Published online Dec 21, 2014. doi: 10.3748/wjg.v20.i47.17877
Revised: April 15, 2014
Accepted: July 24, 2014
Published online: December 21, 2014
Processing time: 287 Days and 6.7 Hours
AIM: To investigate the occurrence and severity of pruritus in chronic hepatitis C patients treated with or without interferon (IFN) therapy.
METHODS: A total of 89 patients with chronic hepatitis C and 55 control (non-hepatitis) patients were asked to rate their experience of diurnal and nocturnal pruritus in the preceding week using a visual analogue scale (VAS) and a five-point scale, respectively. Blood samples were taken and serum thymus and activation-regulated chemokine (TARC) levels were measured by enzyme-linked immunosorbent assay.
RESULTS: A significantly greater proportion of chronic hepatitis C patients experienced nocturnal pruritus compared with control (58.4% vs 5.5%, P < 0.0001). Chronic hepatitis C patients also had more severe pruritus compared with control patients, indicated by the higher mean VAS scores in both the IFN-treated and non-IFN-treated groups. In particular, patients who received combined peginterferon alfa-2b and ribavirin had significantly higher mean VAS scores than those receiving peginterferon alfa-2a or no IFN treatment. Serum TARC levels did not correlate with pruritus scores, and no significant differences in TARC levels were observed between the IFN-treated and non-IFN-treated groups.
CONCLUSION: Patients with chronic hepatitis C experience pruritus more than those without. Serum TARC levels do not correlate with pruritus severity in chronic hepatitis C patients.
Core tip: This is the first paper to evaluate the occurrence and severity of pruritus in chronic hepatitis C patients and to examine the relationship between pruritus and interferon therapy. We found that patients with chronic hepatitis C experience pruritus more than those without chronic hepatitis C.
- Citation: Suzuki K, Tamano M, Katayama Y, Kuniyoshi T, Kagawa K, Takada H, Suzuki K. Study of pruritus in chronic hepatitis C patients. World J Gastroenterol 2014; 20(47): 17877-17882
- URL: https://www.wjgnet.com/1007-9327/full/v20/i47/17877.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i47.17877
Little is known about pruritus associated with interferon (IFN) therapy for chronic hepatitis C[1]. Chronic hepatitis C patients also experience pruritus in the absence of IFN therapy. Serum thymus and activation-regulated chemokine (TARC) levels reflect the activity of atopic dermatitis[2], but the relationship between TARC levels and pruritus in chronic hepatitis C patients has not been studied.
The purpose of this study was to examine the occurrence and severity of pruritus in chronic hepatitis C compared with other gastrointestinal disorders, and to examine the relationship between pruritus and IFN therapy. We also examined the relationship between serum TARC levels and pruritus in chronic hepatitis C patients.
This study was approved by the Ethics Committee of Dokkyo Medical University Koshigaya Hospital and written informed consent was obtained from all participants. This study conformed to the ethical guidelines of the 2008 Declaration of Helsinki. Subjects were 89 chronic hepatitis C patients who were treated in the Department of Gastroenterology of Dokkyo Medical University Koshigaya Hospital between October 2010 and September 2012 (chronic hepatitis C group). This group comprised 44 men and 45 women with a mean age of 57.8 ± 10.6 years (range: 27-78 years). Of these, 54 patients were receiving IFN therapy (IFN group), while the remaining 35 patients did not receive IFN therapy (non-IFN group). Of those treated with IFN, 10 patients were given peginterferon alfa-2a (PEG-IFN α2a) monotherapy (α2a group), while 44 received combined peginterferon alfa-2b (PEG-IFN α2b) and ribavirin (RBV) therapy (α2b + RBV group).
The control group comprised 55 patients treated in our department for non-hepatic disorders during the same period. The group comprised 30 men and 25 women with a mean age of 58.7 ± 13.6 years (range: 24-74 years). Ideally, the control group should have comprised healthy individuals; however, such subjects were not available as the study population was selected from patients in our hospital. There was no statistical difference in the age or sex between these patients and those with chronic hepatitis C. Control patients had the following gastrointestinal disorders: reflux esophagitis (n = 23), atrophic gastritis (n = 15), gastrointestinal ulcers (n = 6), gallbladder polyps (n = 4), and other gastrointestinal disorders (n = 7). Patients with a history of atopic dermatitis or other skin conditions, malignant tumors, or those who used anti-allergic mediation or oral/topical steroids were excluded from the study.
