Published online Dec 28, 2014. doi: 10.3748/wjg.v20.i48.18207
Revised: June 30, 2014
Accepted: September 5, 2014
Published online: December 28, 2014
Processing time: 245 Days and 10.5 Hours
AIM: To investigate whether targeting proteasome might reverse intestinal fibrosis in rats.
METHODS: Chronic colitis was induced in rats by repeated administration of increasing dose of 2,4,6-trinitrobenzene sulfonic acid (TNBS, 15, 30, 45, 60, 60, 60 mg) by rectal injection for 6 wk (from day 0 to day 35), while control rats received the vehicle. TNBS + bortezomib (BTZ) rats received intraperitoneal injections of BTZ twice weekly (from day 37 to day 44) at a dose of 25 mg/kg, whereas the control and TNBS groups received the same amount of the vehicle. Histologic scoring of inflammation and fibrosis was performed. Colonic production of transforming growth factor (TGF)-β was measured by ELISA. Colon fibrosis-related proteins such as phospho-p38, phospho-SMAD2/3, Akt and peroxisome proliferator activated receptor γ (PPARγ) were studied by western blot. Expression of the tight junction proteins, occludin and claudin-1, were assessed by Western blot. Colon proteasome activities (chymotrypsin-like and trypsin-like activities) were assessed.
RESULTS: TNBS-treated rats had a higher colon weight/length ratio compared to control rats (P < 0.01). Furthermore, fibrosis and inflammation scores were higher in TNBS-treated rats compared to control rats (P < 0.01 for both). Colonic production of TGF-β production tended to be higher in TNBS-treated rats (P < 0.06). Fibrosis-related proteins such as phospho-p38, phospho-SMAD2/3, and PPARγ were significantly higher in TNBS-treated rats compared to control rats (all P < 0.05). TNBS rats had a higher expression of Akt compared to control rats (P < 0.01). Tight junction proteins were modified by repeated TNBS challenge: colon occludin expression rose significantly (P < 0.01), whereas claudin-1 expression fell (P < 0.01). Bortezomib inhibition significantly decreased chymotrypsin-like activity (P < 0.05), but had no significant effect on trypsin-like activity (P > 0.05). In contrast, bortezomib had no effect on other studied parameters such as fibrosis score, TGF-β signaling, or tight junction expression (P > 0.05 for all).
CONCLUSION: Rats with TNBS-induced chronic colitis exhibited colon fibrosis associated with higher TGF-β signaling. Proteasome inhibition by bortezomib had no effect on fibrosis in our experimental conditions.
Core tip: Inflammatory bowel disease gives rise to challenging clinical conditions, and many patients have to undergo surgery throughout their life due to irreversible lesions. As no specific treatment is currently available for intestinal fibrosis, we tested an antifibrotic drug that is effective in other chronic inflammatory diseases. In the present study, we found that rats with 2,4,6-trinitrobenzene sulfonic acid-induced chronic colitis exhibited colon fibrosis associated with high expression of transforming growth factor-β signaling, particularly in the Akt pathway. Nevertheless, we failed to inhibit colon fibrosis by proteasome inhibition in our experimental conditions.
- Citation: Loeuillard E, Bertrand J, Herranen A, Melchior C, Guérin C, Coëffier M, Aziz M, Déchelotte P, Savoye G, Marion-Letellier R. 2,4,6-trinitrobenzene sulfonic acid-induced chronic colitis with fibrosis and modulation of TGF-β1 signaling. World J Gastroenterol 2014; 20(48): 18207-18215
- URL: https://www.wjgnet.com/1007-9327/full/v20/i48/18207.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i48.18207
Intestinal fibrosis is a common consequence of inflammatory bowel diseases, giving rise to severe clinical complications such as strictures and intestinal obstructions, and ultimately leading to surgery. Chronic inflammation is also able to activate wound-healing responses including increased extracellular matrix or mesenchymal cell activation that can promote intestinal fibrosis. Crohn’s disease (CD) is the primary example of the fibrosis process. Indeed, CD is characterized by chronic transmural inflammation from the mucosal to the deeper layers of the gut where mesenchymal cells are mainly located. While therapeutic management of such inflammation has progressed markedly, specific treatment for intestinal fibrosis is still unavailable, and surgery is often the only remaining option. Thus, there is a great need for specific medical therapy aimed at preventing or reversing intestinal fibrosis. Antifibrotic drugs that are effective in other chronic inflammatory diseases may be good candidate molecules.
Targeting the proteasome is one potential antifibrotic strategy that might help to reverse fibrosis. The proteasome activates transcription factor nuclear factor (NF)-κB by degrading the inhibitory protein IκB, with consequent induction of genes encoding adhesion molecules and cytokines. Bortezomib (BTZ), which is an inhibitor of proteasomes, has demonstrated antifibrotic properties in skin and lung fibrosis models[1]. The nuclear receptor peroxisome proliferator activated receptor (PPAR)γ has recently appeared as a molecule that is involved in fibrosis and also in proteasomal inhibition by BTZ in lung and skin fibrosis.
Animal models have been developed to better understand the mechanisms underlying intestinal fibrosis and to test therapeutic interventions. Intrarectal administration of the hapten reagent 2,4,6-trinitrobenzene sulfonic acid (TNBS) in ethanol is widely used to investigate acute intestinal inflammation[2-5] and induces CD-like features such as transmural inflammation. Stidham et al[6] developed a chronic model of intestinal inflammation-associated fibrosis by weekly administration of TNBS with ethanol for 6 wk.
We aimed to investigate the signaling pathways involved in a chronic-intestinal inflammation-associated fibrosis model in rats and to test the potential antifibrotic effect of proteasome inhibition by BTZ.
Animal care and experimentation complied with both French regulations and European Community regulations (Official Journal of the European Community L 358, 18/12/1986). In addition, RML and MC are authorized by the French government to use this rat model (authorization: n = 76-106, n = 76-107). Sprague-Dawley male rats (Janvier, Le Genest St Isle, France) weighing 150 g were randomized into three groups: control, control colitic (TNBS), and BTZ-treated colitic rats (TNBS + BTZ). Rats were weighed three times a week. Water and food were provided ad libitum.
Rats were food-deprived for 24 h prior to induction of colitis and were allowed free access to tap water throughout the study. Chronic colitis was induced by weekly administration of increasing concentrations of TNBS (15, 30, 45, 60, 60 and 60 mg; Sigma Aldrich, St. Louis, MO, United States) over 6 wk as previously described by Stidham et al[6]. Colitis was induced in two groups at day 0 by intrarectal injection of TNBS, whereas the control group received the vehicle (0.25 mL of 50% ethanol). Briefly, rats were anesthetized with an intraperitoneal injection of ketamine and chlorpromazine following 24-h food deprivation. TNBS dissolved in 50% (v/v) ethanol was instilled into the colon through a cannula (0.25 mL) to induce chronic colitis. After instillation of TNBS, the rats were then maintained in a head-down position for a few minutes to prevent leakage of the intracolonic instillate. Control groups received the same volume of the vehicle.
TNBS + BTZ rats received intraperitoneal injections of BTZ (Velcade, S1013; Selleck Chemicals, Houston, TX, United States) twice weekly at a dose of 25 mg/kg, whereas the control and TNBS groups received the same amount of the vehicle.
PBS and phosphatase and protease inhibitor cocktails were purchased from Sigma. Bis-Tris gels (4%-12% NuPAGE), Invitrolon, PVDF membranes, and Seeblue multi-colored standard were obtained from Invitrogen (of Thermo Fisher Scientific, Waltham, MA, United States), Hybond membranes were purchased from GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom). The following antibodies were purchased from Santa Cruz Biotechnology (Dallas, TX, United States): anti-PPARγ (sc-7273), anti-Akt (sc-81434), anti-p38 (sc-535), anti-p-p38 (sc-7975-R), anti-p70 ribosomal protein S6 kinase (p70S6K; sc-8418), anti-p-Smad2/3 (sc-11769), anti-cyclooxygenase (COX)-2 (sc-1747), anti-4EBP1 and secondary IgG1 horseradish antibodies. Anti-extracellular regulated kinase (ERK)1/2 (#4665), anti-p-Akt (#5473), and anti-p-ERK1/2 (#9106) were obtained from Cell Signaling Technology (Danvers, MA, United States); anti-occludin (711500) and anti-claudin-1 (374900) were obtained from Life Technologies of Thermo Fisher Scientific and Anti alpha-smooth muscle actin [SMA was obtained by Abcam. The proteasome inhibitor for proteolytic pathway activities MG 132 (Z-Leu-Leu-Leu-al; c2211) and the substrate for trypsin (Boc-Gln-Ala-Arg-7-amido-4-methylcoumarin hydrochloride; B4153) were obtained from Sigma-Aldrich. Chymotrypsin Substrate III, Fluorogenic (539142) was obtained from Calbiochem of Merck KGaA (Darmstadt, Germany).
Frozen colon samples were homogenized in PBS with 1% protease inhibitor cocktail and 1% phosphatase inhibitor cocktail. Homogenates were centrifuged (12000 ×g, 15 min, 4 °C) and the supernatants were collected. Protein concentration was determined using Bradford’s colorimetric method. Aliquots of supernatants containing equal amounts of protein (40 μg) were separated by SDS-PAGE and then transferred to a PVDF or Hybond membrane. After blocking, membranes were incubated with specific primary antibodies at dilutions of 1:250 (for COX-2), 1:500 (for Akt, ERK1/2, p-ERK1/2, p38 and p-p38), 1:1000 (for PPARγ, p-Smad2/3 and P70S6K), and 1:2000 (for α-smooth muscle actin [SMA]). After three washes, the filter was then incubated with secondary horseradish peroxidase linked IgG secondary antibodies. To check equal loading, the blots were analyzed for β-actin expression. Immunodetection was performed using enhanced chemiluminiscence light-detecting kit (Amersham of GE Healthcare). Densitometric data were measured following normalization to the control (housekeeping gene) using an ImageScanner II densitometer and ImageQuant TL analysis software (GE Healthcare).
Immunoprecipitation was performed with 10-μm-cutoff ultrafiltration spin-columns (Pierce of Thermo Fisher Scientific) and 50 μL (50% slurry) protein G-agarose beads (Calbiochem). First, total protein samples were incubated overnight with polyclonal rabbit anti-eIF4E (Santa Cruz Biotechnology). Then, samples were incubated overnight with beads at 4 °C in a tube rotator. Beads were washed two times with 600 μL of ice-cold PBS. Proteins of interest were finally eluted two times with 50 μL of 5 mol/L urea solution. Eluted samples were loaded on SDS-PAGE gels as previously described[7]. Proteins were transferred onto membranes and soaked in TBS-T/bovine serum albumin (BSA; 5% w:v) solution for 1 h at room temperature. Then, blots were incubated overnight at 4 °C in TBS-T/BSA with anti-eIF4E binding protein 1 (4EBP1) antibody (1:500). Membranes were washed three times for 10 min with TBS-T, incubated with horseradish peroxidase conjugated swine anti-rabbit IgG (1:5000; Dako of Agilent Technologies, Glostrup, Denmartk) in TBS-T/BSA for 1 h at room temperature, and then washed three times in TBS-T. Immunocomplexes were detected and analyzed as described above for Western blotting.
Proteolytic pathway activities such as trypsin-like and chymotrypsin-like activities were evaluated as previously described[4]. Briefly, evaluation of their activities was performed by spectrofluorimetry on a microtiter plate fluorometer (Mithras LB 940; Berthold Technologies, Bad Wildbad, Germany) using fluorogenic proteasome substrate in the presence or absence of specific proteasome inhibitors.
Proteins were extracted from frozen colon samples as described above. Samples were then analyzed using the DuoSet ELISA Development System (MB100B; R&D Systems Inc., Minneapolis, MN, United States). Their concentrations were determined by spectrophotometry at wavelength 450 nm with spectrophotometer (Σ960; Metertech Inc., Taipei, Taiwan).
Intestinal tissues were fixed in 4% formaldehyde, embedded in paraffin wax blocks, and 5 μm sections were stained with hematoxylin-eosin-safran to estimate collagen content. Sections were scored by the same pathologist and samples were blinded. Epithelial necrosis, inflammatory cell infiltration and thickness of the mucosa were assessed using semi-quantitative scores that ranged from 0 to 3 for each variable (0, no inflammation; 1, very low level of inflammation; 2, moderate level of leukocyte infiltration; 3, high levels of leukocyte infiltration and vascular density, ulcerations) using Leica QWin analysis software (Leica Microsystems, Bensheim, Germany).
Statistical comparisons were performed using GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA, United States). Data are expressed as mean ± SE. Body weight changes and food intake were analyzed by two-way analysis of variance for repeated measures with Turkey’s post hoc tests. All other variables were analyzed by one-way analyses of variance with Bonferroni’s post hoc test or Kruskal-Wallis test as appropriate. Differences were considered significant at P < 0.05.
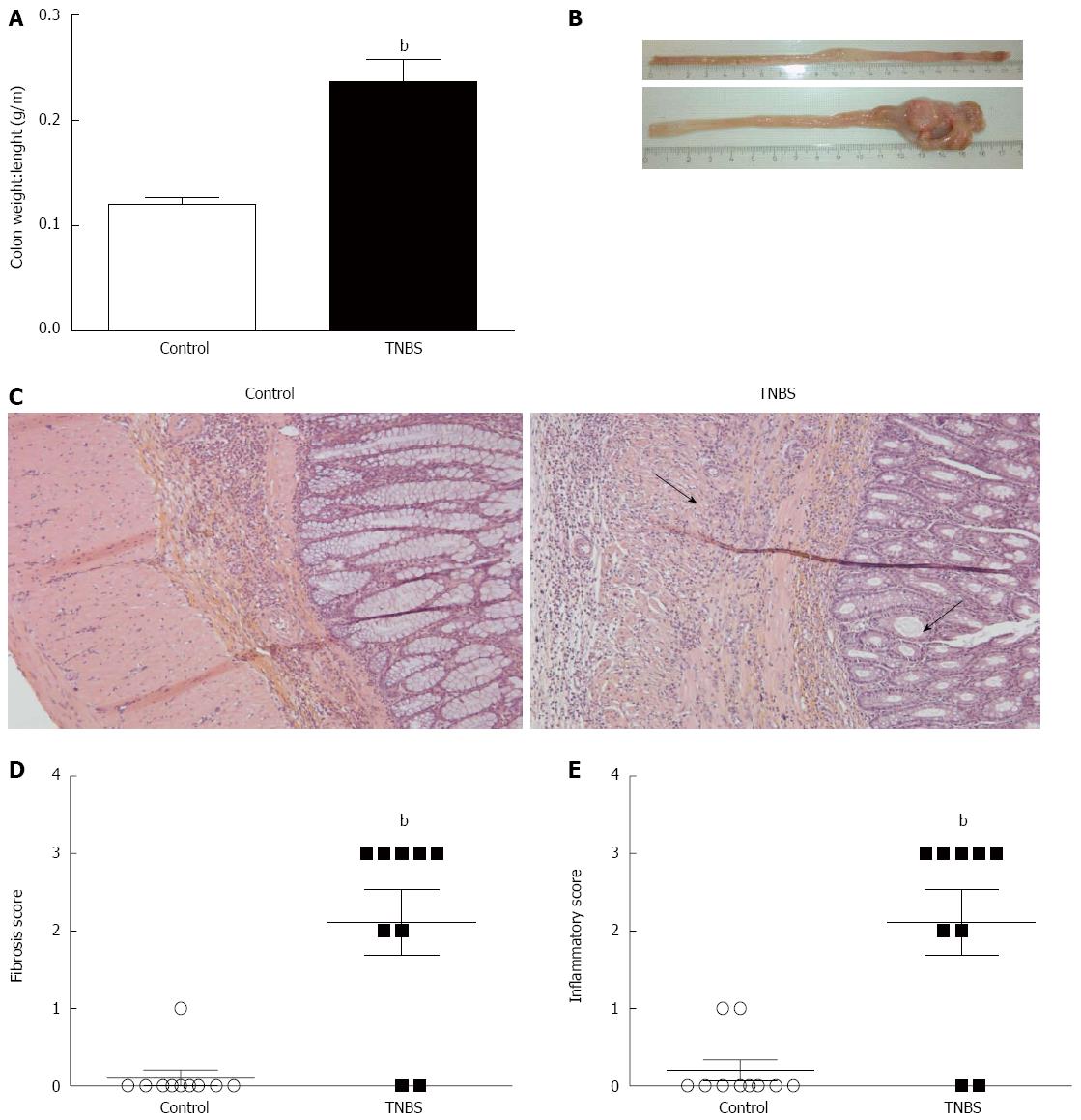
Colon weight/length ratio was significantly higher in TNBS rats compared to control rats (P < 0.05) (Figure 1A). The TNBS group had distal thickening and rigidity of the colon (Figure 1B). Histologic analysis of tissue sections from TNBS rats revealed inflammatory infiltrate, thickening of the mucosa and submucosa, and a higher proportion of collagen deposition compared to the control group (Figure 1C). TNBS rats had a significantly higher fibrosis (P < 0.01) (Figure 1D) and inflammatory (P < 0.01) Figure 1E) scores.
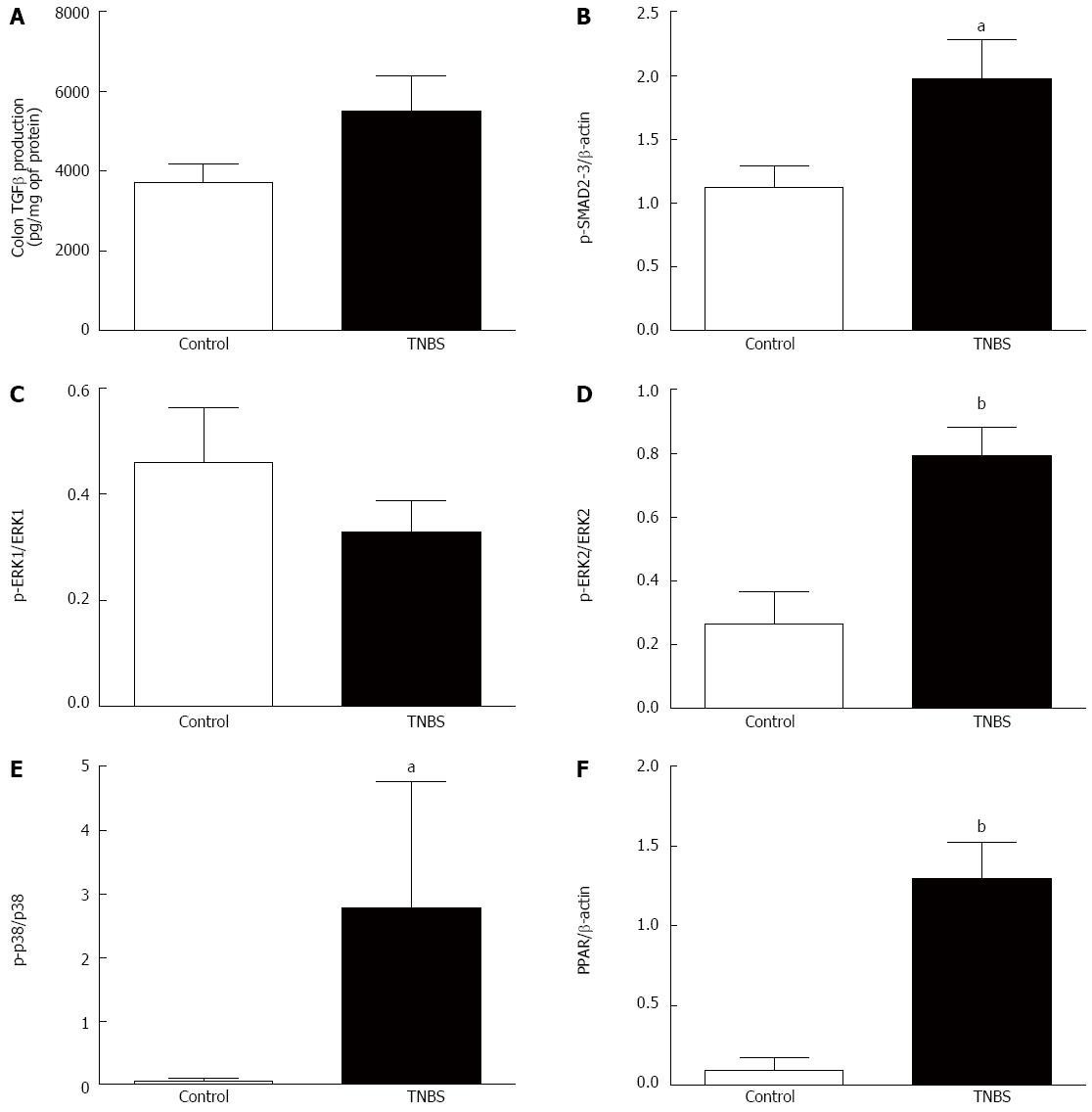
TGF-β is a key cytokine in fibrosis processes and TNBS rats tended to have a higher production of colonic TGF-β compared to control rats (P = 0.06) (Figure 2A). TGF-β receptors interact with Smad signaling pathways, and phospho-Smad2/3 expression was higher in TNBS rats compared to control rats (P < 0.05) (Figure 2B). Non-Smad signaling pathways, such as the ERK-MAPK pathway, were also involved in the TGF-β response. While no significant difference in phospho-ERK1/ERK1 was found among the groups (Figure 2C), TNBS rats had increased expression of phospho-ERK2/ERK2 (P < 0.01) (Figure 2D) and phospho-p38/p38 (P < 0.05) (Figure 2E). PPARγ is a nuclear receptor and has recently been identified as a molecule involved in fibrosis. TNBS rats had a higher colonic expression of PPARγ compared to control rats (P < 0.01) (Figure 2F).
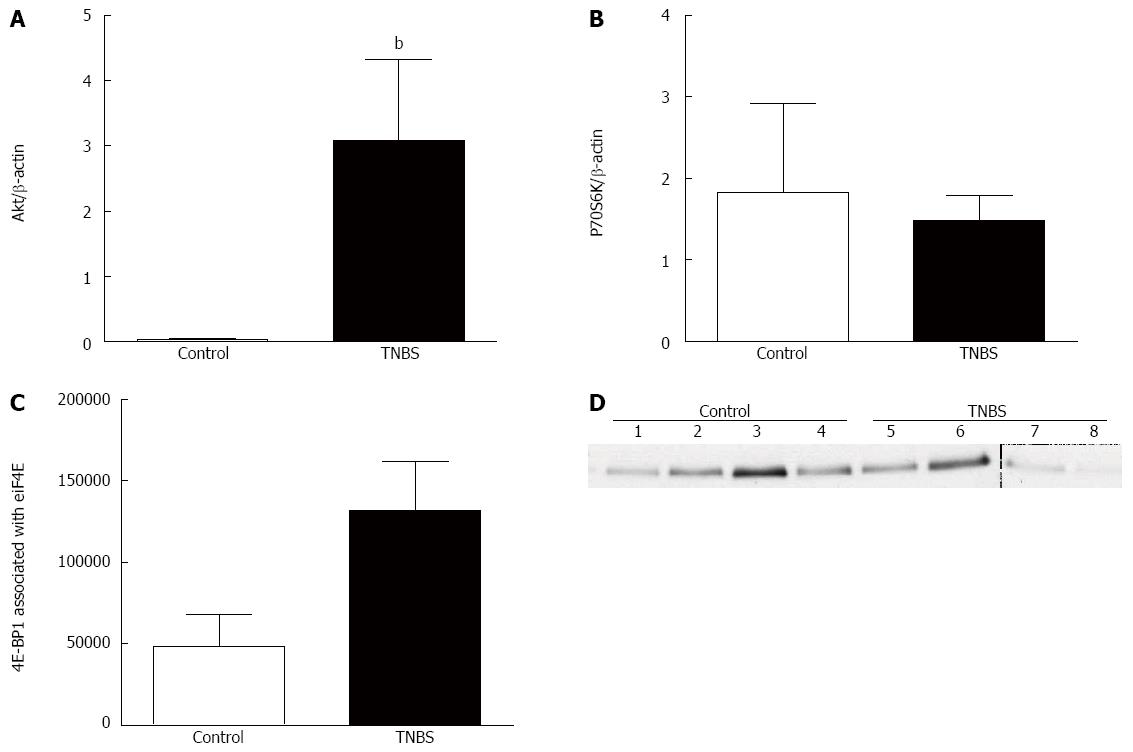
Other TGF-β-induced non-Smad signaling pathways include the Akt-mTOR pathway. TNBS rats had a higher expression of Akt compared to control rats (P < 0.01 (Figure 3A). However, there was no significant change in colonic P70S6K expression among the groups (Figure 3B), though there was an enhanced association of 4EBP1 with eIF4E in rats with chronic TNBS compared to controls (P < 0.05) (Figure 3C, D).
