Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17666
Revised: May 26, 2014
Accepted: July 11, 2014
Published online: December 14, 2014
Processing time: 258 Days and 5.3 Hours
Twin to twin transfusion syndrome (TTTS) is caused by aberrant vascular connections between infant twins and results in high morbidity and mortality in the perinatal period. In donor infants with TTTS and symptoms of intestinal obstruction, small-bowel lesions have been reported in most cases. We report on a 33+6 gestational wk donor infant with TTTS who had intermittent obstructive episodes, including delayed meconium passage and colonic dilatation on abdominal X-ray. The diagnosis of Hirschsprung’s disease was based on a lateral pelvic film with a reversed rectosigmoid ratio. A subsequent barium colon study and rectal suction biopsy indicated a short segment aganglionosis of the colon.
Core tip: Twin to twin transfusion syndrome (TTTS) is caused by an aberrant vascular connection between monochorionic infant twins. Necrotizing enterocolitis, jejunal/ileal atresia, and perforation have previously been reported, but there have been no reports of Hirschsprung’s disease in a donor infant with TTTS. The intrauterine hypoxemia in the case we report here may have inhibited neuroblast cell migration or caused the destruction of ganglion cells in the gut. The patient was a donor baby with symptoms of feeding intolerance and marked colon dilatation who was suffering from Hirschsprung’s disease and TTTS.
- Citation: Park HW, Cho MJ, Kim WY, Kwak BO, Kim MH. Hirschsprung’s disease in twin to twin transfusion syndrome: A case report. World J Gastroenterol 2014; 20(46): 17666-17669
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17666.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17666
Twin to twin transfusion syndrome (TTTS), which occurs in 1%-15% of monochorionic twins[1], has been associated with high perinatal mortality and morbidity[1-3]. Abnormal vascular connections between infant twins and blood volume fluctuations or ischemia cause damage across multiple organs[1-3]. Jejunal or ileal lesions (atresia or perforation) and necrotizing enterocolitis in the gastrointestinal system have been previously reported in donors with TTTS[2-5].
Here, we present the case of a donor baby with TTTS and symptoms of delayed meconium passage and intermittent obstructive episodes with colonic dilatation. Rectal submucosal biopsy and a barium colon study led to a diagnosis of Hirschsprung’s disease.
A male infant was born to a healthy 33-year-old multi-gravid woman. The pregnancy was complicated by polyhydramnios on the recipient side, oligohydramnios on the donor side, a marked difference between the two fetuses in apparent body weight, and pregnancy-induced hypertension. Amnioreductions were performed during pregnancy to alleviate the polyhydramnios in the recipient infant, but laser ablation was not performed. When labor began prematurely at 33+6 wk gestation, the twins were delivered via emergency Caesarean section. The recipient twin weighted 2190 g (50th-75th percentile) and had Apgar scores of 7/10 and 8/10 at 1 and 5 min, respectively. The donor weighed 1250 g < 10th percentile) and had Apgar scores of 2 and 5 at 1 and 5 min, respectively.
At birth, the donor baby was pale and exhibited weak muscle tone and poor spontaneous respiratory effort. Therefore, he required immediate intubation and assisted ventilation. His face showed compression deformities attributable to the oligohydramnios. The difference in birth weight between the infants was 42.9%. The hemoglobin levels of the recipient and donor were 20.3 and 3.4 g/dL, respectively (a difference of 16.9 g/dL). On the first day of life, the donor baby was transfused with a pack of red blood cells (10 mL/kg, three times by 3 d of age), after which the hematocrit level stabilized at more than 30%. In the recipient baby, partial exchange transfusion was performed because of polycythemia and respiratory symptoms, including tachypnea.
Head ultrasound of both babies showed increased echogenicity of the periventricular area, which decreased by the time of the follow-up exam. An echocardiogram showed a 2.5 mm atrial septal defect (ASD) in the recipient and a 2.5 mm ASD, 3.5 mm patent ductus arteriosus (PDA) in the donor. No other abnormal findings, such as ventricular hypertrophy, tricuspid valve regurgitation, or right ventricular outflow tract obstruction, were observed in the recipient.
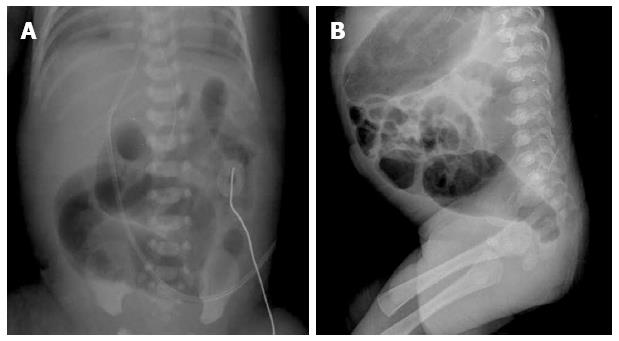
The recipient baby was able to received full enteral feeding on 10 d of life, and he was discharged from the newborn intensive care unit at 18 d of age. In the donor baby, oral ibuprofen was started at 2 d of age to treat the symptomatic PDA that caused respiratory symptoms, which required ventilator support. He showed marked dilatation of the sigmoid colon (Figure 1A) and no meconium passage was observed by the age of 4 d. Enema and rectal decompression were performed with the insertion of a rubber catheter and normal saline at 4 d of life. The first meconium passage after birth was observed only after normal saline enemas. Oral ibuprofen administration was ceased owing to abdominal distension, but was restarted at 10 d of life. After one cycle of ibuprofen had been completed, closure of the ductus arteriosus was demonstrated via echocardiography.
During the period of hospitalization, the donor infant showed feeding intolerance with marked dilatation of the sigmoid colon that required intermittent rectal decompression and glycerin enemas. We suspected congenital megacolon because of the dilatation of the sigmoid colon with an inversed sigmoid-rectum diameter ratio on lateral pelvic film at the age of 61 d (Figure 1B). The anus was located as usual on physical examination, and a 9 mm Hegar dilator could be inserted easily when the body weight was 2070 g. Therefore, we could rule out anal stenosis. A barium enema study revealed the transition zone in the recto-sigmoid region, with an inversed recto-sigmoid ratio.
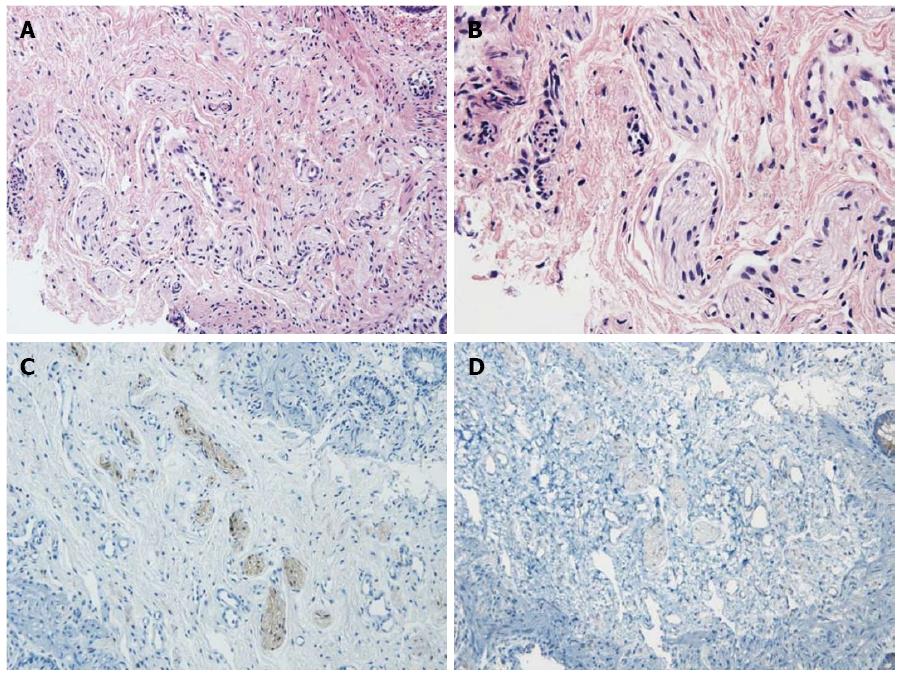
Retained barium contrast in the bowel could be seen on delayed film, obtained 48 h after the colon study, as well as 24 h after. Increased numbers of nerve fibers in the submucosal layer were observed, but no ganglion cells were found in a biopsy specimen obtained via rectal suction biopsy (Figure 2). The results of the rectal submucosal biopsy and barium colon study supported a diagnosis of Hirschsprung’s disease. Colostomy surgery was performed at 81 d of age. The operative finding showed marked dilatation immediately above the rectum, which indicated a short segment of an aganglionic lesion. After the operation, no further obstructive symptoms or findings of colonic dilatation on abdominal X-ray were observed.
TTTS is a severe complication of monochorionic twins that carries high mortality and morbidity[1-3]. Abnormal vascular connections between donor and recipient twin babies through the placenta can result in various morbidities, along with blood-volume fluctuations or ischemia[1-3]. The morbidities in twins with TTTS have been associated with volume overload in the recipient twin and with volume or oxygen depletion in the donor twin. Other consequences of TTTS in recipient infants include arterial cerebral stroke due to hyperviscosity, ventricular hypertrophy or right ventricular outflow-tract obstruction (pulmonary atresia or stenosis) associated with volume overload or an elevated level of endothelin-1, hepatic infarction, and intestinal injury[3,6].
A donor infant may experience hypoxic-ischemic injury to the brain, heart, kidneys, liver, or intestine[3]. Necrotizing enterocolitis, jejunal/ileal atresia, and perforation in the donor twin have been reported in previous studies[1-4]. It has been postulated that some intestinal abnormalities are caused by hypoperfusion and ischemic injury to intestine or embolic phenomena after laser ablation[3,4]. Laser ablation of aberrant connecting vessels between the twins can produce emboli by coagulating the vessels of the placenta. If these emboli settle in the mesenteric vascular bed, they can cause gangrene, resulting in stenosis, atresia, or perforation of the involved intestine[3-5]. It has also been reported that the incidence of non-cardiac structural anomalies is higher in donors[1]. Anomalies of the gastrointestinal system have included enteric duplication cysts, jejunal or ileal atresia, gastroschisis, and the absence of a gallbladder. Although there have been no previous reports of Hirschsprung’s disease in donor infants with TTTS, it may be one of the anomalies that accompany the symptom. Touloukian[7] described a premature infant with an aganglionic segment in the distal colon who experienced hypoxic damage. We postulated that the intrauterine hypoxemia in our patient might have caused the failure of neuroblast cell migration[7] or the destruction of ganglion cells in the gut[8].
The coexistence of Hirschsprung’s disease and anal stenosis has also been reported[8,9]. Anal stenosis can present with symptoms similar to those of short-segment Hirschsprung’s disease, including marked colonic dilatation and obstructive episodes. We ruled out the possibility of anal stenosis by inserting Hegar dilators larger than the normal anus of a newborn (1.3 + 3* birth weight)[10].
The clinical features of segmental dilatation of the colon are similar to those of Hirschsprung’s disease, including constipation and abdominal distension[11,12], but the histological findings are different, particularly the presence of ganglion cells in a circular layer of the intestine on the segmental dilation of the colon. In this case, no ganglion cells were observed in a submucosal biopsy specimen of the rectum.
In summary, we report a donor baby with symptoms of feeding intolerance and marked colon dilatation diagnosed with Hirschsprung’s disease and TTTS. In donor infants with TTTS who show delayed passage of meconium and intermittent obstructive episodes with feeding intolerance and colon dilatation on abdominal X-ray, Hirschsprung’s disease should be considered.
A donor baby with symptoms of feeding intolerance and marked colon dilatation was diagnosed with Hirschsprung’s disease and twin to twin transfusion syndrome (TTTS).
The patient was diagnosed with delayed meconium passage and colonic dilatation on abdominal X-ray.
The differential diagnosis included anal stenosis and other causes of delayed meconium passage and colonic dilatation.
Hemoglobin 3.4 g/dL on the first day of life.
Dilatation of the sigmoid colon with an inversed sigmoid-rectum diameter ratio on lateral pelvic film at the age of 61 d.
No ganglion cells were found in a rectal suction biopsy specimen. Elevated numbers of nerve bundles were noted in the submucosal layer.
Colostomy surgery.
In infants with TTTS who show symptoms such as feeding intolerance, delayed passage of meconium, and marked colon dilatation, Hirschsprung’s disease should be considered.
This report is well-organized and systemically analyzes the relationship between ischemia and pathogenesis of Hirschsprung’s disease in twin to twin transfusion syndrome. These findings are important to those with closely-related research interests. The results suggest that clinicians should consider Hirschsprung’s disease in infants with TTTS who show delayed passage of meconium and intermittent obstructive episodes.
P- Reviewer: Lee HW S- Editor: Ding Y L- Editor: Rutherford A E- Editor: Zhang DN
| 1. | Patel S, Randolph LM, Benirschke K, Llanes A, Yedigarova L, Chmait RH. Prevalence of noncardiac structural anomalies in twin-twin transfusion syndrome. J Ultrasound Med. 2012;31:555-560. [PubMed] |
| 2. | Tsukimori K, Yumoto Y, Masumoto K, Taguchi T, Kondo H, Sueishi K, Wake N. Ischemic ileal perforation in the donor of monochorionic twins complicated by twin-twin transfusion syndrome. Fetal Diagn Ther. 2009;26:173-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 3. | Lopriore E, Oepkes D, Walther FJ. Neonatal morbidity in twin-twin transfusion syndrome. Early Hum Dev. 2011;87:595-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Arul GS, Carroll S, Kyle PM, Soothill PW, Spicer RD. Intestinal complications associated with twin-twin transfusion syndrome after antenatal laser treatment: Report of two cases. J Pediatr Surg. 2001;36:301-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 5. | Saura L, Muñoz ME, Castañón M, Eixarch E, Corradini M, Aguilar C, Ma Ribó J. Intestinal complications after antenatal fetoscopic laser ablation in twin-to-twin transfusion syndrome. J Pediatr Surg. 2010;45:E5-E8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 6. | Mari G, Roberts A, Detti L, Kovanci E, Stefos T, Bahado-Singh RO, Deter RL, Fisk NM. Perinatal morbidity and mortality rates in severe twin-twin transfusion syndrome: results of the International Amnioreduction Registry. Am J Obstet Gynecol. 2001;185:708-715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 127] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Touloukian RJ. Acquired aganglionic megacolon in a premature infant: report of a case. Pediatrics. 1975;56:459-462. [PubMed] |
| 8. | Earlam RJ. A vascular cause for aganglionic bowel. A new hypothesis. Am J Dig Dis. 1972;17:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Das K, Alladi A, Kini U, Babu MK, D’Cruz AJ. Hirschsprung’s disease, associated rare congenital anomalies. Indian J Pediatr. 2001;68:835-837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | Devadas D, Curry J. Don’t be fooled by meconium. Arch Dis Child Educ Pract Ed. 2007;92:ep135-ep138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Mahadevaiah SA, Panjwani P, Kini U, Mohanty S, Das K. Segmental dilatation of sigmoid colon in a neonate: atypical presentation and histology. J Pediatr Surg. 2011;46:e1-e4. [PubMed] [DOI] [Full Text] |
| 12. | al-Salem AH, Grant C. Segmental dilatation of the colon. Report of a case and review of the literature. Dis Colon Rectum. 1990;33:515-518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 0.3] [Reference Citation Analysis (0)] |