Pruritus was defined as generalized itching in the absence of a rash or erythema; localized itching at IFN injection sites, insect bites or hives were not included in the analysis. Two types of patient-based instruments were used to assess pruritus. In the first instrument, nocturnal pruritus severity was assessed using a scale described by Kawashima et al[3] in which nocturnal pruritus in the preceding week was rated on a five-point scale from 0 (none) to 5 (very severe) (Table 1). In the second instrument, the diurnal pruritus severity in the preceding week was recorded on a visual analogue scale (VAS) and converted to a numerical score (Figure 1). For patients undergoing IFN therapy, assessment of nocturnal and diurnal pruritus severity was conducted during the 4- to 8-wk period after initiation of IFN therapy.
| Score | Nocturnal pruritus |
| 4 | Very severe, interfering with sleep |
| 3 | Severe, very annoying, substantially interfering with sleep |
| 2 | Moderate, annoying and troublesome, may interfere with sleep |
| 1 | Mild, not annoying or interfering with sleep |
| 0 | None |
Blood was taken from patients in the chronic hepatitis C group at the time when the pruritus assessment was performed. The serum was stored at -40 °C, and TARC levels were measured by enzyme-linked immunosorbent assay. The following laboratory parameters were also measured in the chronic hepatitis C group: alanine aminotransferase (ALT), alkaline phosphatase (ALP), serum γ-glutamyltransferase (GGT), total bilirubin and albumin, platelet count, and prothrombin activity.
Pruritus and VAS scores, TARC levels, and laboratory parameter values are expressed as mean ± SE. The Mann-Whitney U test was used to compare differences between two groups, and the Kruskal-Wallis test was used to compare multiple groups. P < 0.05 was regarded as statistically significant.
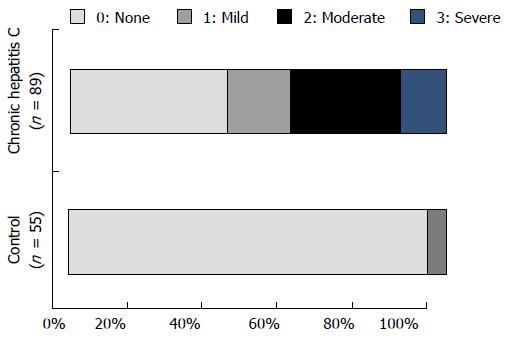
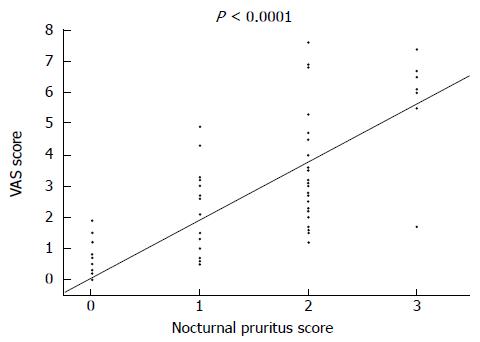
Figure 2 shows the nocturnal pruritus scores of the chronic hepatitis C and control groups. Patients in the chronic hepatitis C group had the following scores: 37 had a score of 0; 15 had a score of 1; 26 had a score of 2; 11 had a score 3; and none of them had a score of 4. Thus, 52 of 89 patients (58.4%) in the chronic hepatitis C group had a nocturnal pruritus score ≥ 1. Patients in the control group had the following scores: 52 patients had a score of 0; 3 patients had a score of 1; and none of them had a score ≥ 2. Thus, only 3 of 55 patients (5.5%) in the control group had pruritus; the proportion of patients with pruritus in the control group was significantly lower than that in the chronic hepatitis C group (P < 0.0001). Figure 3 shows the relationship between nocturnal pruritus scores and VAS scores of the 144 subjects. Nocturnal pruritus scores of 0, 1, 2, and 3 corresponded to VAS scores of 0.09 ± 0.04 cm, 2.32 ± 0.54 cm, 3.50 ± 0.37 cm, and 6.23 ± 0.55 cm, respectively, with a good correlation between the pruritus score and VAS score (P < 0.0001). Based on this result, we used the VAS score to evaluate pruritus.
Table 2 shows the characteristics of chronic hepatitis C patients treated with or without IFN. No significant difference in the age or sex between the two groups was noted. The mean duration of IFN treatment was 22.3 wk, and HCV RNA was significantly lower in the IFN group than the non-IFN group. ALT and ALP were significantly higher in the non-IFN group than the IFN group, but other clinical parameters were not significantly different.
| IFN (-),n = 35 | IFN (+),n = 54 | P value | |
| Age | 60.0 ± 12.3 | 56.4 ± 9.2 | 0.1112 |
| Sex | M:17; F:18 | M:27; F:27 | 0.6351 |
| Duration of IFN therapy (wk) | NA | 22.3 ± 17.4 | NA |
| HCV RNA (Log IU/mL) | 6.0 ± 1.3 | 1.2 ± 2.0 | < 0.0001 |
| ALT (U/L) | 60.5 ± 52.6 | 33.4 ± 26.2 | 0.0017 |
| ALP (U/L) | 307.0 ± 108.0 | 265.2 ± 77.2 | 0.0360 |
| GGT (U/L) | 48.9 ± 37.41 | 45.4 ± 44.3 | 0.6981 |
| T-Bil (mg/dL) | 0.86 ± 0.28 | 0.85 ± 0.44 | 0.9323 |
| Alb (g/dL) | 4.19 ± 0.40 | 4.15 ± 0.44 | 0.6315 |
| PLT (× 104/μL) | 14.5 ± 5.1 | 13.1 ± 6.9 | 0.2938 |
| PT | 84.2% ± 7.5% | 86.0 ± 10.1 | 0.7966 |
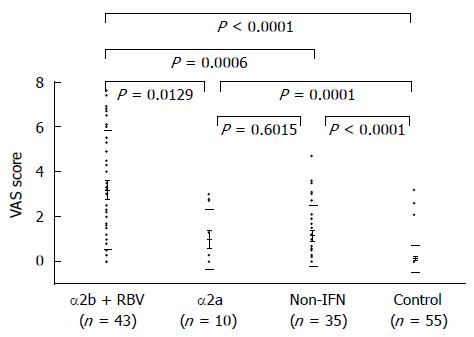
Figure 4 shows the VAS scores for the chronic hepatitis C and control groups. The VAS score of the control group was 0.32 ± 0.26. With respect to chronic hepatitis C patients, the non-IFN group, α2a group, and α2b + RBV group had VAS scores of 1.13 ± 0.29, 1.01 ± 0.56, and 3.20 ± 0.27, respectively, all of which were significantly higher than that of the control group. The VAS score was significantly different between the non-IFN group and α2b + RBV group, and between the α2a group and α2b + RBV group, but the VAS score was not significantly different between the non-IFN group and α2a group.
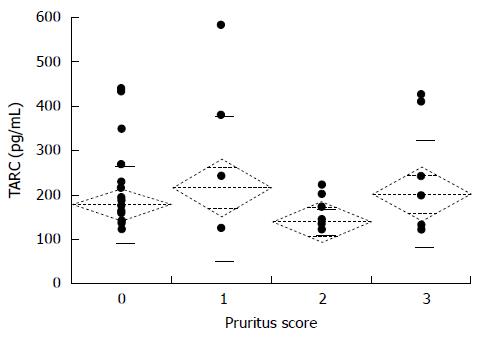
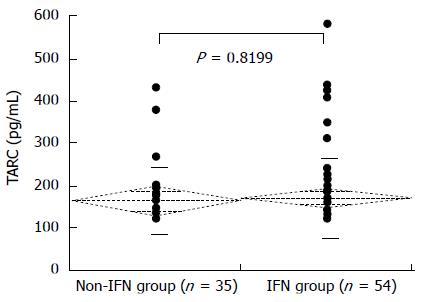
Figure 5 shows the nocturnal pruritus scores and serum TARC levels of the 89 chronic hepatitis C patients. The mean TARC levels for the pruritus scores 0, 1, 2, and 3 were 180.0 ± 16.4 pg/mL, 217.8 ± 54.3 pg/mL, 140.8 ± 7.0 pg/mL, and 203.7 ± 38.0 pg/mL, respectively. The pruritus score did not correlate with TARC levels. The mean serum TARC levels of the non-IFN and IFN groups were 166.8 ± 79.1 pg/mL and 173.3 ± 92.1 pg/mL, respectively; the difference in TARC levels between the two groups was not significant (Figure 6). The mean serum TARC levels of the α2a and α2b + RBV groups were 165.1 ± 30.9 pg/mL and 174.7 ± 12.8 pg/mL, respectively; the difference in TARC levels between the two groups was not significant (Figure 7).
Pruritus occurs in 2.5%-23% of patients with chronic hepatitis C[4-7]. The cause of pruritus in chronic hepatitis C is not well understood, although it has been associated with liver fibrosis, bile duct lesions and cholestasis[8-11]. In addition, hepatitis C may cause a primary skin disorder with pruritus as a symptom[5]. In this study, 58.4% of chronic hepatitis C patients experienced pruritus, which was higher than the 5.5% of control patients. There was no difference in the age or sex between the two groups, and patients were enrolled in the study at the same time, so we do not expect any differences in environmental factors such as temperature and humidity that might have influenced the results. Forty-four percent of chronic hepatitis C patients who did not receive IFN had pruritus, which was significantly higher than that seen in control patients (data not shown).
Adverse skin reactions and pruritus during IFN treatment for chronic hepatitis C may be caused by either immune changes or an excessive immune reaction[12,13]. Peginterferon induces injection site reactions in 36%-59% of cases[14,15] and can sometimes cause generalized dermatitis[13,16,17]. In our study of chronic hepatitis C patients, we only assessed the presence of pruritus and excluded injection site reactions and atopic dermatitis from the analysis.
The severity of pruritus as indicated by the VAS score was examined in subgroups of chronic hepatitis C patients. No significant difference in the VAS score between the non-IFN group and the α2a group was observed. Significant differences in VAS scores were seen between the non-IFN and α2b + RBV groups (P = 0.0006), and between the α2a and α2b + RBV groups (P = 0.0129). PEG-IFN α2a has a branched 40-kDa PEG chain, whereas PEG-IFN α2b has a linear 12-kDa PEG chain. Although PEG-IFN α2a and PEG-IFN α2b are expected to have similar side effect profiles[18], the difference in molecular weight might affect the development of pruritus due to these agents, but this is difficult to prove experimentally. Comparison between PEG-IFN α2a and PEG-IFN α2b monotherapies would have been preferable; however, this was not possible because the Japanese health insurance system stipulates that PEG-IFN α2b must be administered with RBV.
RBV itself has been reported to cause pruritus[13,19]. When combined with PEG-IFN, a synergistic effect may result in a delayed-type hypersensitivity reaction, with a shift in the helper T (Th) cell balance towards a Th1-dominant response due to the influence of IFN. This may explain the higher prevalence of pruritus in the α2b + RBV group[20].
Chemokines serve as chemotactic factors for immune cells particularly leukocytes. Chemokines are categorized into four types-namely, C, CC, CXC and CX3C-depending on the position of cysteine in their amino acid chains. The normal level of TARC (a CC chemokine) in healthy adults is ≤ 450 pg/mL. In patients with moderate atopic dermatitis, the serum TARC level is ≥ 750 pg/mL and reflects the disease activity of this condition[2]. One study reported that HCV-induced TARC expression attracted Treg cells to the liver, and TARC expression was normalized in patients with a sustained virological response after IFN therapy[21]. Based on these results, we selected TARC as a marker for pruritus in hepatitis C patients in the present study. Although the development of dermatitis in atopic patients treated with IFN for chronic hepatitis C has been reported[22], our study is the first to investigate the relationship between TARC levels and pruritus in chronic hepatitis C patients. Our findings revealed a lack of correlation between serum TARC levels and pruritus scores in the 89 patients with chronic hepatitis C. Also, no significant differences in the serum TARC levels between the non-IFN and IFN groups, or between the α2a and α2b + RBV groups, were noted. Notably, 3 of 54 patients (5.6%) treated with IFN had pruritus and skin lesions such as erythema, eczema, and erosion at non-injection sites (data not shown). Although the VAS score was higher in these patients, only one patient had an elevated TARC level. Despite the unknown association between pruritus in chronic hepatitis C patients and atopic dermatitis, our results suggest that serum TARC levels do not reflect the severity of skin symptoms in chronic hepatitis C patients during IFN treatment.
Patients with chronic hepatitis C experience pruritus more than those without chronic hepatitis C, and pruritus is particularly worse in patients undergoing combined PEG-IFN α2b and RBV therapy. Serum TARC levels do not correlate with pruritus severity in chronic hepatitis C patients.
Little is known about pruritus associated with interferon (IFN) therapy for chronic hepatitis C. Chronic hepatitis C patients also experience pruritus in the absence of IFN therapy. Serum thymus and activation-regulated chemokine (TARC) levels reflect the activity of atopic dermatitis, but the relationship between TARC levels and pruritus in chronic hepatitis C patients has not been studied.
The normal level of TARC (a CC chemokine) in healthy adults is ≤ 450 pg/mL. In patients with moderate atopic dermatitis, the serum TARC level is ≥ 750 pg/mL and reflects the disease activity of this condition. One study reported that HCV-induced TARC expression attracted Treg cells to the liver, and TARC expression was normalized in patients with a sustained virological response after IFN therapy. Based on these results, we selected TARC as a marker for pruritus in hepatitis C patients in the present study. Although the development of dermatitis in atopic patients treated with IFN for chronic hepatitis C has been reported, our study is the first to investigate the relationship between TARC levels and pruritus in chronic hepatitis C patients.
In this study, 58.4% of chronic hepatitis C patients experienced pruritus, which was higher than the 5.5% of control patients. The severity of pruritus as indicated by the VAS score was examined in subgroups of chronic hepatitis C patients. No significant difference in the VAS score between the non-IFN group and the α2a group was observed. Significant differences in VAS scores were seen between the non-IFN and α2b + RBV groups (P = 0.0006), and between the α2a and α2b + RBV groups (P = 0.0129). This paper revealed a lack of correlation between serum TARC levels and pruritus scores in the 89 patients with chronic hepatitis C. Also, no significant differences in the serum TARC levels between the non-IFN and IFN groups, or between the α2a and α2b + RBV groups, were noted. Patients with chronic hepatitis C experience pruritus more than those without chronic hepatitis C, and pruritus is particularly worse in patients undergoing combined PEG-IFN α2b and RBV therapy. Serum TARC levels do not correlate with pruritus severity in chronic hepatitis C patients.
The study results showed the actual situation of pruritus in the hepatitis C patient.
Chemokines serve as chemotactic factors for immune cells particularly leukocytes. Chemokines are categorized into four types-namely, C, CC, CXC and CX3C-depending on the position of cysteine in their amino acid chains. TARC is CC chemokine.
The authors of this manuscript discuss pruritus in chronic hepatitis C and tried to correlate this symptom with serum TARC levels. Unfortunately however, TARC levels were not correlated with pruritus in chronic hepatitis C with or without IFN treatment. They found a strong correlation between pruritus and Peg-IFNα2b + RBV combination therapy. The obtained results in this study are in accordance with our experience in daily clinical practice.
P- Reviewer: Mihaila RG, Takahashi T S- Editor: Ma N L- Editor: A E- Editor: Wang CH
| 1. | Hoffman CJ, Ray SC. Severe pruritus after completing pegylated interferon for hepatitis C. AIDS Read. 2008;18:562-565. [PubMed] |
| 2. | Kakinuma T, Nakamura K, Wakugawa M, Mitsui H, Tada Y, Saeki H, Torii H, Asahina A, Onai N, Matsushima K. Thymus and activation-regulated chemokine in atopic dermatitis: Serum thymus and activation-regulated chemokine level is closely related with disease activity. J Allergy Clin Immunol. 2001;107:535-541. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 403] [Cited by in RCA: 417] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 3. | Kawashima M, Tango T, Noguchi T, Inagi M, Nakagawa H, Harada S. Addition of fexofenadine to a topical corticosteroid reduces the pruritus associated with atopic dermatitis in a 1-week randomized, multicentre, double-blind, placebo-controlled, parallel-group study. Br J Dermatol. 2003;148:1212-1221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 4. | Bonacini M. Pruritus in patients with chronic human immunodeficiency virus, hepatitis B and C virus infections. Dig Liver Dis. 2000;32:621-625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Cordel N, Chosidow O, Francès C. Cutaneous disorders associated with hepatitis C virus infection. Ann Med Interne (Paris). 2000;151:46-52. [PubMed] |
| 6. | Sène D, Limal N, Cacoub P. Hepatitis C virus-associated extrahepatic manifestations: a review. Metab Brain Dis. 2004;19:357-381. [PubMed] |
| 7. | Al-Ali J, Al-Mutari N, Ahmed el-SF. Hepatitis C virus and the skin. Hepatogastroenterology. 2011;58:880-886. [PubMed] |
| 8. | Lebovics E, Seif F, Kim D, Elhosseiny A, Dworkin BM, Casellas A, Clark S, Rosenthal WS. Pruritus in chronic hepatitis C: association with high serum bile acids, advanced pathology, and bile duct abnormalities. Dig Dis Sci. 1997;42:1094-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Chia SC, Bergasa NV, Kleiner DE, Goodman Z, Hoofnagle JH, Di Bisceglie AM. Pruritus as a presenting symptom of chronic hepatitis C. Dig Dis Sci. 1998;43:2177-2183. [PubMed] |
| 10. | Kumar KS, Saboorian MH, Lee WM. Cholestatic presentation of chronic hepatitis C: a clinical and histological study with a review of the literature. Dig Dis Sci. 2001;46:2066-2073. [PubMed] |
| 11. | Raslan HM, Ezzat WM, Abd El Hamid MF, Emam H, Amre KS. Skin manifestations of chronic hepatitis C virus infection in Cairo, Egypt. East Mediterr Health J. 2009;15:692-700. [PubMed] |
| 12. | Paoletti V, Parlapiano C, Labbadia G, Cavina G, Marziali M, Donnarumma A, Paoletti F. Skin diseases as extrahepatic manifestations of HCV. Review of some clinical cases. Minerva Gastroenterol Dietol. 2002;48:277-283. [PubMed] |
| 13. | Hashimoto Y, Kanto H, Itoh M. Adverse skin reactions due to pegylated interferon alpha 2b plus ribavirin combination therapy in a patient with chronic hepatitis C virus. J Dermatol. 2007;34:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358:958-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4736] [Cited by in RCA: 4558] [Article Influence: 189.9] [Reference Citation Analysis (0)] |
| 15. | Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36:S237-S244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 208] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 16. | Moore MM, Elpern DJ, Carter DJ. Severe, generalized nummular eczema secondary to interferon alfa-2b plus ribavirin combination therapy in a patient with chronic hepatitis C virus infection. Arch Dermatol. 2004;140:215-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 17. | Shen Y, Pielop J, Hsu S. Generalized nummular eczema secondary to peginterferon Alfa-2b and ribavirin combination therapy for hepatitis C infection. Arch Dermatol. 2005;141:102-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 18. | Foster GR. Pegylated interferons for the treatment of chronic hepatitis C: pharmacological and clinical differences between peginterferon-alpha-2a and peginterferon-alpha-2b. Drugs. 2010;70:147-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 75] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 19. | Khakoo S, Glue P, Grellier L, Wells B, Bell A, Dash C, Murray-Lyon I, Lypnyj D, Flannery B, Walters K. Ribavirin and interferon alfa-2b in chronic hepatitis C: assessment of possible pharmacokinetic and pharmacodynamic interactions. Br J Clin Pharmacol. 1998;46:563-570. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 110] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 20. | Souvignet C, Zarski JP. Combination treatment for chronic hepatitis C: what is the role of ribavirin? Fundam Clin Pharmacol. 2000;14:321-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Riezu-Boj JI, Larrea E, Aldabe R, Guembe L, Casares N, Galeano E, Echeverria I, Sarobe P, Herrero I, Sangro B. Hepatitis C virus induces the expression of CCL17 and CCL22 chemokines that attract regulatory T cells to the site of infection. J Hepatol. 2011;54:422-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 22. | Berger L, Descamps V, Marck Y, Dehen L, Grossin M, Crickx B, Marcellin P, Belaich S. [Alpha interferon-induced eczema in atopic patients infected by hepatitis C virus: 4 case reports]. Ann Dermatol Venereol. 2000;127:51-55. [PubMed] |