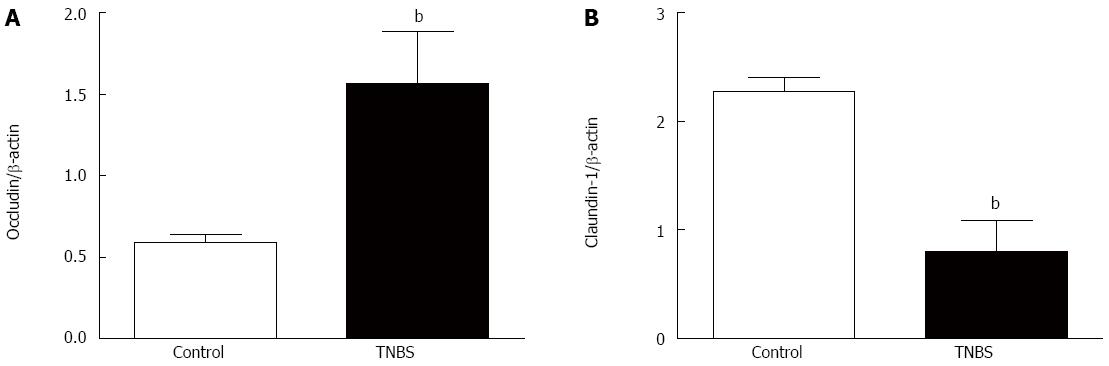
Chronic administration of TNBS significantly increased occludin expression (P < 0.01) (Figure 4A) and decreased claudin-1 expression (P < 0.01) (Figure 4B).
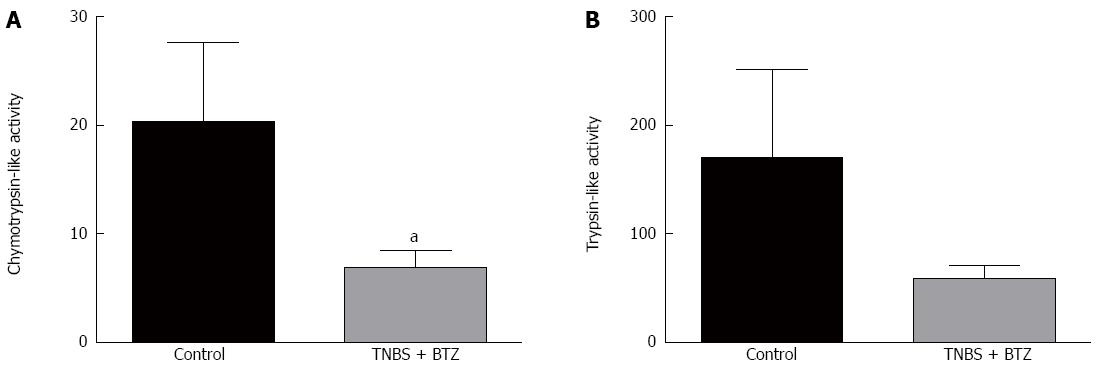
BTZ inhibition of proteasomes significantly decreased chymotrypsin-like activity (P < 0.05) (Figure 5A), but had no significant effect on trypsin-like activity (Figure 5B). BTZ also had no effect on other studied parameters such as fibrosis score, TGF-β signaling, or tight junction expression.
We aimed to characterize a model of chronic colitis that reflects human CD. By administration of weekly doses of intrarectal TNBS, rats developed increasing fibrosis of the colonic lamina propria. Although repeated administration of TNBS has been widely evaluated in mice, resistance to mouse strain-dependent fibrosis has led to unacceptably high mortality in mice[8,9]. In contrast, rats are more susceptible to fibrosis, and we found that 7/9 rats showed fibrosis with chronic TNBS-induced colitis. A rat model with chronic TNBS has already been used to investigate the feasibility of ultrasound elasticity imaging to evaluate intestinal fibrosis[6]. An appropriate animal model of intestinal fibrosis will add to understanding of the fibrosis process. The present study investigated signaling involved in rats with chronic TNBS and tested the potential of targeting the proteasome as a therapeutic intervention.
TGF-β is known to play a key role in intestinal fibrosis by inducing collagen production in fibroblasts and intestinal smooth-muscle cells. In an acute model of TNBS colitis, Medina et al[10] observed a significant increase in colonic TGF-β production. In the present study, TNBS rats had a higher level of colonic TGF-β production, but did not reach statistical significance. The mechanisms behind TGF-β effects have not yet been elucidated and are difficult to study in humans. TGF-β signaling involves the transduction of signals to the nucleus through the phosphorylation of Smad2 and Smad3 proteins. We found that expression of these proteins was increased in rats with chronic TNBS. As non-Smad signaling is also involved in the TGF-β response, we also studied the expression of Akt/mTOR signaling. mTOR is a key regulator of protein synthesis, and plays important roles in other biologic processes, such as cell growth, angiogenesis and autophagy. mTOR exists in two functionally distinct complexes: mTORC1 and mTORC2. mTORC1 activates P70S6K1 and inactivates 4E-BP1, which promotes protein translation and cell growth as well as autophagy and fibrosis. mTOR regulation is best achieved through activation of the PI3K/Akt pathway, but mTOR receives input from multiple signaling pathways. In the present study, we found that chronic TNBS upregulates Akt expression without affecting P70S6K1. As we observed an enhanced association between 4E-BP1 and eIF4E, mTORC1 does not seem to be involved in the fibrosis process in the present model.
We studied expression of PPARγ, as this nuclear receptor has recently emerged as a molecule involved in fibrosis. PPARγ can be activated by nutrients[11] and regulates intestinal inflammation. In addition, proteasomal inhibition by BTZ prevents lung and skin fibrosis after injury, in part by increasing the abundance and activity of PPARγ[1].
Occludin and claudin-1 are major tight junction proteins of gut epithelial cells. In the present study, we observed increased expression of occludin with concomitant altered expression of claudin-1. A similar alteration in junctional proteins, and reduced expression of claudin-1 with increased occludin, has been observed in eosinophilic esophagitis[12], a disease leading to sub-epithelial fibrosis. Eosinophils are a source of TGF-β in fibrosis processes[13]. To induce a pro-fibrotic phenotype, fibroblasts are exposed to TGF-β, and it has been demonstrated that TGF-β-treated fibroblasts exhibit a higher expression of occludin[14]. An identical tight junction pattern has been observed in intestinal epithelial cell line Caco-2 monolayers upon tumor necrosis factor α stimulation: down-regulation of claudin-1 with increased occludin expression[15].
Although we had assumed that proteasome inhibition might hold promise for intestinal fibrosis, no therapeutic effect of BTZ was observed in the present study. Indeed, BTZ inhibits chymotrypsin-like activity without affecting fibrosis. As intestinal inflammation drives intestinal fibrosis, we chose to test the therapeutic effect of BTZ in preexisting tissue fibrosis. As proteasome inhibition by BTZ attenuates experimental colitis[16,17], we speculated that testing the preventive effect of BTZ might result in inhibition of colitis and subsequently partial development of fibrosis in an animal model. Analyzing fibrosis in experimental models is particularly challenging because distinguishing altered inflammation from direct antifibrotic effects is tricky[18]. In murine TNBS colitis, treatment with NF-κB antisense downregulated fibrosis parameters[8], but it has also been reported as an anti-inflammatory agent in colitis[19]. It is thus difficult to determine whether antifibrotic effects are independent of the anti-inflammatory properties of tested molecules. A recent model of murine Salmonella typhimurium-induced intestinal fibrosis showed that early elimination of inflammation with levofloxacin led to eradication of infection with concomitant reduced intestinal fibrosis[20].
Although BTZ prevents development of dermal fibrosis, it had no therapeutic effect once fibrogenesis was set in motion, and was unable to reverse the process[21].
In summary, repeated injections of TNBS can induce chronic colitis with fibrosis in rats. We observed an upregulation of fibrosis score with increased fibrosis-related protein expression that characterized a reliable model of intestinal fibrosis. However, our attempt to target the proteasome to inhibit or reverse the fibrosis process by BTZ administration failed. Identification of relevant therapeutic targets remains crucial in this field of intestinal fibrosis where treatment needs remain unmet.
Intestinal fibrosis is a common consequence of inflammatory bowel diseases, giving rise to severe clinical complications. Chronic inflammation is also able to activate wound-healing responses including increased extracellular matrix or mesenchymal cell activation that can promote intestinal fibrosis. While management of inflammation has progressed markedly, specific treatment for intestinal fibrosis is still unavailable and surgery is often the only remaining option. Thus, there is a great need for specific medical therapy aimed at preventing or reversing intestinal fibrosis. Antifibrotic drugs that are effective for other chronic inflammatory diseases may be good candidate molecules.
Animal models have been developed to better understand the mechanisms underlying intestinal fibrosis and to test therapeutic interventions. Intrarectal administration of the hapten reagent 2,4,6-trinitrobenzene sulfonic acid in ethanol is widely used to investigate acute intestinal inflammation and exhibits Crohn’s disease-like features such as transmural inflammation.
This paper investigates the signaling pathways involved in a chronic intestinal inflammation-associated fibrosis model in rats and tests the potential antifibrotic effect of proteasome inhibition by bortezomib.
Targeting the proteasome is one potential antifibrotic strategy that might help to reverse fibrosis.
The proteasome activates transcription factor nuclear factor-κB by degrading the inhibitory protein IκB, with consequent induction of genes encoding adhesion molecules and cytokines.
This manuscript provides an interesting view for the subset of patients with Crohn’s disease characterized by fibrotic changes. This is an essential topic as the authors continue to try to understand this disease and target therapeutic interventions. Any therapeutic intervention that moderates or reverses fibrosis will be a major step forward in those disease management. Your project does a very nice job simulating fibrosis and characterizing the basic science pathways involved in the fibrotic process.
P- Reviewer: Feuerstadt P S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Zhang DN
| 1. | Mutlu GM, Budinger GR, Wu M, Lam AP, Zirk A, Rivera S, Urich D, Chiarella SE, Go LH, Ghosh AK. Proteasomal inhibition after injury prevents fibrosis by modulating TGF-β(1) signalling. Thorax. 2012;67:139-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 2. | Andoh A, Tsujikawa T, Ishizuka I, Araki Y, Sasaki M, Koyama S, Fujiyama Y. N-3 fatty acid-rich diet prevents early response of interleukin-6 elevation in trinitrobenzene sulfonic acid-induced enteritis. Int J Mol Med. 2003;12:721-725. [PubMed] |
| 3. | Charpentier C, Marion-Letellier R, Savoye G, Nicol L, Mulder P, Aziz M, Vera P, Déchelotte P, Savoye-Collet C. Magnetic resonance colonography in rats with TNBS-induced colitis: a feasibility and validation study. Inflamm Bowel Dis. 2012;18:1940-1949. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Hassan A, Ibrahim A, Mbodji K, Coëffier M, Ziegler F, Bounoure F, Chardigny JM, Skiba M, Savoye G, Déchelotte P. An α-linolenic acid-rich formula reduces oxidative stress and inflammation by regulating NF-κB in rats with TNBS-induced colitis. J Nutr. 2010;140:1714-1721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 5. | Mbodji K, Charpentier C, Guérin C, Querec C, Bole-Feysot C, Aziz M, Savoye G, Déchelotte P, Marion-Letellier R. Adjunct therapy of n-3 fatty acids to 5-ASA ameliorates inflammatory score and decreases NF-κB in rats with TNBS-induced colitis. J Nutr Biochem. 2013;24:700-705. [PubMed] |
| 6. | Stidham RW, Xu J, Johnson LA, Kim K, Moons DS, McKenna BJ, Rubin JM, Higgins PD. Ultrasound elasticity imaging for detecting intestinal fibrosis and inflammation in rats and humans with Crohn’s disease. Gastroenterology. 2011;141:819-826.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 129] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 7. | Bertrand J, Goichon A, Chan P, Azhar S, Lecleire S, Donnadieu N, Vaudry D, Cailleux AF, Déchelotte P, Coëffier M. Enteral glutamine infusion modulates ubiquitination of heat shock proteins, Grp-75 and Apg-2, in the human duodenal mucosa. Amino Acids. 2014;46:1059-1067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Lawrance IC, Wu F, Leite AZ, Willis J, West GA, Fiocchi C, Chakravarti S. A murine model of chronic inflammation-induced intestinal fibrosis down-regulated by antisense NF-kappa B. Gastroenterology. 2003;125:1750-1761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 187] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 9. | Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol. 2007;13:5581-5593. [PubMed] |
| 10. | Medina C, Santos-Martinez MJ, Santana A, Paz-Cabrera MC, Johnston MJ, Mourelle M, Salas A, Guarner F. Transforming growth factor-beta type 1 receptor (ALK5) and Smad proteins mediate TIMP-1 and collagen synthesis in experimental intestinal fibrosis. J Pathol. 2011;224:461-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Marion-Letellier R, Déchelotte P, Iacucci M, Ghosh S. Dietary modulation of peroxisome proliferator-activated receptor gamma. Gut. 2009;58:586-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 12. | Abdulnour-Nakhoul SM, Al-Tawil Y, Gyftopoulos AA, Brown KL, Hansen M, Butcher KF, Eidelwein AP, Noel RA, Rabon E, Posta A. Alterations in junctional proteins, inflammatory mediators and extracellular matrix molecules in eosinophilic esophagitis. Clin Immunol. 2013;148:265-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Wight TN, Potter-Perigo S. The extracellular matrix: an active or passive player in fibrosis? Am J Physiol Gastrointest Liver Physiol. 2011;301:G950-G955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 226] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 14. | Karagiannis GS, Schaeffer DF, Cho CK, Musrap N, Saraon P, Batruch I, Grin A, Mitrovic B, Kirsch R, Riddell RH. Collective migration of cancer-associated fibroblasts is enhanced by overexpression of tight junction-associated proteins claudin-11 and occludin. Mol Oncol. 2014;8:178-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 15. | Fischer A, Gluth M, Pape UF, Wiedenmann B, Theuring F, Baumgart DC. Adalimumab prevents barrier dysfunction and antagonizes distinct effects of TNF-α on tight junction proteins and signaling pathways in intestinal epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2013;304:G970-G979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Schmidt N, Gonzalez E, Visekruna A, Kühl AA, Loddenkemper C, Mollenkopf H, Kaufmann SH, Steinhoff U, Joeris T. Targeting the proteasome: partial inhibition of the proteasome by bortezomib or deletion of the immunosubunit LMP7 attenuates experimental colitis. Gut. 2010;59:896-906. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 144] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Yanaba K, Asano Y, Tada Y, Sugaya M, Kadono T, Sato S. Proteasome inhibitor bortezomib ameliorates intestinal injury in mice. PLoS One. 2012;7:e34587. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Macdonald TT. A mouse model of intestinal fibrosis? Gastroenterology. 2003;125:1889-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Neurath MF, Pettersson S, Meyer zum Büschenfelde KH, Strober W. Local administration of antisense phosphorothioate oligonucleotides to the p65 subunit of NF-kappa B abrogates established experimental colitis in mice. Nat Med. 1996;2:998-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 618] [Cited by in RCA: 631] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 20. | Johnson LA, Luke A, Sauder K, Moons DS, Horowitz JC, Higgins PD. Intestinal fibrosis is reduced by early elimination of inflammation in a mouse model of IBD: impact of a “Top-Down” approach to intestinal fibrosis in mice. Inflamm Bowel Dis. 2012;18:460-471. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 21. | Koca SS, Ozgen M, Dagli F, Tuzcu M, Ozercan IH, Sahin K, Isik A. Proteasome inhibition prevents development of experimental dermal fibrosis. Inflammation. 2012;35:810-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |