wasting disease and colon inflammation. Moreover, the shorting of the overall colon length, which is usually observed in colitis, was not found in intravenous MSC-treated mice. Histological examination showed that intravenous MSC treatment resulted in minimal ulceration and lower levels of inflammation that was confined to the mucosal layer. However, intraperitoneal MSC treatment showed no improvement. Duijvestein et al[28] previously treated DSS-induced colitis in mice using IFN-γ pre-stimulated MSCs transplanted intraperitoneally. Their study demonstrated that mice treated with pre-stimulated MSCs displayed colitis recovery, while unstimulated MSCs showed no immunosuppressive effect. This is consistent with our data using unstimulated or naive MSCs.
The only experimental study that has used different routes of administration to evaluate the effects of MSC transplantation in IBD showed better results with intraperitoneal administration when compared to an intravenous route of delivery[29]. There are many differences between this study and our own. We used cultivated MSCs and not cryopreserved cells, and the DSS animal model used in our study presents a distinct cytokine and pathology profile from the TNBS model[30]. The MSC administration route and migration to the inflamed colon might vary based on the experimental model being used. For these reasons, our results contribute a different view regarding the importance of the delivery route for MSCs.
In respect of the anti-inflammatory action of exogenous MSCs, these cells are known to regulate the immune response in IBD pathogenesis. MSCs reduce colonic inflammation by downregulating the production of inflammatory mediators by mucosal immune cells, and by increasing the levels of the anti-inflammatory cytokine IL-10[5]. Cytokines produced by infiltrating cells and macrophages play a critical role in colonic tissue destruction. They modulate important biological cellular functions, and mediate immune cell proliferation and differentiation. In IBD, the immunologic response is reflected by the imbalance in T helper 1 (Th1) and T helper 2 (Th2) cells, and thus the cytokine production at different stages of disease. In this sense, UC has been primarily associated with a Th2 response[30,31]. However, studies have also indicated Th1 profiles in UC, as well as Th17 in the manifestation of chronic intestinal inflammation[32-34]. Our experimental model of DSS-induced colitis appears to have both a Th1 and Th2 response. In addition, we found that the levels of Th17 cytokines in serum from untreated DSS mice were similar to those in Naive mice. These results suggest that in an acute DSS-colitis model, colonic Th cells exhibit a Th1 and Th2 profile, but not a Th17 profile[35]. Intravenous treatment with MSCs increased the levels of the anti-inflammatory cytokines IL-10 and IL-4, and decreased the levels of the pro-inflammatory cytokine IL-6, although these differences were not significant. Sheng et al[36] demonstrated that high levels of IFN produced by T cells in contact with MSCs lead to activation of the immunosuppressive effect of MSC. Our results demonstrated that intravenous MSC treatment increased IFN serum levels, in contrast with the results from the intraperitoneal MSC-treated group. These results might potentially support the idea that an intravenous route enhances contact between MSCs and T cells, which creates a favorable environment for IFN production and, consequently, MSC activation. Thus, the intraperitoneal route may not provide for the activation of the immunosuppressive properties. Recent studies have shown that MSCs can function in either an anti-inflammatory or a pro-inflammatory role, depending on their interaction with other cell types and/or soluble factors[25,37].
In the present study, we analyzed the rate of apoptosis in the inflamed colon. It is known that there is a substantial reduction in T cell apoptosis in patients with IBD[6,38]. Increased IL-10 and/or blockage of IL-6 signaling can induce apoptosis in T cells[39,40]. Although the exact mechanisms underlying MSC-mediated suppression of lymphocyte proliferation remain essentially unknown, it is possible that MSCs can accelerate apoptosis of active inflammatory cells. Akiyama et al[13] showed that the systemic infusion of MSCs induced T cell apoptosis via the Fas ligand (FasL)-dependent Fas pathway, reducing symptoms of DSS-induced colitis. Our data are in agreement with these studies. We also showed that fewer apoptotic cells are present in the untreated DSS and intraperitoneal MSC-treated groups.
To our knowledge, this is the first study to evaluate different routes of MSC administration for the treatment of DSS-induced colitis in an experimental model. We found that intravenous delivery is the best route of MSC administration reducing signs of colon inflammation. This fact may be related to MSC migratory capacity, as well as the activation of the immunosuppressive properties of the transplanted MSCs and, consequently, their immunomodulatory and tissue repair activity. In conclusion, the successful treatment of experimental colitis through intravenous administration of MSCs supports the concept of using this route in cell therapy to treat intestinal inflammation.
The authors extend a special thanks to Dr. Mario Delgado for his guidance and instructions about animal models and the immunomodulatory potential of MSCs, as well as Dr. Flávia Carneiro for proofreading this manuscript.
Inflammatory bowel disease such as ulcerative colitis (UC) is a chronic disease characterized by severe T cell inflammation and immune disorder. After receiving appropriate biological signals during injury or tissue inflammation, mesenchymal stem cells (MSCs) can migrate to the affected site and modulate inflammatory responses and tissue regeneration. Currently, little is known regarding the optimal delivery strategy for MSCs to treat UC. Therefore, we report a comparison between intravenous and intraperitoneal routes of cell transplantation. Clarifying the best methodology for cell therapy may enhance the success of UC treatment.
Mounting evidence suggests that MSCs have properties including low immunogenicity, immunomodulatory activity and anti-inflammatory activity in experimental colitis. However, so far, no studies have shown which method of cell transplantation is the best to treat colon inflammation.
This study demonstrated that intravenous administration is the best route for cell therapy in UC, showing that this route was effective with regards to reducing colon inflammation.
The successful treatment of experimental colitis through intravenous administration of MSCs supports the concept of using this route in cell therapy to treat intestinal inflammation in the future research.
Dextran sulfate sodium (DSS): sulfated polysaccharide that induces acute or chronic colitis in experimental models, triggering specific inflammation in the colon and rectum.
This paper is well-written and experimental data is reliable. Although focus of this paper is limited, it is interesting to identify the best route for MSC therapy.
P- Reviewer: Nakase H, Perakath B S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat Rev Immunol. 2003;3:521-533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1309] [Cited by in RCA: 1348] [Article Influence: 61.3] [Reference Citation Analysis (0)] |
| 2. | Zhou Q, Price DD, Dreher KL, Pronold B, Callam CS, Sharma J, Verne GN. Localized colonic stem cell transplantation enhances tissue regeneration in murine colitis. J Cell Mol Med. 2012;16:1900-1915. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Anderson P, Souza-Moreira L, Morell M, Caro M, O’Valle F, Gonzalez-Rey E, Delgado M. Adipose-derived mesenchymal stromal cells induce immunomodulatory macrophages which protect from experimental colitis and sepsis. Gut. 2013;62:1131-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 155] [Cited by in RCA: 171] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 4. | Hisamatsu T, Mikami Y, Matsuoka K, Kanai T, Hibi T. Imunological Abnormalities in the Pathogenesis of Inflammatory Bowel Disease. Intes Res. 2011;10:317-323. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 5. | Gonzalez-Rey E, Anderson P, González MA, Rico L, Büscher D, Delgado M. Human adult stem cells derived from adipose tissue protect against experimental colitis and sepsis. Gut. 2009;58:929-939. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 497] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 6. | Mudter J, Neurath MF. Apoptosis of T cells and the control of inflammatory bowel disease: therapeutic implications. Gut. 2007;56:293-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 100] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 7. | Tanaka F, Tominaga K, Ochi M, Tanigawa T, Watanabe T, Fujiwara Y, Ohta K, Oshitani N, Higuchi K, Arakawa T. Exogenous administration of mesenchymal stem cells ameliorates dextran sulfate sodium-induced colitis via anti-inflammatory action in damaged tissue in rats. Life Sci. 2008;83:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 105] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 8. | Beyer Nardi N, da Silva Meirelles L. Mesenchymal stem cells: isolation, in vitro expansion and characterization. Handb Exp Pharmacol. 2006;249-282. [PubMed] |
| 9. | Bielby R, Jones E, McGonagle D. The role of mesenchymal stem cells in maintenance and repair of bone. Injury. 2007;38 Suppl 1:S26-S32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 157] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 10. | Caplan AI. Review: mesenchymal stem cells: cell-based reconstructive therapy in orthopedics. Tissue Eng. 2005;11:1198-1211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 602] [Cited by in RCA: 541] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 11. | Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006;177:2080-2087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 545] [Cited by in RCA: 524] [Article Influence: 27.6] [Reference Citation Analysis (0)] |
| 12. | Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105:1815-1822. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3271] [Cited by in RCA: 3279] [Article Influence: 156.1] [Reference Citation Analysis (0)] |
| 13. | Akiyama K, Chen C, Wang D, Xu X, Qu C, Yamaza T, Cai T, Chen W, Sun L, Shi S. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 570] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 14. | Ko IK, Kim BG, Awadallah A, Mikulan J, Lin P, Letterio JJ, Dennis JE. Targeting improves MSC treatment of inflammatory bowel disease. Mol Ther. 2010;18:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 145] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 15. | da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204-2213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1715] [Cited by in RCA: 1707] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 16. | Gebler A, Zabel O, Seliger B. The immunomodulatory capacity of mesenchymal stem cells. Trends Mol Med. 2012;18:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 242] [Cited by in RCA: 267] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 17. | Abdi R, Fiorina P, Adra CN, Atkinson M, Sayegh MH. Immunomodulation by mesenchymal stem cells: a potential therapeutic strategy for type 1 diabetes. Diabetes. 2008;57:1759-1767. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 374] [Cited by in RCA: 353] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 18. | Hao L, Sun H, Wang J, Wang T, Wang M, Zou Z. Mesenchymal stromal cells for cell therapy: besides supporting hematopoiesis. Int J Hematol. 2012;95:34-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Horwitz EM, Le Blanc K, Dominici M, Mueller I, Slaper-Cortenbach I, Marini FC, Deans RJ, Krause DS, Keating A. Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy. 2005;7:393-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1345] [Cited by in RCA: 1374] [Article Influence: 72.3] [Reference Citation Analysis (0)] |
| 20. | Gonçalves Fda C, Paz AH, Lora PS, Passos EP, Cirne-Lima EO. Dynamic culture improves MSC adhesion on freeze-dried bone as a scaffold for bone engineering. World J Stem Cells. 2012;4:9-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 21. | Gonçalves FC, Schneider N, Mello HF, Passos EP, Meurer L, Cirne-Lima EO, Paz AH. Characterization of acute murine dextran sodium sulfate (DSS) colitis: severity of inflammation is dependent on the DSS molecular weight and concentration. Acta Sci Vet. 2013;41:1142. |
| 22. | Dieleman LA, Palmen MJ, Akol H, Bloemena E, Peña AS, Meuwissen SG, Van Rees EP. Chronic experimental colitis induced by dextran sulphate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immunol. 1998;114:385-391. [PubMed] |
| 23. | He XW, He XS, Lian L, Wu XJ, Lan P. Systemic infusion of bone marrow-derived mesenchymal stem cells for treatment of experimental colitis in mice. Dig Dis Sci. 2012;57:3136-3144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 75] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 24. | Yabana T, Arimura Y, Tanaka H, Goto A, Hosokawa M, Nagaishi K, Yamashita K, Yamamoto H, Adachi Y, Sasaki Y. Enhancing epithelial engraftment of rat mesenchymal stem cells restores epithelial barrier integrity. J Pathol. 2009;218:350-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 76] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 25. | Fan H, Zhao G, Liu L, Liu F, Gong W, Liu X, Yang L, Wang J, Hou Y. Pre-treatment with IL-1β enhances the efficacy of MSC transplantation in DSS-induced colitis. Cell Mol Immunol. 2012;9:473-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 178] [Article Influence: 13.7] [Reference Citation Analysis (1)] |
| 26. | Xu X, Chen C, Akiyama K, Chai Y, Le AD, Wang Z, Shi S. Gingivae contain neural-crest- and mesoderm-derived mesenchymal stem cells. J Dent Res. 2013;92:825-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 27. | Nemoto Y, Kanai T, Takahara M, Oshima S, Nakamura T, Okamoto R, Tsuchiya K, Watanabe M. Bone marrow-mesenchymal stem cells are a major source of interleukin-7 and sustain colitis by forming the niche for colitogenic CD4 memory T cells. Gut. 2013;62:1142-1152. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 28. | Duijvestein M, Wildenberg ME, Welling MM, Hennink S, Molendijk I, van Zuylen VL, Bosse T, Vos AC, de Jonge-Muller ES, Roelofs H. Pretreatment with interferon-γ enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells. 2011;29:1549-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 29. | Castelo-Branco MT, Soares ID, Lopes DV, Buongusto F, Martinusso CA, do Rosario A, Souza SA, Gutfilen B, Fonseca LM, Elia C. Intraperitoneal but not intravenous cryopreserved mesenchymal stromal cells home to the inflamed colon and ameliorate experimental colitis. PLoS One. 2012;7:e33360. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 30. | Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341-352. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 616] [Cited by in RCA: 623] [Article Influence: 38.9] [Reference Citation Analysis (0)] |
| 31. | Bamias G, Kaltsa G, Ladas SD. Cytokines in the pathogenesis of ulcerative colitis. Discov Med. 2011;11:459-467. [PubMed] |
| 32. | Feng T, Qin H, Wang L, Benveniste EN, Elson CO, Cong Y. Th17 cells induce colitis and promote Th1 cell responses through IL-17 induction of innate IL-12 and IL-23 production. J Immunol. 2011;186:6313-6318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 161] [Cited by in RCA: 154] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 33. | Fuss IJ. Is the Th1/Th2 paradigm of immune regulation applicable to IBD? Inflamm Bowel Dis. 2008;14 Suppl 2:S110-S112. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 34. | Yen D, Cheung J, Scheerens H, Poulet F, McClanahan T, McKenzie B, Kleinschek MA, Owyang A, Mattson J, Blumenschein W. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J Clin Invest. 2006;116:1310-1316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1153] [Cited by in RCA: 1235] [Article Influence: 65.0] [Reference Citation Analysis (0)] |
| 35. | Kim YS, Lee MH, Ju AS, Rhee KJ. Th17 responses are not induced in dextran sodium sulfate model of acute colitis. Immune Netw. 2011;11:416-419. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 36. | Sheng H, Wang Y, Jin Y, Zhang Q, Zhang Y, Wang L, Shen B, Yin S, Liu W, Cui L. A critical role of IFNgamma in priming MSC-mediated suppression of T cell proliferation through up-regulation of B7-H1. Cell Res. 2008;18:846-857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 274] [Cited by in RCA: 303] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 37. | Danese S, Rutella S, Vetrano S. Mesenchymal stromal cells in inflammatory bowel disease: conspirators within the ‘colitogenic niche’? Gut. 2013;62:1098-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 38. | Bu P, Keshavarzian A, Stone DD, Liu J, Le PT, Fisher S, Qiao L. Apoptosis: one of the mechanisms that maintains unresponsiveness of the intestinal mucosal immune system. J Immunol. 2001;166:6399-6403. [PubMed] |
| 39. | Atreya R, Mudter J, Finotto S, Müllberg J, Jostock T, Wirtz S, Schütz M, Bartsch B, Holtmann M, Becker C. Blockade of interleukin 6 trans signaling suppresses T-cell resistance against apoptosis in chronic intestinal inflammation: evidence in crohn disease and experimental colitis in vivo. Nat Med. 2000;6:583-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 945] [Cited by in RCA: 1028] [Article Influence: 41.1] [Reference Citation Analysis (0)] |
| 40. | Bailey DP, Kashyap M, Bouton LA, Murray PJ, Ryan JJ. Interleukin-10 induces apoptosis in developing mast cells and macrophages. J Leukoc Biol. 2006;80:581-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |