Published online Dec 21, 2014. doi: 10.3748/wjg.v20.i47.17985
Revised: August 24, 2014
Accepted: September 29, 2014
Published online: December 21, 2014
Processing time: 210 Days and 7.1 Hours
AIM: To establish and validate a simple quantitative assessment method for nonalcoholic fatty liver disease (NAFLD) based on a combination of the ultrasound hepatic/renal ratio and hepatic attenuation rate.
METHODS: A total of 170 subjects were enrolled in this study. All subjects were examined by ultrasound and 1H-magnetic resonance spectroscopy (1H-MRS) on the same day. The ultrasound hepatic/renal echo-intensity ratio and ultrasound hepatic echo-intensity attenuation rate were obtained from ordinary ultrasound images using the MATLAB program.
RESULTS: Correlation analysis revealed that the ultrasound hepatic/renal ratio and hepatic echo-intensity attenuation rate were significantly correlated with 1H-MRS liver fat content (ultrasound hepatic/renal ratio: r = 0.952, P = 0.000; hepatic echo-intensity attenuation r = 0.850, P = 0.000). The equation for predicting liver fat content by ultrasound (quantitative ultrasound model) is: liver fat content (%) = 61.519 × ultrasound hepatic/renal ratio + 167.701 × hepatic echo-intensity attenuation rate -26.736. Spearman correlation analysis revealed that the liver fat content ratio of the quantitative ultrasound model was positively correlated with serum alanine aminotransferase, aspartate aminotransferase, and triglyceride, but negatively correlated with high density lipoprotein cholesterol. Receiver operating characteristic curve analysis revealed that the optimal point for diagnosing fatty liver was 9.15% in the quantitative ultrasound model. Furthermore, in the quantitative ultrasound model, fatty liver diagnostic sensitivity and specificity were 94.7% and 100.0%, respectively, showing that the quantitative ultrasound model was better than conventional ultrasound methods or the combined ultrasound hepatic/renal ratio and hepatic echo-intensity attenuation rate. If the 1H-MRS liver fat content had a value < 15%, the sensitivity and specificity of the ultrasound quantitative model would be 81.4% and 100%, which still shows that using the model is better than the other methods.
CONCLUSION: The quantitative ultrasound model is a simple, low-cost, and sensitive tool that can accurately assess hepatic fat content in clinical practice. It provides an easy and effective parameter for the early diagnosis of mild hepatic steatosis and evaluation of the efficacy of NAFLD treatment.
Core tip: The quantitative ultrasound model is a simple, low-cost, and sensitive tool that can accurately assess hepatic fat content in clinical practice. It provides an easy and effective parameter for early diagnosis of mild hepatic steatosis and evaluation of the efficacy of non-alcoholic fatty liver disease treatment.
- Citation: Zhang B, Ding F, Chen T, Xia LH, Qian J, Lv GY. Ultrasound hepatic/renal ratio and hepatic attenuation rate for quantifying liver fat content. World J Gastroenterol 2014; 20(47): 17985-17992
- URL: https://www.wjgnet.com/1007-9327/full/v20/i47/17985.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i47.17985
Nonalcoholic fatty liver disease (NAFLD) is one of the most important causes of chronic liver disease. At present, the prevalence of NAFLD has been increasing year by year, reaching 30% in Europe and the United States and about 15% in Asia[1]. NAFLD is closely associated with the occurrence of many chronic diseases, such as diabetes and cardiovascular diseases. NAFLD was also reported to be associated with increased insulin resistance[2]. A meta-analysis showed that the prevalence of diabetes significantly increased in patients with NAFLD, and was 2-3 times higher than normal[3]. If the disease is not diagnosed early, abnormal glucose metabolism and cardiovascular disease could easily manifest in patients with NAFLD. Hence, early diagnosis of NAFLD could help predict the occurrence of diabetes and cardiovascular disease, which is important in preventing these chronic diseases from developing further.
At present, the gold standard for diagnosing NAFLD is liver biopsy. However, liver biopsy is invasive and may cause serious complications - a method not suitable for screening NAFLD. 1H magnetic resonance spectroscopy (1H-MRS) is a noninvasive technique that can quantitatively detect NAFLD with high sensitivity. However, using this technique is not practical for screening NAFLD, due to equipment requirements and high cost[4]. Therefore, it appears that there is an urgent need for screening early NAFLD by using simpler and more efficient methods.
Liver ultrasonic scanning is one of the common methods for diagnosing NAFLD, but this method can be easily affected by subjective factors; hence, this method cannot accurately detect liver fat content. As a result, it is difficult to diagnose NAFLD at an early stage. However, with the development of computer technology, it is now possible to quantitatively determine liver fat content by the ultrasound hepatic/renal ratio. Xia et al[5] found that the ultrasound hepatic/renal ratio significantly correlated with liver biopsy and 1H-MRS in determining liver fat content, which implied that the ultrasound hepatic/renal ratio could partly reflect NAFLD development. In addition, the hepatic echo-intensity attenuation rate is an ultrasonic quantitative indicator; and Kwon et al[6] found that the hepatic echo-intensity attenuation rate showed a correlation with the degree of NAFLD in animal experiments; however, results were obtained by applying these two methods had low accuracy. Szczepaniak et al[7] found that the sensitivity and specificity of the ultrasound hepatic/renal ratio (62.7%) and the hepatic echo-intensity attenuation rate (68.0%) in fatty liver diagnosis were 64.7% and 70.0%, respectively. Sensitivity and specificity were still not able to achieve the requirements for clinical diagnosis of NAFLD. Physicians need a mature quantitative ultrasound model that can provide simple, accurate, and stable results in evaluating liver fat content.
Hence, in our hypothesis, we propose a combined ultrasound hepatic/renal ratio and hepatic echo-intensity attenuation rate evaluation; compensating for their shortcomings and improving the accuracy of detecting NAFLD. This study aims to explore a simple, low-cost, and sensitive model that can detect liver fat content. The specificity and sensitivity of this new evaluation model were determined.
From December 2012 to October 2013, 129 patients with NAFLD in our hospital were enrolled according to the following inclusion criteria: (1) diagnosed with fatty liver by ultrasound; (2) no recent heavy drinking history; (3) did not take drugs that could affect liver functions and liver fat; and (4) no serious liver or kidney disease.
The 129 NAFLD patients had a mean age of 45.3 ± 7.1 years; 83 cases were diagnosed with mild fatty liver and 46 cases were diagnosed with severe fatty liver. The 41 healthy subjects diagnosed without fatty liver were enrolled in the control group.
Anthropometric measurement and biochemical detection: The height, weight, waistline, and hipline measurements were taken from the patients; body mass index (BMI) and waist/hip ratios were then calculated. Triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), and liver enzyme, alanine and aspartate aminotransferase (AST, ALT) serum levels were recorded.
Fat content detection by 1H-MRS: The liver fat content of all subjects were detected by 1H-MRS. The right lobe of the liver was located when patients were lying in the supine position. Areas under the water peak and fat peak were recorded. Liver fat content was calculated as [liver fat content (%) = area under the fat peak × 100/(area under the fat peak + area under the water peak)]. Liver fat content ≥ 5.56% was defined as fatty liver[7-9].
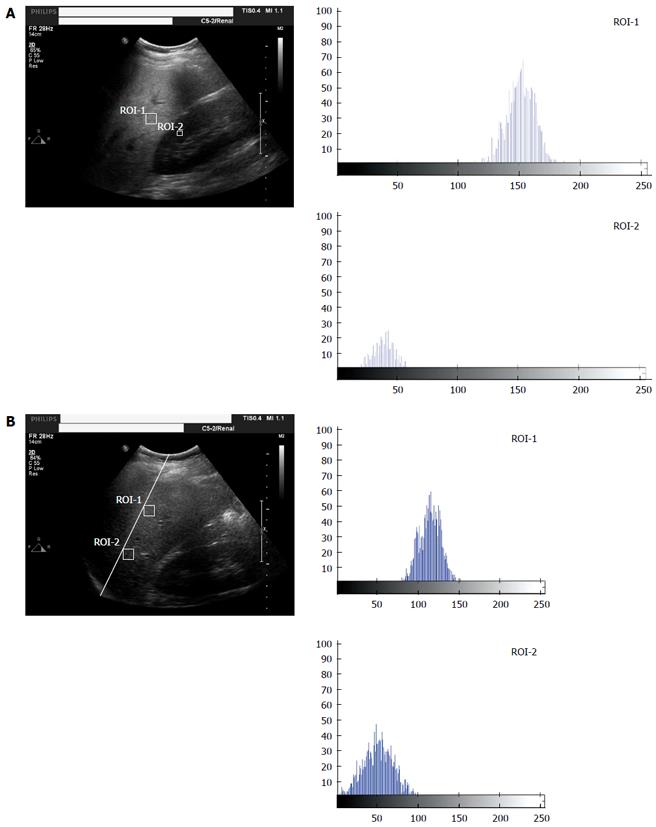
Ultrasound hepatic/renal ratio and hepatic echo-intensity attenuation rate analysis: All subjects underwent color Doppler ultrasound examinations. Ultrasonic images were analyzed by MATLAB software. Average gray-scale intensities were determined in liver and renal cortex regions of interest (ROIs). The hepatic/renal ratio was calculated according to the equation: hepatic/renal ratio = mean gray-scale intensity of the liver/mean gray-scale intensity of the renal cortex (Figure 1A). Two liver ROI samples were selected at a depth of 4-6 cm from the near-field of the same beam. The distance between the two ROIs were measured (Figure 1B). The hepatic echo-intensity attenuation rate was calculated according to the equation: hepatic echo-intensity attenuation rate = (lnAn-lnAf)/(∆d × f); where An and Af represent the mean echo intensity of the near-field and far-field ROIs, respectively; ∆d is the line distance between the two ROIs and f is the ultrasonic transducer frequency.
Data was analyzed using SPSS 18.0 statistical software (SPSS Inc., Chicago, IL, United States). Numerical variable data with normal distribution were recorded as (mean ± SD). Spearman correlation analysis was performed between the quantitative ultrasound parameters and 1H-MRS liver fat content. The optimum qualitative diagnosis point of the NAFLD and quantitative fat content estimation by ultrasound were determined by receiver operating characteristic (ROC) curve analysis. The optimum ultrasonic quantity point was confirmed by the Youden index [maximum value of (sensitivity + specificity-1)]. Logistic regression analysis was used for the quantitative ultrasound parameters to determine the equation for predicting liver fat content. P < 0.05 indicates that the differences were statistically significant.
Based on the diagnostic standard (liver fat content ≥ 5.56% defined as fatty liver) determined by 1H-MRS, the subjects were divided into two groups, the fatty liver group and the non-fatty liver group. The general data of the two groups was compared. BMI, waist/hip ratio, and serum ALT, AST, and TC levels of the fatty liver group were significantly higher than the non-fatty liver group, while serum HDL-C levels were significantly lower. The differences were statistically significant (P < 0.05). The age, gender, TG, and LDL-C differences between the two groups were not statistically significant (P > 0.05). The ultrasound hepatic/renal ratio and hepatic echo-intensity attenuation rate of the fatty liver group were significantly higher than the non-fatty liver group; and the differences were statistically significant (P < 0.05) (Table 1).
| Classification | Non-fatty liver group (n = 41) | Fatty liver group (n = 129) | P |
| Age (yr) | 54.32 ± 6.24 | 53.72 ± 5.81 | 0.182 |
| Gender (male/female) | 20/21 | 67/62 | 0.613 |
| BMI (kg/m2) | 24.11± 0.73 | 27.38 ± 0.64 | 0.005 |
| Waist/hip ratio | 0.86 ± 0.02 | 0.93 ± 0.01 | 0.001 |
| ALT (IU/L) | 18 (11-22) | 35 (18-41) | 0.001 |
| AST (IU/L) | 18 (14-25) | 24 (18-39) | 0.001 |
| TC (mmol/L) | 1.03 (0.75-1.22) | 1.72 (1.35-2.43) | 0.000 |
| TG (mmol/L) | 4.87 ± 0.27 | 5.02 ± 0.18 | 0.532 |
| HDL-C (mmol/L) | 1.42 ± 0.05 | 1.18 ± 0.04 | 0.003 |
| LDL-C (mmol/L) | 3.01 ± 0.23 | 2.98 ± 0.15 | 0.672 |
| Ultrasound hepatic/renal ratio | 0.55 ± 0.01 | 0.80 ± 0.04 | 0.000 |
| Hepatic echo-intensity attenuation rate (cm-1·MHz-1) | -0.0193 ± 0.0031 | 0.0027 ± 0.0016 | 0.000 |
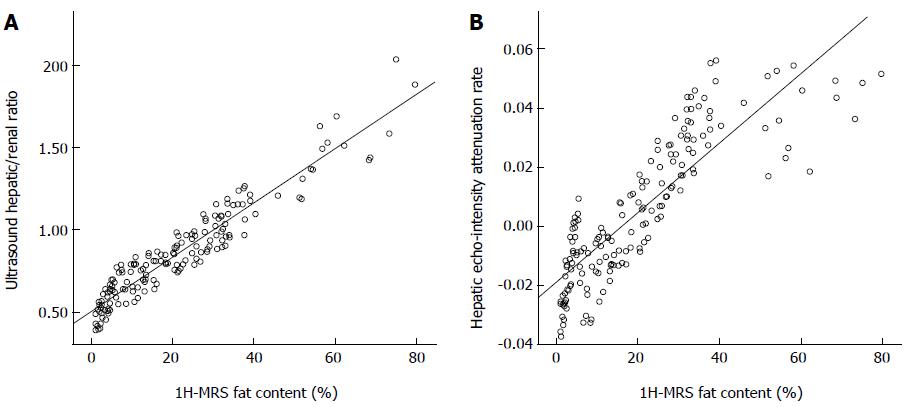
Correlation analysis revealed that the ultrasound hepatic/renal ratio and hepatic echo-intensity attenuation rate were significantly correlated with 1H-MRS liver fat content (ultrasound hepatic/renal ratio: r = 0.952, P = 0.000; hepatic echo-intensity attenuation r = 0.850, P = 0.000) (Figure 2).
1H-MRS liver fat content was set as a dependent variable. Multiple linear regression analysis was utilized to screen the main quantitative parameters for liver fat content estimation. The ultrasound hepatic/renal ratio was the strongest predictor of liver fat content (corrective R2 = 79.0%, P = 0.000). When combined with the hepatic echo-intensity attenuation rate, a higher estimation accuracy can be achieved (corrective R2 = 80.1%, P = 0.000). The equation for liver fat content prediction by ultrasound (quantitative ultrasound model) was: liver fat content (%) = 61.519 × ultrasound hepatic/renal ratio + 167.701 × hepatic echo-intensity attenuation rate -26.736 (Table 2).
| Model | Regression coefficients ± SD | P value | Corrective R2 | RMSE | ||||
| Ultrasound hepatic/renal ratio | Hepatic echo-intensity attenuation rate | Constant | ||||||
| Liver fat content | 1 | 72.012 ± 3.445 | - | -34.704 ± 2.302 | -34.704 ± 2.302 | 0.000 | 79.0% | 6.12 |
| 2 | 61.519 ± 4.311 | 167.701 ± 42.115 | -26.736 ± 3.012 | -26.736 ± 3.012 | 0.000 | 80.1% | 5.33 | |
Spearman correlation analysis revealed that the liver fat content quantitative ultrasound model was positively correlated with serum ALT, AST, and TG, but negatively correlated with HDL-C; however, it was not correlated with serum LDL-C and TC (Table 3).
| AST | ALT | TC | TG | HDL-C | LDL-C | |
| r | 0.301 | 0.411 | 0.015 | 0.512 | -0.331 | -0.079 |
| P | 0.001 | 0.000 | 0.721 | 0.000 | 0.001 | 0.332 |
ROC analysis revealed that the optimum point of fatty liver diagnosis was 9.15%, using the quantitative ultrasound model. When 1H-MRS was set as the gold standard for diagnosing fatty liver by the quantitative ultrasonic model, the sensitivity and specificity for fatty liver diagnosis were 94.7% and 100.0%, respectively. These results were better than using the conventional ultrasound method, or single use of the ultrasound hepatic/renal ratio and the hepatic echo-intensity attenuation rate. A subgroup analysis was also performed, where 1H-MRS liver fat content was < 15%; the results for the sensitivity and specificity of the quantitative ultrasound model were 81.4% and 100%, respectively. These results are still better than applying other methods (Table 4).
| Group | 1H-MRS diagnosis of fatty liver | |||
| Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | |
| All subjects | ||||
| Quantitative ultrasound model | 94.7 | 100.0 | 100.0 | 82.6 |
| Conventional ultrasound | 83.7 | 70.7 | 90.0 | 58.0 |
| Ultrasound hepatic/renal ratio | 88.3 | 84.5 | 94.2 | 72.4 |
| Hepatic echo-intensity attenuation rate | 86.7 | 78.7 | 92.0 | 67.0 |
| Subjects with fatty content < 15% (1H-MRS) | ||||
| Quantitative ultrasound model | 81.4 | 100.0 | 100.0 | 84.5 |
| Conventional ultrasound | 47.6 | 70.3 | 73.1 | 62.7 |
| Ultrasound hepatic/renal ratio | 67.5 | 81.7 | 87.6 | 70.3 |
| Hepatic echo-intensity attenuation rate | 55.6 | 74.3 | 82.1 | 68.7 |
In this study, the ultrasound hepatic/renal ratio and hepatic echo-intensity attenuation rate significantly correlated with 1H-MRS liver fat content. The sensitivity and specificity of fatty liver diagnosis by the novel quantitative ultrasound model (hepatic/renal ratio and hepatic echo-intensity attenuation rate combination) were significantly higher than by conventional ultrasound; which implies that the quantitative ultrasound model can conveniently and accurately determine liver fat content. We hope that this model could provide a convenient and effective method for diagnosing NAFLD and mild fatty liver degeneration.
Currently, 1H-MRS is considered the gold standard for determining liver fat content[10,11]. In this study, the hepatic/renal ratio and hepatic echo-intensity attenuation rate were highly correlated with 1H-MRS liver fat content; suggesting that these methods could also accurately assess liver fatty content as well. Compared with 1H-MRS, these methods are more convenient, economic, and easy to operate[3,12,13], which can be used as new tools for clinical NAFLD diagnosis.
This study shows that the sensitivity and specificity of fatty liver diagnosis, by the ultrasound hepatic/renal ratio and hepatic echo-intensity attenuation rate, were significantly better than the conventional ultrasound method. Furthermore, combining the two methods and the quantitative ultrasound model resulted in better sensitivity and specificity, which implies that the quantitative ultrasound model could significantly improve the sensitivity and specificity of fatty liver diagnosis. A possible reason for this observation is that the traditional standard for ultrasound fatty liver diagnosis is relatively subjective and can easily result in misdiagnosis[14-16]. However, a computer-assisted quantitative ultrasound model makes the fatty liver objective and quantitative, which cannot easily be influenced by subjective factors and avoids subjective deviation[5,17,18]. Meanwhile, this method can accurately provide quantitative liver fat content information; overcoming the limitations of a qualitative-only diagnosis performed by conventional ultrasound.
The result shows that the sensitivity and specificity of mild fatty liver diagnosis by quantitative ultrasound model is significantly higher than by ultrasound hepatic/renal ratio, hepatic echo-intensity attenuation rate, or conventional ultrasound. The result also indicates that even with a slightly echo-enhanced ultrasound image of the liver, fatty liver still could not be distinguished by the naked eye; but fatty liver could be identified early by using the indices of the quantitative ultrasound model. Hence, patients with early fatty liver can be diagnosed by using the quantitative ultrasound model[19,20]. Moreover, the quantitative ultrasound model is more convenient and easier to operate than the 1H-MRS, especially for early fatty liver screening in a large population.
The study showed that liver fat content determined by quantitative ultrasound model was positively correlated with serum ALT, AST, and TG, but negatively correlated with HDL-C; suggesting a correlation with liver function and blood lipids. The result was similar to the previous studies on determining liver fat content by 1H-MRS[21-23]. Most patients diagnosed with fatty liver have liver function damage and dyslipidemia. The quantitative ultrasound model can detect this correlation, and we hope that this method could be a useful tool for detecting fatty liver in clinical practice.
There are still some deficiencies in this study. At present, the strictest standard for diagnosing fatty liver is liver biopsy. In our study, 1H-MRS detection was set as the golden standard for diagnosing NAFLD and not liver biopsy, because of the high risk of complications. We believe that 1H-MRS is a relatively accurate and reliable method for diagnosing fatty liver, because this method was used as the diagnostic criteria for several large-scale studies. Many studies have also shown that 1H-MRS was consistent with liver biopsy, in terms of diagnosing fatty liver[24-28].
In conclusion, the study successfully established the quantitative ultrasound model for liver fat content; by combining the ultrasound hepatic/renal ratio and hepatic echo-intensity attenuation rate, liver fat content can be accurately determined. The study also provides a simple and effective method for the early diagnosis of mild NAFLD degeneration.
Nonalcoholic fatty liver disease (NAFLD) is one of the most important causes of chronic liver disease. This disease is closely associated with the occurrence of many chronic diseases, such as diabetes and cardiovascular diseases. A meta-analysis showed that abnormal glucose metabolism and cardiovascular disease could easily manifest in patients with NAFLD, if the disease could not be diagnosed in a timely manner. Hence, early diagnosis of NAFLD could predict the occurrence of diabetes and cardiovascular disease: an important factor in preventing the development of these chronic diseases.
The gold standard for NAFLD diagnosis is liver biopsy. However, this method is invasive and may cause serious complications. Furthermore, this method is not suitable for screening NAFLD. We established that liver fat content could be quantitatively detected by the ultrasound hepatic/renal ratio. Moreover, the hepatic echo-intensity attenuation rate is a quantitative ultrasound indicator. However, its sensitivity and specificity did not meet the requirement for clinically diagnosing NAFLD. Therefore, physicians need a mature quantitative ultrasound model that can provide a simple, accurate, and stable method for evaluating liver fat content.
This study successfully established the quantitative ultrasound model for determining liver fat content by combining the ultrasound hepatic/renal ratio and hepatic echo-intensity attenuation rate; which can accurately reflect liver fat content. The study also provides a simple and effective method for diagnosing early mild NAFLD degeneration.
The quantitative ultrasound model can conveniently and accurately determine liver fat content. We hope it can provide a convenient and effective method for diagnosing NAFLD and mild fatty liver degeneration.
This is a very interesting manuscript about ultrasound hepatic/renal ratio and hepatic attenuation rate. In this paper, the authors establish and validate a simple method for quantitative assessment of NAFLD based on the combination of ultrasound hepatic/renal ratio and hepatic attenuation rate.
P- Reviewer: Nakada K S- Editor: Qi Y L- Editor: Cant MR E- Editor: Liu XM
| 1. | Vajro P, Lenta S, Socha P, Dhawan A, McKiernan P, Baumann U, Durmaz O, Lacaille F, McLin V, Nobili V. Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee. J Pediatr Gastroenterol Nutr. 2012;54:700-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 382] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 2. | Orešič M, Hyötyläinen T, Kotronen A, Gopalacharyulu P, Nygren H, Arola J, Castillo S, Mattila I, Hakkarainen A, Borra RJ. Prediction of non-alcoholic fatty-liver disease and liver fat content by serum molecular lipids. Diabetologia. 2013;56:2266-2274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 113] [Cited by in RCA: 134] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 3. | Li X, Xia M, Ma H, Hu Y, Yan H, He W, Lin H, Zhao N, Gao J, Gao X. Liver fat content, evaluated through semi-quantitative ultrasound measurement, is associated with impaired glucose profiles: a community-based study in Chinese. PLoS One. 2013;8:e65210. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (1)] |
| 4. | Cacciapuoti F, Scognamiglio A, Palumbo R, Forte R, Cacciapuoti F. Silymarin in non alcoholic fatty liver disease. World J Hepatol. 2013;5:109-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (3)] |
| 5. | Xia MF, Yan HM, He WY, Li XM, Li CL, Yao XZ, Li RK, Zeng MS, Gao X. Standardized ultrasound hepatic/renal ratio and hepatic attenuation rate to quantify liver fat content: an improvement method. Obesity (Silver Spring). 2012;20:444-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 111] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 6. | Kwon HJ, Kim KW, Lee SJ, Kim SY, Lee JS, Kim HJ, Song GW, Kim SA, Yu ES, Lee J. Value of the ultrasound attenuation index for noninvasive quantitative estimation of hepatic steatosis. J Ultrasound Med. 2013;32:229-235. [PubMed] [DOI] [Full Text] |
| 7. | Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, Hobbs HH, Dobbins RL. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462-E468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1119] [Cited by in RCA: 1183] [Article Influence: 59.2] [Reference Citation Analysis (0)] |
| 8. | Wang JH, Hung CH, Kuo FY, Eng HL, Chen CH, Lee CM, Lu SN, Hu TH. Ultrasonographic quantification of hepatic-renal echogenicity difference in hepatic steatosis diagnosis. Dig Dis Sci. 2013;58:2993-3000. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Xia MF, Lin HD, Li XM, Yan HM, Bian H, Chang XX, He WY, Jeekel J, Hofman A, Gao X. Renal function-dependent association of serum uric acid with metabolic syndrome and hepatic fat content in a middle-aged and elderly Chinese population. Clin Exp Pharmacol Physiol. 2012;39:930-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Mancini M, Prinster A, Annuzzi G, Liuzzi R, Giacco R, Medagli C, Cremone M, Clemente G, Maurea S, Riccardi G. Sonographic hepatic-renal ratio as indicator of hepatic steatosis: comparison with (1)H magnetic resonance spectroscopy. Metabolism. 2009;58:1724-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 70] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | He WY, Jin YJ, Wang WP, Li CL, Ji ZB, Yang C. Tissue elasticity quantification by acoustic radiation force impulse for the assessment of renal allograft function. Ultrasound Med Biol. 2014;40:322-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 50] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 12. | Borges VF, Diniz AL, Cotrim HP, Rocha HL, Andrade NB. Sonographic hepatorenal ratio: a noninvasive method to diagnose nonalcoholic steatosis. J Clin Ultrasound. 2013;41:18-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 13. | Martín-Rodríguez JL, Arrebola JP, Jiménez-Moleón JJ, Olea N, González-Calvin JL. Sonographic quantification of a hepato-renal index for the assessment of hepatic steatosis in comparison with 3T proton magnetic resonance spectroscopy. Eur J Gastroenterol Hepatol. 2014;26:88-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 14. | Dou J, Ma X, Fang Q, Hao Y, Yang R, Wang F, Zhu J, Bao Y, Jia W. Relationship between serum osteocalcin levels and non-alcoholic fatty liver disease in Chinese men. Clin Exp Pharmacol Physiol. 2013;40:282-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 15. | Magosso E, Ansari MA, Gopalan Y, Shuaib IL, Wong JW, Khan NA, Abu Bakar MR, Ng BH, Yuen KH. Tocotrienols for normalisation of hepatic echogenic response in nonalcoholic fatty liver: a randomised placebo-controlled clinical trial. Nutr J. 2013;12:166. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Sogabe M, Okahisa T, Hibino S, Yamanoi A. Usefulness of differentiating metabolic syndrome into visceral fat type and subcutaneous fat type using ultrasonography in Japanese males. J Gastroenterol. 2012;47:293-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 17. | Dhadly P, McDonald MC, Cockerill G, Hinds CJ, Miller NE, Thiemermann C. Pre-treatment with high density lipoprotein (HDL) attenuates the renal and hepatic dysfunction caused by endotoxin in rats. British J Anaesthesia. 2000;688. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 18. | von Volkmann HL, Havre RF, Løberg EM, Haaland T, Immervoll H, Haukeland JW, Hausken T, Gilja OH. Quantitative measurement of ultrasound attenuation and Hepato-Renal Index in Non-Alcoholic Fatty Liver Disease. Med Ultrason. 2013;15:16-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Ballestri S, Lonardo A, Romagnoli D, Carulli L, Losi L, Day CP, Loria P. Ultrasonographic fatty liver indicator, a novel score which rules out NASH and is correlated with metabolic parameters in NAFLD. Liver Int. 2012;32:1242-1252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 151] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 20. | Shannon A, Alkhouri N, Carter-Kent C, Monti L, Devito R, Lopez R, Feldstein AE, Nobili V. Ultrasonographic quantitative estimation of hepatic steatosis in children With NAFLD. J Pediatr Gastroenterol Nutr. 2011;53:190-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 214] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 21. | Mahaling DU, Basavaraj MM, Bika AJ. Comparison of lipid profile in different grades of non-alcoholic fatty liver disease diagnosed on ultrasound. Asian Pacific Trop Biomed. 2013;3:907-912. [RCA] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1522] [Cited by in RCA: 1618] [Article Influence: 115.6] [Reference Citation Analysis (1)] |
| 23. | Cocciolillo S, Parruti G, Marzio L. CEUS and Fibroscan in non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. World J Hepatol. 2014;6:496-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 24. | Springer F, Machann J, Claussen CD, Schick F, Schwenzer NF. Liver fat content determined by magnetic resonance imaging and spectroscopy. World J Gastroenterol. 2010;16:1560-1566. [PubMed] |
| 25. | Narasimhan S, Gokulakrishnan K, Sampathkumar R, Farooq S, Ravikumar R, Mohan V, Balasubramanyam M. Oxidative stress is independently associated with non-alcoholic fatty liver disease (NAFLD) in subjects with and without type 2 diabetes. Clin Biochem. 2010;43:815-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 84] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Musso G, Gambino R, Cassader M, Pagano G. Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med. 2011;43:617-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 917] [Article Influence: 65.5] [Reference Citation Analysis (0)] |
| 27. | Koot BG, van der Baan-Slootweg OH, Tamminga-Smeulders CL, Rijcken TH, Korevaar JC, van Aalderen WM, Jansen PL, Benninga MA. Lifestyle intervention for non-alcoholic fatty liver disease: prospective cohort study of its efficacy and factors related to improvement. Arch Dis Child. 2011;96:669-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Dagnelie PC, Leij-Halfwerk S. Magnetic resonance spectroscopy to study hepatic metabolism in diffuse liver diseases, diabetes and cancer. World J Gastroenterol. 2010;16:1577-1586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |