Published online Dec 21, 2014. doi: 10.3748/wjg.v20.i47.18048
Revised: October 7, 2014
Accepted: November 18, 2014
Published online: December 21, 2014
Processing time: 117 Days and 7.2 Hours
Large cell neuroendocrine carcinoma (LCNEC) in the biliary system is a poorly differentiated, high-grade neuroendocrine tumor. These tumors exhibit aggressive behavior and an increased tendency for early nodal and distant metastases. Herein, we report an unusual case of a pure primary LCNEC of the common bile duct (CBD). A 75-year-old female presented with nausea and jaundice. The patient underwent a CBD excision with lymph node dissection. Upon histological and immunohistochemical examination, the tumor exhibited pure large cell-type neuroendocrine features. Metastases were noted in two of the eight lymph nodes. The patient was administered adjuvant chemotherapy. The patient’s cancer recurred 7 mo after surgery, and the patient died from liver failure 5 mo after recurrence. The prognosis of LCNEC of CBD remains poor despite curative resection and adjuvant chemotherapy. The role of additional therapies, such as multimodal treatment including radiation therapy, must be further studied to improve the prognoses of patients.
Core tip: Primary neuroendocrine carcinomas of the common bile duct rarely occur; among these tumors, large cell neuroendocrine carcinoma is extremely rare. Large cell neuroendocrine carcinoma (LCNEC) is a high-grade malignant neuroendocrine neoplasm. This case report represents a rare case of a primary neuroendocrine carcinoma of the common bile duct. More clinicopathological data and further studies with multimodal treatment are required to identify the prognostic indicators and histogenesis of LCNEC of common bile duct.
- Citation: Park SB, Moon SB, Ryu YJ, Hong J, Kim YH, Chae GB, Hong SK. Primary large cell neuroendocrine carcinoma in the common bile duct: First Asian case report. World J Gastroenterol 2014; 20(47): 18048-18052
- URL: https://www.wjgnet.com/1007-9327/full/v20/i47/18048.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i47.18048
Although neuroendocrine neoplasms are frequently observed in the gastrointestinal tract, primary neuroendocrine carcinoma (NEC) of the common bile duct (CBD) rarely occurs. Among these tumors, large cell NEC (LCNEC) is extremely rare. LCNEC is a high-grade malignant neuroendocrine neoplasm. LCNEC of the CBD was first described by Sato et al[1], and only two additional cases of LCNEC of the CBD have been reported to date[2,3]. The pathogeneses of NEC of the CBD remains unclear. The diagnosis of CBD LCNEC is rarely made preoperatively because this tumor generally appears with nonspecific symptoms. Furthermore, the preoperative differentiation between cholangiocarcinoma and LCNEC is not possible using current imaging techniques. Histopathologically, reported LCNECs have been noted as pure forms or combined with adenocarcinoma or other tumors[2]. Given the invasive nature and aggressive biology of the tumor, the prognosis of patients with LCNEC of the CBD is poor[1]. Herein, we present a rare case of LCNEC of the CBD.
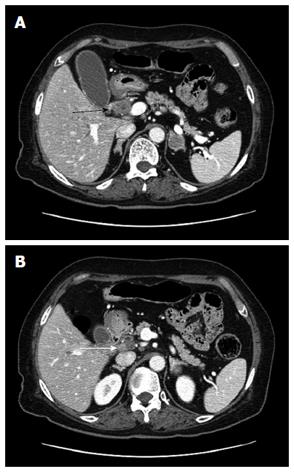
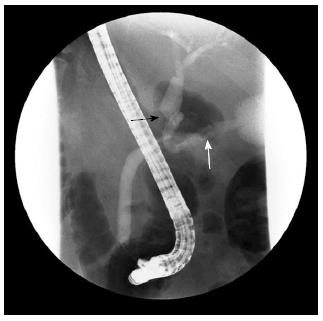
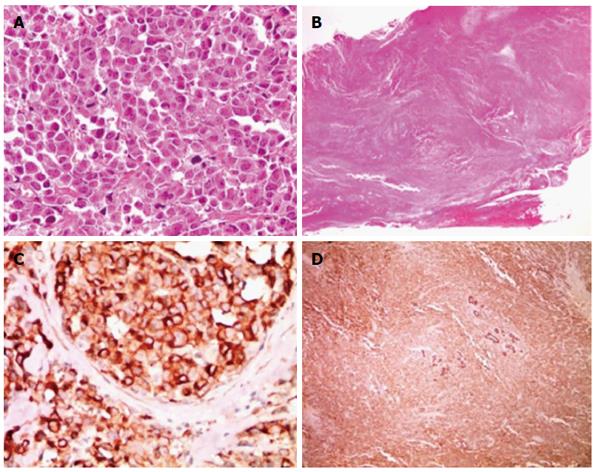
A 75-year-old female presented to our hospital with a 5-d history of nausea and jaundice. A physical examination revealed an icteric sclera, and the patient had neither abdominal tenderness nor a palpable mass in the right upper quadrant of the abdomen. She had hypertension and diabetes mellitus, and both were well controlled with medication. The patient had no family history of cancer. On the date of admission, the complete blood count and serum biochemical parameters were as follows: white blood cells, 10500 × 103/μL (3.8-10.0 × 103/μL); total bilirubin, 6.4 mg/dL (0.3-1.2 mg/dL); direct bilirubin, 5.6 mg/dL (0.1-0.5 mg/dL); aspartate aminotransaminase, 151 U/L (normal 15-41 U/L); alanine aminophosphatase, 302 U/L (14-54 U/L); aspartate transferase, 240 U/L (38-126 U/L); and gamma-glutamyltranspeptidase, 939 U/L (7-50 U/L). Carbohydrate antigen 19-9 (CA19-9) was 118.1 U/mL (0-37 U/mL). Carcinoembryonic antigen and alpha fetoprotein levels were within normal limits. The chest X-ray was within normal limits. An abdominal computed tomography (CT) scan revealed a mass that was approximately 2.7 cm in size located in the mid common bile duct and a regional metastatic node in the hepatoduodenal ligament (Figure 1). The endoscopic retrograde cholangiopancreatography demonstrated an asymmetric filling defect in the mid common bile duct (Figure 2). The cystic duct was well visualized. After the endoscopic sphincterotomy, an endoscopic nasobiliary drainage catheter was inserted. The patient underwent an operation with the presumed diagnosis of CBD cancer. The tumor of the bile duct was resected via cholecystectomy. The patient exhibited enlarged lymph nodes in the hepatoduodenal ligament and porta hepatis, which were also resected. No evidence of distant metastasis and organ invasion was noted. The resection margin of the proximal and distal bile duct frozen biopsy was tumor negative. Reconstruction was performed with a Roux-en Y hepaticojejunostomy. On histopathological examination, the tumor exhibited high cellularity and invasion throughout the entire CBD wall without serosal penetration. The tumor cells are monotonous, with relatively abundant cytoplasm and hyperchromatic nuclei that exhibited course chromatin and prominent nucleoli (Figure 3A, B). Immunohistochemical findings indicated that tumor cells are immunopositive for neuroendocrine markers, including synaptophysin and chromogranin. No evidence of adenocarcinoma or other tumor components were noted (Figure 3C, D). Metastases were noted in two of eight lymph nodes. No postoperative complications occurred, and the patient was discharged. The patient was administered adjuvant chemotherapy consisting of 5-fluorouracil, epirubicin, and cisplatin. CT scans 7 mo after surgery indicated recurrence at the hepaticojejunostomy site as well as in liver and portocaval area. The patient died of liver failure five mo after recurrence.
Carcinoma of the CBD accounts for less than 2% of all cancers, and the lesions identified in the CBD are predominantly cholangiocarcinomas (80%)[4]. The bile ducts is one of the rarest primary sites for neuroendocrine tumors (NETs), accounting for 0.2% to 2.0% of all such tumors[4]. Based on the WHO classification, neuroendocrine neoplasms are classified into five general categories, including NET, NEC, mixed adenoneuroendocrine carcinoma (MANEC), goblet cell carcinoid, and tubular carcinoid. In addition, NECs are classified as either LCNEC or small cell neuroendocrine carcinoma (SCNEC)[5]. Neuroendocrine neoplasms of the CBD are rare; carcinoid tumors represent the majority of these lesions.
A primary NEC of the CBD is extremely rare because normal CBD mucosa does not contain neuroendocrine cells. The origin of NEC of the CBD remains unclear. Neuroendocrine cells can be detected at sites of intestinal metaplasia induced by chronic inflammation, including long-standing chronic inflammation due to cholelithiasis and congenital anomalies, which may be the initial step in the development of neuroendocrine tumors of the CBD[6].
The symptoms of LCNEC of the CBD are non-specific. The preoperative diagnosis of LCNEC is difficult because it is not possible to differentiate LCNEC from an adenocarcinoma of the CBD using imaging studies, such as ultrasonography, CT, and abdominal angiography. Tumor markers, such as alpha fetoprotein, carcinoembryonic antigen, CA19-9 and CA125, are also non-specific.
LCNEC is a rare type of CBD, and to our knowledge, only three previous cases have been described in the English-language literature (Table 1)[1-3]. Thus, this is the fourth reported case of LCNEC occurring in the extra bile duct. The cases described by Sato et al[1] and Demoreuil et al[2] were MANECs (adenocarcinoma and LCNEC). Therefore, strictly speaking, our case is the second to report a pure LCNEC arising in the extra bile duct followed; the report of Sasatomi et al[3] was the first to report this type of lesion. Our study was the first report of an LCNEC in the CBD in an Asian individual.
| Ref. | Sex/age (yr) | Tumor histology | Location size (cm) | Treatment | Survival duration (post OP) |
| Sato et al[1] | M/68 | LCNEC + AD | D 2 | Resection and chemotherapy | DD 3 mo |
| Demoreuil et al[2] | M/73 | LCNEC + AD | P 3 | Resection and chemotherapy | DD 12 mo |
| Sasatomi et al[3] | M/76 | LCNEC | P 5 | Resection | DD 21 d |
| Present report | F/75 | LCNEC | M 3 | Resection and chemotherapy | DD 12 mo |
More specific staining markers are required to accurately diagnose NECs of the CBD (i.e., immunohistochemistry). The diagnosis of NEC should be confirmed by positive immunohistochemical staining for at least one neuroendocrine marker, such as chromogranin, synaptophysin, or neuron-specific enolase[7]. Microscopically, NET is defined as a well-differentiated neuroendocrine neoplasm with mild to moderate nuclear atypia and a low proliferation fraction (≤ 20 mitoses per 10 high-power fields or ≤ 20% Ki-67 index). Unlike NET, LCNEC is a poorly differentiated, high-grade malignant neuroendocrine neoplasm that is composed of large cells with marked nuclear atypia and a high proliferation fraction (> 20 mitoses per 10 high-power fields or > 20% Ki-67 index)[7].
Therapeutic interventions have not been well defined, and no optimal postoperative adjuvant therapy, such as chemotherapy and radiation therapy, is available due to the rarity of LCNEC of the CBD and its poor prognosis. LCNEC is treated in a similar manner as other cancers in the CBD. The best treatment option is surgical excision. In most cases, adequate clearance can be achieved by an excision of the CBD via portal lymphadenectomy and Roux-en-Y hepaticojejunostomy reconstruction, but some distal CBD tumors are best treated by pancreaticoduodenectomy. The role of adjuvant therapy remains controversial, and most studies have failed to demonstrate a survival advantage[8].
The prognosis of patient with LCNEC of the CBD is reportedly poor[8]. The survival duration of previously reported LCNEC of the CBD cases ranged from only 21 d to 12 mo after surgery (Table 1, cases 1-3). In our case, recurrence was noted 7 mo after surgery, and the patient died 5 mo after this recurrence. SCNEC of the CBD, another NEC type, has a poor prognosis, similar to LCNES. Edakuni et al[9] reported the longest survival of SCNEC of the CBD as 45 mo after surgery. In this case, the tumor was a MANEC (40% adenocarcinoma and 60% SCNEC) and exhibited a reduced proliferative fraction (9.6% Ki-67-positive tumor cell). Shimono et al[10] reported a long survival (69 mo after surgery) in a gallbladder patient with LCNEC who underwent an operation and adjuvant chemotherapy as well as radiation therapy after surgery[10]. Though this case involves a single LCNEC of a gallbladder patient, the multimodal treatment appears to offer enhanced treatment, resulting in increased survival for LCNEC of the CBD.
LCNEC of the CBD is a poorly differentiated, rare tumor that exhibits high-grade NETs with aggressive behavior and has a high tendency for early lymph node and distant metastases. More clinicopathological data and further studies with multimodal treatment are required to identify the prognostic indicators and the histogenesis of LCNEC of the CBD.
A 75-year-old female presented with a history of nausea, increasing yellowish discoloration of the face and dark-colored urine.
Icteric sclera. The patient did not exhibit abdominal tenderness or a palpable mass in the right upper quadrant of the abdomen.
Cholangiocarcinoma and other common bile duct (CBD) cancers.
White blood cells, 10500 103/μL (3.8-10.0 103/μL); serum albumin, 3.1 g/dL (3.5-4.8 g/dL); total bilirubin, 6.4 mg/dL (0.3-1.2 mg/dL); direct bilirubin, 5.6 mg/dL (0.1-0.5 mg/dL); aspartate aminotransaminase, 151 U/L (normal 15-41 U/L); alanine aminophosphatase, 302 U/L (14-54 U/L); aspartate transferase, 240 U/L (38-126 U/L); and gammaglutamyl transpeptidase, 939 U/L (7-50 U/L). The carbohydrate antigen 19-9 level was 118.1 U/mL (0-37 U/mL).
A computed tomography scan revealed a mass approximately 2.7 cm in size in the mid common bile duct and a regional metastatic node in the hepatoduodenal ligament. The endoscopic retrograde cholangiopancreatography demonstrated an asymmetric filling defect in the mid common bile duct.
The patient underwent a CBD excision with lymph node dissection. The patient was administered adjuvant chemotherapy consisting of 5-fluorouracil, epirubicin, and cisplatin.
Tumor cells are monotonous and exhibit relatively abundant cytoplasm and prominent hyperchromatic nuclei with course chromatin. Immunohistochemical findings indicated that tumor cells are immunopositive to neuroendocrine markers, including synaptophysin and chromogranin.
This case report presents a rare case of a primary neuroendocrine carcinoma of the common bile duct. More clinicopathological data and further studies with multimodal treatment are required to identify the prognostic indicators and the histogenesis of large cell neuroendocrine carcinoma of the CBD.
This article applies information about a rare case of a primary neuroendocrine carcinoma of the common bile duct.
P- Reviewer: Athanasopoulos PG, Campagnacci R, Cheng NS, Harada R, Zhang XW S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
| 1. | Sato K, Waseda R, Tatsuzawa Y, Fujinaga H, Wakabayashi T, Ueda Y, Katsuda S. Composite large cell neuroendocrine carcinoma and adenocarcinoma of the common bile duct. J Clin Pathol. 2006;59:105-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 2. | Demoreuil C, Thirot-Bidault A, Dagher C, Bou-Farah R, Benbrahem C, Lazure T, Gayral F, Buffet C. [Poorly differentiated large cell endocrine carcinoma of the extrahepatic bile ducts]. Gastroenterol Clin Biol. 2009;33:194-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Sasatomi E, Nalesnik MA, Marsh JW. Neuroendocrine carcinoma of the extrahepatic bile duct: case report and literature review. World J Gastroenterol. 2013;19:4616-4623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Komminoth P, Arnold R, Capella C, Klimstra DS, Kloppel G, Rindi G, Albores-Saavedra J, Solcia E. Neuroendocrine neoplasms of the gallbladder and extrahepatic bile ducts. WHO classification of tumours of the digestive system. Lyon: IARC Press 2010; 274-276. |
| 5. | Klimstra DS, Modlin IR, Coppola D, Lloyd RV, Suster S. The pathologic classification of neuroendocrine tumors: a review of nomenclature, grading, and staging systems. Pancreas. 2010;39:707-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 776] [Cited by in RCA: 752] [Article Influence: 50.1] [Reference Citation Analysis (2)] |
| 6. | Albores-Saavedra J, Nadji M, Henson DE, Ziegels-Weissman J, Mones JM. Intestinal metaplasia of the gallbladder: a morphologic and immunocytochemical study. Hum Pathol. 1986;17:614-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 55] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Cavazza A, Gallo M, Valcavi R, De Marco L, Gardini G. Large cell neuroendocrine carcinoma of the ampulla of vater. Arch Pathol Lab Med. 2003;127:221-223. [PubMed] |
| 8. | Saltz L, Kemeny N, Schwartz G, Kelsen D. A phase II trial of alpha-interferon and 5-fluorouracil in patients with advanced carcinoid and islet cell tumors. Cancer. 1994;74:958-961. [PubMed] |
| 9. | Edakuni G, Sasatomi E, Satoh T, Tokunaga O, Miyazaki K. Composite glandular-endocrine cell carcinoma of the common bile duct. Pathol Int. 2001;51:487-490. [PubMed] |
| 10. | Shimono C, Suwa K, Sato M, Shirai S, Yamada K, Nakamura Y, Makuuchi M. Large cell neuroendocrine carcinoma of the gallbladder: long survival achieved by multimodal treatment. Int J Clin Oncol. 2009;14:351-355. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 24] [Article Influence: 1.5] [Reference Citation Analysis (0)] |