Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17558
Revised: June 9, 2014
Accepted: July 11, 2014
Published online: December 14, 2014
Processing time: 274 Days and 22.5 Hours
AIM: To evaluate the feasibility of 3-Tesla magnetic resonance elastography (MRE) for hepatic fibrosis and to compare that with diffusion-weighted imaging (DWI) and gadoxetic acid-enhanced magnetic resonance (MR) imaging.
METHODS: Forty-two patients were included in the study. On MRE, mean stiffness values were measured on the elastograms in kilopascals. The apparent diffusion coefficient (ADC) of the liver was measured using DWI. On gadoxetic acid enhanced MR, the contrast enhancement index (CEI) was calculated as signal intensity (SI)post/SIpre, where SIpost is liver-to-muscle SI ratio on hepatobiliary phase images and SIpre is that on nonenhanced images. Correlation between aspartate aminotransferase to the platelet ratio index (APRI) and three MR parameters was assessed. Each MR parameter was compared between a hepatic fibrosis (HF) group and non-hepatic fibrosis (nHF) group.
RESULTS: Liver stiffness showed strong positive correlation with APRI [Spearman correlation coeffiecient (r) = 0.773, P < 0.0001], while ADC and CEI showed weak or prominent negative correlation (r = -0.28 and -0.321, respectively). In the HF group, only liver stiffness showed strong correlation with APRI (r = 0.731, P < 0.0001). Liver stiffness, ADC, and APRI were significantly different between the HF group and nHF group.
CONCLUSION: MRE at 3-Tesla could be a feasible method for the assessment of hepatic fibrosis.
Core tip: Magnetic resonance elastography at 3-Tesla could be a feasible method for the assessment of hepatic fibrosis and may have a role as a potential noninvasive modality which would replace liver biopsy.
- Citation: Park HS, Kim YJ, Yu MH, Choe WH, Jung SI, Jeon HJ. Three-Tesla magnetic resonance elastography for hepatic fibrosis: Comparison with diffusion-weighted imaging and gadoxetic acid-enhanced magnetic resonance imaging. World J Gastroenterol 2014; 20(46): 17558-17567
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17558.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17558
Hepatic fibrosis is a consequence of a wound-healing response to various types of chronic liver disease and injury[1]. Fibrosis can lead to cirrhosis, portal venous hypertension, and to development of hepatocellular carcinoma, which cause increased morbidity and mortality[2]. Information on the presence and degree of hepatic fibrosis will be useful in patients with chronic liver disease for therapeutic decisions or disease outcome predictions[1,3]. In this regard, until now, diagnosis and follow-up of chronic liver diseases has long relied on semiquantitative pathologic scoring systems such as the Metavir score, through liver biopsy[4]. However, biopsy has several well-known limitations including sampling error due to small sample volume, interobserver and intraobserver variabilities, invasiveness with associated morbidity, and low patient tolerance[5-7]. Owing to the aforementioned limitations of liver biopsy, there arises a clinical need for noninvasive alternative tools for assessment of hepatic fibrosis. Several noninvasive methods for staging liver fibrosis have been proposed. Biochemical tests include composite scores such as aspartate aminotransferase to the platelet ratio index (APRI) or FibroTest (BioPredictive, Paris, France), and serologic markers such as hyaluronic acid[8,9].
Recent advances in magnetic resonance (MR) imaging facilitated various functional MR imaging techniques and contributed to hepatic fibrosis evaluation[4]. Such MR imaging methods are diffusion-weighted imaging (DWI), perfusion-weighted imaging, hepatobiliary phase of gadoxetic acid-enhanced MR imaging, and MR elastography (MRE)[4,10]. DWI produces representative apparent diffusion coefficient (ADC) values by measuring random motion of water molecules[4,11]. Prior studies using DWI demonstrated lower ADC values in patients with liver fibrosis and inflammation compared with normal liver tissue, and therefore ADC values may have a role as an index for predicting advanced hepatic fibrosis[12-14]. Gadoxetic acid (Primovist; Bayer Schering Pharma, Berlin, Germany) is a liver specific MR contrast agent which carries combined perfusion and hepatocyte-selective properties[15-17]. Decreased hepatic parenchymal enhancement on hepatobiliary phase images suggests impaired gadoxetic acid uptake by the liver, which may be caused by a decreased number of functioning hepatocytes[15-17]. A recent study demonstrated the strong correlation of contrast enhancement degree at hepatobiliary phase images of gadoxetic acid enhanced MR with hepatic fibrosis stage[15-17].
MRE is a new noninvasive technique for quantitative imaging of the direct consequence of hepatic viscoelastic properties, based on the observation that fibrosis leads to increased tissue stiffness[4,9,18,19]. Studies performed so far have demonstrated that MRE is a promising method for the evaluation of hepatic fibrosis. Stiffness value measured by MRE showed at least moderate correlation with hepatic fibrosis stage determined by pathology, and diagnostic performance of MRE was at least equal or superior to other modalities such as gadoxetic acid-enhanced MR imaging, serum marker (APRI), or DWI[11,18,20-26].
Reports of previous MRE studies with regard to hepatic fibrosis were performed using 1.5-Tesla (T) MR machines[11,18,20-26]. Because MRE acquisition uses gradient echo sequences which are prone to susceptibility artifact, elastography imaging at a high tesla MR unit may be challenging. However, a recent study on MRE at 3-T in healthy volunteers demonstrated that liver stiffness measurements could be made without modifying the approach used at 1.5-T MR, and the obtained stiffness values were in agreement to those reported in studies at 1.5-T[27]. If so, the purpose of our study is to evaluate the feasibility of MRE at 3-T for the assessment of hepatic fibrosis based on APRI, and compare that with DWI and gadoxetic acid-enhanced MR imaging.
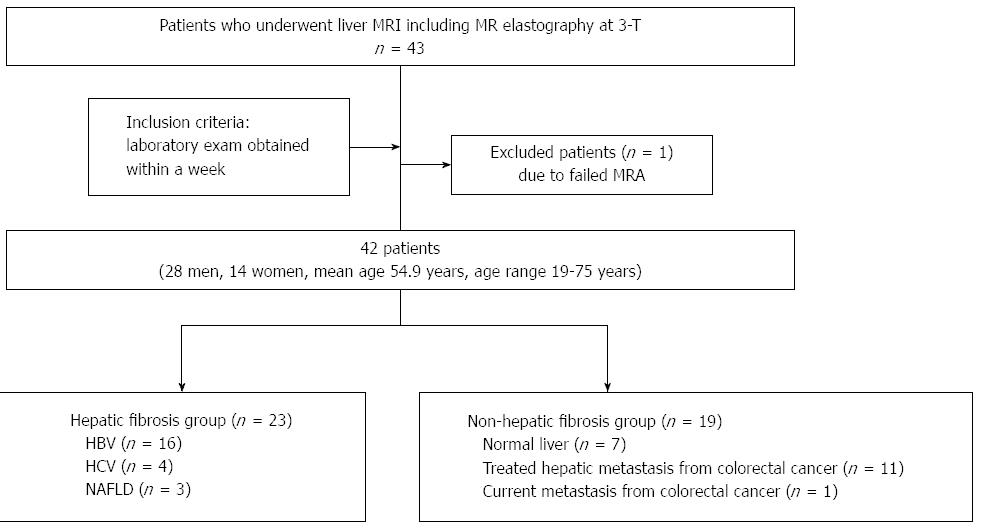
This study was approved by the Institutional Review Board of our hospital (KUH1140078), and patient informed consent was waived. Forty-three consecutive patients with suspected focal or diffuse liver disease underwent gadoxetic acid-enhanced MR imaging and DWI at 3-T. MRE exam was also performed as part of routine liver MR examination. All patients had laboratory exam data including serum aspartate aminotransferase and platelet count obtained within a week. One patient was excluded because of MRE signal intensity which was too low. The final study group consisted of 42 patients. There were 28 male and 14 female patients with a mean age of 54.9 years and age range from 19 to 75 years. Among the 42 patients, 23 patients had chronic liver disease or liver cirrhosis. The causes of chronic liver diseases or cirrhosis included chronic hepatitis B (n = 16), chronic hepatitis C (n = 4), and nonalcoholic fatty liver disease (n = 3). Twelve patients had treated (n = 11) or current (n = 1) hepatic metastasis from colorectal cancer, and the remaining 7 patients had normal liver parenchyma. The patients were classified into two groups. One was the hepatic fibrosis (HF) group including patients with viral hepatitis or nonalcoholic fatty liver disease (n = 23), and the other was the non-hepatic fibrosis (nHF) group, comprising the patients having a past or present history of hepatic metastasis, and normal liver parenchyma (n = 19). A flow chart of the profile based on recommended standards for reporting diagnostic accuracy is presented in Figure 1.
Gadoxetic acid-enhanced MR imaging: All MR examinations were performed on a 3-T MR unit (Magnetom Skyra, Siemens Medical Solutions, Erlangen, Germany) using 18-channel coils. The patients were examined in the supine position, and the receiver coil was positioned to cover the upper abdomen. Patients underwent a routine clinical imaging protocol of the liver, including breath-hold axial and coronal T2-weighted half-Fourier acquisition single shot turbo-spin echo (or single shot fast-spin echo), axial in- and opposed-phase chemical shift imaging, breath-hold T2-weighted fast-spin echo with fat suppression, and T1-weighted gradient-recalled echo fat-suppressed sequences before and after the injection of contrast agent. Arterial phase T1-weighted MR images were acquired during a single breath-hold of 20-35 s. Portal and equilibrium phase images were obtained 60 and 180 s after injecting contrast medium, and delayed hepatobiliary imaging was performed at 20 min. A bolus (0.025 mmol/kg body weight) of gadoxetic acid (Primovist) was administered into the antecubital vein at a rate of 1.5 mL/s. Parameters for T2-weighted fast-spin echo imaging protocol were as follows: acquisition method, breath-hold; repetition time/echo time, 3000/104 ms; flip angle, 136°; slice thickness, 5 mm; interslice gap, 1 mm; field of view, 380 mm; matrix size, 320 × 200. Parameters for T1-weighted three-dimensional gradient-recalled echo MR imaging protocol were as follows: technique, VIBE (Volumetric Interpolated Breath-hold Examination); repetition time/echo time, 3.52/1.37 ms; flip angle, 9°; slice thickness, 2.7 mm; interslice gap, none; field of view, 380 mm; matrix size, 480 × 263.
MRE: The 60 Hz acoustic wave was used as an active driver. A passive longitudinal shear-wave driver of 19-cm-diameter, 1.5-cm-thick cylindrical shape was placed against the right chest wall over the liver at the xiphoid process level. Then, by transmitting continuous acoustic vibration from the active driver to the passive driver through a flexible vinyl tube, propagation of shear waves in the liver was produced. The measurement parameters for MR elastographic gradient echo sequence were as follows: repetition time/echo time, 50/22.49 ms; flip angle, 25°; field of view, 30 cm; matrix size, 128 × 102; slice thickness, 5 mm; interslice gap, 1 mm. Four slices of MRE were obtained for each patient. Each slice took 22 s, and the patients were asked to hold their breath at the end-expiratory period to obtain a consistent position of the liver for each phase offset. When the acquisition was completed, the wave images were automatically processed by the MR scanner and images depicting tissue stiffness (elastograms) were generated. These quantitative images represented shear stiffness in units of kilopascals (kPa) and were displayed in a gray scale or with a color scale[19]. In addition, the elastogram was reviewed automatically by the intrinsic software for artifacts, such as significant wave interference or oblique wave propagation, and elastograms of 95% confidence mapping was produced by the exclusion of such area[28].
DWI: Diffusion-weighted single-shot echo-planar images were acquired with simultaneous use of free breathing method. Specific sequence parameters for DWI were as follows: b values, 50, 400, 800; repetition time/echo time, 5600/50 s; receiver bandwidth, 2442 Hz/pixel; matrix size, 84 × 128; slice thickness, 5 mm; interslice gap, 1 mm; number of slices acquired, 4; field of view, 380 × 309 mm2; aquisition time, 218 s; number of slices, 35; parallel imaging factor, 2.
MRE: The mean shear stiffness of the liver was calculated by placing the manually specified region of interest (ROI) into the stiffness map of MRE images. ROIs were drawn in the hepatic parenchyma excluding major blood vessels such as hepatic veins or portal veins and their large branches, liver edges, and motion artifacts. The stiffness values of the liver parenchyma were calculated by placing multiple ROIs (at least four), of circular shape and 1-2 cm diameter each[20]. ROI placement was performed by an abdominal radiologist who was blinded to the patient’s clinical and biochemical data. The mean stiffness values were measured in kPa.
DWI: For ADC value measurement, ROIs were placed on the ADC maps. At least four circular shaped ROIs of 1-2 cm were placed in the right lobe of the liver, almost the same location as those in MRE, and those multiple values were averaged. In addition, motion artifacts and liver tissue with poor signal noise were carefully avoided[11]. ADC values were automatically calculated on ADC maps using the SI within the manually drawn ROI and the following equation: ADC = [ln(S50) - ln(S400)]/(400 - 50), where S50 is the SI on DW images obtained with a b value of 50 s/mm2, and S400 is the SI on DW images obtained with a b value of 400 s/mm2[15].
CEI: The liver SI ratio was calculated as a ratio of the hepatic parenchyma SI to the paraspinal muscle SI on nonenhanced images [liver-to-muscle SI ratio (SIpre)] and the ratio of the hepatic SI to the paraspinal muscle SI on hepatobiliary phase images [liver-to-muscle SI ratio (SIpost)]. Contrast-enhancement index (CEI) was calculated as SIpost/SIpre[15].
APRI was calculated as follows: APRI= {[aspartate aminotransferase (AST) level (IU/L)/upper limit of AST level]/platelet count (× 109/L)} × 100[29].
Three MR parameters (liver stiffness, ADC and CEI) and APRI score was compared between the HF group and nHF group using Mann-Whitney U test. For correlation between the three MR parameters and APRI score in the HF group and nHF group separately, Spearman’s correlation was used. A P-value < 0.05 was considered statistically significant. Results of the statistical analysis were obtained using commercially available software (MedCalc, version 10.1.0.0; MedCalc Software, Mariakierke, Belgium).
Comparison of three MR parameters (liver stiffness, ADC, and CEI) with APRI score between the HF group and nHF group is shown in Table 1. The mean stiffness values of the liver, as measured by MRE, were 4.05 ± 2.06 kPa in the HF group and 2.1 ± 0.44 kPa in the nHF group. The HF group showed significantly higher stiffness values compared with those of the nHF group (P < 0.0001). ADC values measured on DWI were (1.04 ± 0.11) × 10-3 mm2/s in the HF group and (1.21 ± 0.12) × 10-3 mm2/s in the nHF group. ADC values were significantly lower in the HF group compared with those in the nHF group (P = 0.0001). CEI values measured on hepatobiliary phase images on gadoxetic acid-enhanced MR imaging were 1.58 ± 0.38 in the HF group and 1.70 ± 0.22 in the nHF group. CEI values were lower in the HF group compared with those of the nHF group, but the difference did not reach statistical significance (P = 0.3002). APRI score was 0.99 ± 0.67 in the HF group and 0.42 ± 0.36 in the nHF group. APRI score was significantly higher in the HF group (P = 0.0002).
| Liver stiffness (kPa) | ADC (× 10-3 mm2/s) | CEI | APRI | |||||
| HF | nHF | HF | nHF | HF | nHF | HF | nHF | |
| Sample size | 23 | 19 | 23 | 19 | 23 | 19 | 23 | 19 |
| Value range | 1.44-10.46 | 1.46-3.28 | 0.80-1.32 | 0.96-1.46 | 0.44-2.04 | 1.33-2.02 | 0.18-3.00 | 0.12-1.52 |
| Median | 3.79 | 2.04 | 1.04 | 1.20 | 1.63 | 1.71 | 0.77 | 0.33 |
| Mean ± SD | 4.05 ± 2.06 | 2.1 ± 0.44 | 1.04 ± 0.11 | 1.21 ± 0.12 | 1.57 ± 0.38 | 1.7 ± 0.22 | 0.99 ± 0.67 | 0.42 ± 0.36 |
| P value1 | < 0.0001 | 0.0001 | 0.3002 | 0.0002 | ||||
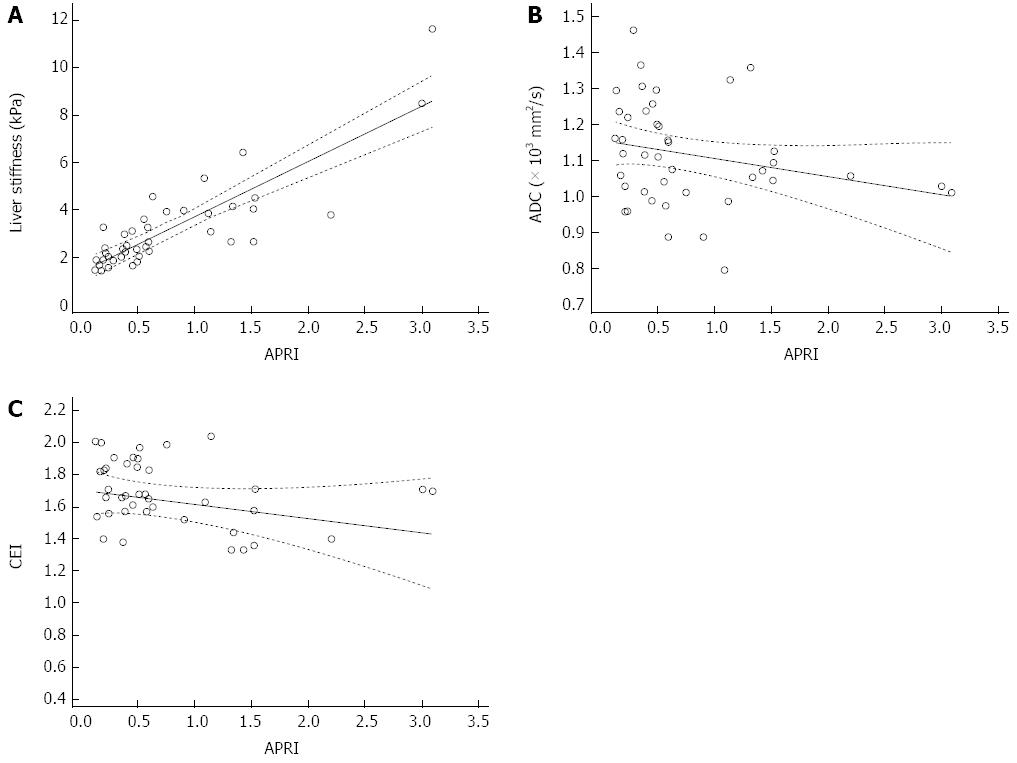
Liver stiffness showed strong positive correlation between APRI score [Spearman’s coefficient of rank correlation rho (r) = 0.773] and the correlation was statistically significant (P < 0.0001) (Table 2, Figure 2). ADC values and CEI showed weak negative correlation with APRI score (Spearman’s coefficient of rank correlation r = -0.28 and -0.321, respectively), and the correlation was significant in CEI and APRI (P = 0.038).
| Variable Y | Liver stiffness | ADC | CEI |
| Variable X | APRI | APRI | APRI |
| Sample size | 42 | 42 | 42 |
| Spearman's coefficient of rank correlation (r) | 0.773 | -0.28 | -0.321 |
| 95%CI for r | 0.613-0.872 | -0.538-0.026 | -0.570-(-0.019) |
| P value1 | < 0.0001 | 0.0723 | 0.038 |
When the correlation test was conducted between the three MR parameters and APRI score in each group separately, liver stiffness and APRI in the HF group showed strong correlation (r = 0.731) (P = 0.0001). Correlation between stiffness and APRI in the nHF group was moderate (r = 0.411) (P = 0.081). Correlation between ADC or CEI and APRI was weak in both groups (Table 3, Figures 3 and 4).
| Variable Y | Liver stiffness | ADC | CEI | |||
| Variable X | APRI | APRI | APRI | |||
| Group | HF | nHF | HF | nHF | HF | nHF |
| Sample size | 23 | 19 | 23 | 19 | 23 | 19 |
| Spearman's coefficient of rank correlation (r) | 0.731 | 0.411 | -0.128 | -0.0404 | -0.217 | -0.132 |
| 95%CI | 0.457-0.879 | -0.054-0.729 | -0.3-0.513 | -0.422-0.486 | -0.578-0.214 | -0.553-0.343 |
| for r | ||||||
| P value1 | 0.0001 | 0.081 | 0.561 | 0.870 | 0.319 | 0.591 |
This study using MRE at 3-T demonstrated that liver stiffness evaluation was feasible at 3-T MR in patients having various stages of hepatic fibrosis as well as in those having normal liver, without significant modification of the MRE protocol at 1.5-T. Our study also confirmed the previous preliminary result of a feasibility study done in a healthy population at 3-T[27]. In our study, liver stiffness measured by MRE was 4.05 ± 2.06 kPa in the HF group and 2.1 ± 0.44 kPa in the nHF group, and these measurements fell in the range of those reported in the previous MRE studies using 1.5-T and 3-T MR[20,24-27,30-32].
Three imaging parameters (liver stiffness, ADC and CEI) and one serologic test (APRI) in our study showed the capability of differentiating the HF group from the nHF group. The difference was statistically significant for liver stiffness, ADC and APRI. Particularly, the difference was most significant in the liver stiffness value measured by MRE (P < 0.0001). The superiority of MRE in the hepatic fibrosis evaluation, over other noninvasive methods such as various imaging studies or serologic tests is reported in many previous studies[11,18,22]. Previous studies of MRE in hepatic fibrosis demonstrated that MRE is accurate in the discrimination of moderate degree fibrosis (F2 or F3 and higher), and this ability is useful in determining candidates for antiviral treatment in patients having hepatitis C, and furthermore liver stiffness values increased in parallel with the fibrosis degree[11,12,20,26,33].
Compared with MRE, DWI showed limited ability in distinguishing fibrosis stage[11]. Even though DWI was comparable to MRE in the differentiation of the HF and the nHF group as shown in our study, and hepatic fibrosis stage ≥ F2 or ≥ F3 was predictable by ADC value measurement, ADC values did not significantly decrease with increasing histologic fibrosis stage[11,14,34]. Substantial overlap of ADC values between cirrhosis and no-to-moderate cirrhosis was also reported[11]. The exact mechanism of diffusion restriction in chronic liver disease is not clearly elucidated yet and is likely to be multifactorial, possibly related to the increased connective tissue lacking in proton and diminished capillary perfusion[4].
In our study, the degree of hepatic parenchymal enhancement on hepatobiliary phase images after gadoxetic acid administration (CEI) was lower in the HF group compared with that in the nHF group, but without statistical significance. Increased hepatic SI after gadoxetic acid enhancement is known to be associated with several factors such as hepatic function, clearance of indocyanine green, and serum bilirubin levels[15]. On the other hand, decreased hepatic enhancement on hepatobiliary phase suggests impaired uptake of gadoxetic acid by the liver, and impaired gadoxetic acid uptake may be due to decreased number of functioning hepatocytes or disturbed gadoxetic acid excretion due to hepatocytes dysfunction[15]. Previous study results of hepatic enhancement after gadoxetic acid on hepatic fibrosis evaluation indicated that this simple method is useful in the differentiation of mild fibrosis from no fibrosis[15,16].
Serum tests for staging hepatic fibrosis such as hyaluronic acid or N-terminal collagen III propeptide are available but their usefulness is limited because fibrosis is not a liver-specific phenomenon[18]. FibroTest (Biopredictive, Paris, France) and APRI methods rather depend on statistical approach for the prediction of fibrosis stage, because biochemical markers used in these tests have no direct relationship with the fibrosis[29]. APRI had a reliability for the prediction of severe fibrosis and cirrhosis and was superior to CEI for the discrimination of minimal and advanced fibrosis, although it was less accurate than MRE in the fibrosis staging[16,18,29]. Besides, it is a simple index using readily available laboratory results[18,29].
We used APRI score as a reference tool in comparison of three MR parameters in the hepatic fibrosis assessment in this study. Promisingly, among the three MR parameters, significant positive correlation was observed with liver stiffness measured by MRE and APRI score (correlation coefficient r = 0.773). When those two parameters were compared in the HF group and the nHF group separately, the HF group showed significant positive correlation (correlation coefficient r = 0.731), and these results are in accord to the previous comparison study of MRE and APRI score in hepatic fibrosis staging[18].
Even though plenty of reports on MRE in hepatic fibrosis staging have been or are being published, few use the 3-T MR machine and therefore the experiences of MRE in 3-T MR is limited so far. Because 3-T MR in abdominal imaging is widely used in many institutions nowadays owing to improved resolution, application of MRE in the 3-T should be positively considered. Despite the concern of susceptibility artifacts caused by gradient echo sequence in 3-T, several preliminary studies, including our study, showed the feasibility of MRE studies in 3-T MR, in various degrees of hepatic fibrosis patients as well as healthy volunteers[27].
Several limitations and comments in this study should be mentioned. First, because the categorization of patients as the HF group or the nHF group was performed on the basis of clinical diagnosis rather than histopathologic results, either stratification of fibrosis staging or performance study of the MR parameters was not achieved. There was no information available, therefore, on the relationship between mild or moderate fibrosis and MR imaging parameters. Instead, we used APRI score as a reference subject. Second, some patients who had received chemotherapy for colorectal cancers were included in the nHF group. Although those patients did not show liver function test abnormalities, the possible influence of chemotherapeutic agents on hepatic parenchymal elasticity cannot be excluded. Third, there were partial losses of elastogram in some patients in our study, probably due to artifacts caused by susceptibility on 3-T. We need to present quantitative results with regard to the image quality of MRE on 3-T, in comparison with that on 1.5-T MR in the future study. Fourth, interobserver variation regarding the measurement of MR parameters used in this study was not assessed because all of the measurement was performed by one radiologist. Fortunately, however, high reproducibility and repeatability of the stiffness measurement by MRE was proved in the prior study, even though the study was done in 1.5-T MR[32,35]. Also, ROI selection by one radiologist may be prone to subjectivity although it was done according to rules to avoid major hepatic vessels, liver edges, and motion artifact. The small sample size of the study population must be another drawback lastly, which limited the power of data analysis.
In conclusion, based on the results of our preliminary study, MRE on 3-T MR is a feasible imaging method in the evaluation of patients having various degrees of hepatic fibrosis. Further study accompanied by a histopathologic reference standard would consolidate the usefulness of MRE in 3-T MR as a potential noninvasive modality which would replace liver biopsy in hepatic fibrosis.
This work was supported by Konkuk University.
Information on the presence and degree of hepatic fibrosis will be useful in patients with chronic liver disease for therapeutic decisions or disease outcome predictions. Magnetic resonance elastography (MRE) is a new noninvasive technique for quantitative imaging of direct consequence of hepatic viscoelastic properties, based on the observation that fibrosis leads to increased tissue stiffness.
Studies performed so far have demonstrated that MRE is a promising method for the evaluation of hepatic fibrosis. A stiffness value measured by MRE showed at least moderate correlation with hepatic fibrosis stage determined by pathology and diagnostic performance of MRE was at least equal or superior to other modalities such as gadoxetic acid enhanced magnetic resonance (MR) imaging, serum marker such as aspartate aminotransferase to the platelet ratio index (APRI), or diffusion weighted imaging (DWI). In this study, the authors investigated the feasibility of MRE at 3-Tesla (T) for the assessment of hepatic fibrosis based on APRI, and compared that with DWI and gadoxetic acid-enhanced MR imaging.
Even though plenty of reports on MRE in the hepatic fibrosis staging have been or are being published, those using 3-T MR machines are few and therefore the experiences of MRE in the 3-T MR is limited so far. Because 3-T MR in abdominal imaging is widely used in many institutions nowadays owing to improved resolution, application of MRE in the 3-T should be positively considered. Despite the concern of susceptibility artifacts caused by gradient echo sequence in 3-T, several preliminary studies including this study showed the feasibility of MRE studies in 3-T MR, in the various degrees of hepatic fibrosis patients as well as healthy volunteers.
Based on the results of our preliminary study, MRE on 3-T MR is a feasible imaging method in the evaluation of patients having various degree of hepatic fibrosis. Further study accompanied by a histopathologic reference standard would consolidate usefulness of MRE in 3-T MR as a potential noninvasive modality which would replace liver biopsy in the hepatic fibrosis.
This is a highly interesting study demonstrating the utility of 3-T MR imaging for assessing patients with hepatic fibrosis. I have enjoyed reading this article because the authors wrote a very nice paper documenting their cross-sectional study in 42 patients. They presented solid data on the group separation and pairwise correlations based on regional parameters derived from MRE, Diffusion-weighted and gadoxetic acid-enhanced MR imaging. The results supported the conclusion that MRE at 3-T may be a feasible method for the assessment of hepatic fibrosis and provides a potential noninvasive modality which would replace liver biopsy in the future.
P- Reviewer: Ma Y S- Editor: Nan J L- Editor: O’Neill M E- Editor: Liu XM
| 1. | Rockey DC. Hepatic fibrosis, stellate cells, and portal hypertension. Clin Liver Dis. 2006;10:459-479, vii-viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 2. | Afdhal NH, Nunes D. Evaluation of liver fibrosis: a concise review. Am J Gastroenterol. 2004;99:1160-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 422] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 3. | Ghany MG, Strader DB, Thomas DL, Seeff LB. Diagnosis, management, and treatment of hepatitis C: an update. Hepatology. 2009;49:1335-1374. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2320] [Cited by in RCA: 2240] [Article Influence: 140.0] [Reference Citation Analysis (1)] |
| 4. | Taouli B, Ehman RL, Reeder SB. Advanced MRI methods for assessment of chronic liver disease. AJR Am J Roentgenol. 2009;193:14-27. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 150] [Cited by in RCA: 133] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 5. | Bravo AA, Sheth SG, Chopra S. Liver biopsy. N Engl J Med. 2001;344:495-500. [PubMed] |
| 6. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1569] [Article Influence: 68.2] [Reference Citation Analysis (0)] |
| 7. | Cadranel JF, Rufat P, Degos F. Practices of liver biopsy in France: results of a prospective nationwide survey. For the Group of Epidemiology of the French Association for the Study of the Liver (AFEF). Hepatology. 2000;32:477-481. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 731] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 8. | Rockey DC, Bissell DM. Noninvasive measures of liver fibrosis. Hepatology. 2006;43:S113-S120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 243] [Cited by in RCA: 249] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 9. | Huwart L, Sempoux C, Vicaut E, Salameh N, Annet L, Danse E, Peeters F, ter Beek LC, Rahier J, Sinkus R. Magnetic resonance elastography for the noninvasive staging of liver fibrosis. Gastroenterology. 2008;135:32-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 547] [Cited by in RCA: 539] [Article Influence: 31.7] [Reference Citation Analysis (0)] |
| 10. | Faria SC, Ganesan K, Mwangi I, Shiehmorteza M, Viamonte B, Mazhar S, Peterson M, Kono Y, Santillan C, Casola G. MR imaging of liver fibrosis: current state of the art. Radiographics. 2009;29:1615-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 192] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 11. | Wang Y, Ganger DR, Levitsky J, Sternick LA, McCarthy RJ, Chen ZE, Fasanati CW, Bolster B, Shah S, Zuehlsdorff S. Assessment of chronic hepatitis and fibrosis: comparison of MR elastography and diffusion-weighted imaging. AJR Am J Roentgenol. 2011;196:553-561. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 12. | Fujimoto K, Tonan T, Azuma S, Kage M, Nakashima O, Johkoh T, Hayabuchi N, Okuda K, Kawaguchi T, Sata M. Evaluation of the mean and entropy of apparent diffusion coefficient values in chronic hepatitis C: correlation with pathologic fibrosis stage and inflammatory activity grade. Radiology. 2011;258:739-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 93] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Taouli B, Chouli M, Martin AJ, Qayyum A, Coakley FV, Vilgrain V. Chronic hepatitis: role of diffusion-weighted imaging and diffusion tensor imaging for the diagnosis of liver fibrosis and inflammation. J Magn Reson Imaging. 2008;28:89-95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 171] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 14. | Sandrasegaran K, Akisik FM, Lin C, Tahir B, Rajan J, Saxena R, Aisen AM. Value of diffusion-weighted MRI for assessing liver fibrosis and cirrhosis. AJR Am J Roentgenol. 2009;193:1556-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 15. | Watanabe H, Kanematsu M, Goshima S, Kondo H, Onozuka M, Moriyama N, Bae KT. Staging hepatic fibrosis: comparison of gadoxetate disodium-enhanced and diffusion-weighted MR imaging--preliminary observations. Radiology. 2011;259:142-150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 155] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 16. | Motosugi U, Ichikawa T, Oguri M, Sano K, Sou H, Muhi A, Matsuda M, Fujii H, Enomoto N, Araki T. Staging liver fibrosis by using liver-enhancement ratio of gadoxetic acid-enhanced MR imaging: comparison with aspartate aminotransferase-to-platelet ratio index. Magn Reson Imaging. 2011;29:1047-1052. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 17. | Goshima S, Kanematsu M, Watanabe H, Kondo H, Kawada H, Moriyama N, Bae KT. Gd-EOB-DTPA-enhanced MR imaging: prediction of hepatic fibrosis stages using liver contrast enhancement index and liver-to-spleen volumetric ratio. J Magn Reson Imaging. 2012;36:1148-1153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Huwart L, Sempoux C, Salameh N, Jamart J, Annet L, Sinkus R, Peeters F, ter Beek LC, Horsmans Y, Van Beers BE. Liver fibrosis: noninvasive assessment with MR elastography versus aspartate aminotransferase-to-platelet ratio index. Radiology. 2007;245:458-466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 287] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 19. | Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37:544-555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 500] [Article Influence: 41.7] [Reference Citation Analysis (0)] |
| 20. | Kim BH, Lee JM, Lee YJ, Lee KB, Suh KS, Han JK, Choi BI. MR elastography for noninvasive assessment of hepatic fibrosis: experience from a tertiary center in Asia. J Magn Reson Imaging. 2011;34:1110-1116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 21. | Chen J, Talwalkar JA, Yin M, Glaser KJ, Sanderson SO, Ehman RL. Early detection of nonalcoholic steatohepatitis in patients with nonalcoholic fatty liver disease by using MR elastography. Radiology. 2011;259:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 336] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 22. | Rustogi R, Horowitz J, Harmath C, Wang Y, Chalian H, Ganger DR, Chen ZE, Bolster BD, Shah S, Miller FH. Accuracy of MR elastography and anatomic MR imaging features in the diagnosis of severe hepatic fibrosis and cirrhosis. J Magn Reson Imaging. 2012;35:1356-1364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 23. | Motosugi U, Ichikawa T, Koshiishi T, Sano K, Morisaka H, Ichikawa S, Enomoto N, Matsuda M, Fujii H, Araki T. Liver stiffness measured by magnetic resonance elastography as a risk factor for hepatocellular carcinoma: a preliminary case-control study. Eur Radiol. 2013;23:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 24. | Venkatesh SK, Wang G, Lim SG, Wee A. Magnetic resonance elastography for the detection and staging of liver fibrosis in chronic hepatitis B. Eur Radiol. 2014;24:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 25. | Kim D, Kim WR, Talwalkar JA, Kim HJ, Ehman RL. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology. 2013;268:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 183] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 26. | Yin M, Talwalkar JA, Glaser KJ, Manduca A, Grimm RC, Rossman PJ, Fidler JL, Ehman RL. Assessment of hepatic fibrosis with magnetic resonance elastography. Clin Gastroenterol Hepatol. 2007;5:1207-1213.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 771] [Cited by in RCA: 714] [Article Influence: 39.7] [Reference Citation Analysis (1)] |
| 27. | Mannelli L, Godfrey E, Graves MJ, Patterson AJ, Beddy P, Bowden D, Joubert I, Priest AN, Lomas DJ. Magnetic resonance elastography: feasibility of liver stiffness measurements in healthy volunteers at 3T. Clin Radiol. 2012;67:258-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 28. | Dzyubak B, Glaser K, Yin M, Talwalkar J, Chen J, Manduca A, Ehman RL. Automated liver stiffness measurements with magnetic resonance elastography. J Magn Reson Imaging. 2013;38:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 29. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3245] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 30. | Motosugi U, Ichikawa T, Amemiya F, Sou H, Sano K, Muhi A, Enomoto N, Araki T. Cross-validation of MR elastography and ultrasound transient elastography in liver stiffness measurement: discrepancy in the results of cirrhotic liver. J Magn Reson Imaging. 2012;35:607-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 31. | Yoon JH, Lee JM, Woo HS, Yu MH, Joo I, Lee ES, Sohn JY, Lee KB, Han JK, Choi BI. Staging of hepatic fibrosis: comparison of magnetic resonance elastography and shear wave elastography in the same individuals. Korean J Radiol. 2013;14:202-212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 32. | Lee YJ, Lee JM, Lee JE, Lee KB, Lee ES, Yoon JH, Yu MH, Baek JH, Shin CI, Han JK. MR elastography for noninvasive assessment of hepatic fibrosis: reproducibility of the examination and reproducibility and repeatability of the liver stiffness value measurement. J Magn Reson Imaging. 2014;39:326-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 84] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 33. | Martínez SM, Crespo G, Navasa M, Forns X. Noninvasive assessment of liver fibrosis. Hepatology. 2011;53:325-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 314] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 34. | Taouli B, Tolia AJ, Losada M, Babb JS, Chan ES, Bannan MA, Tobias H. Diffusion-weighted MRI for quantification of liver fibrosis: preliminary experience. AJR Am J Roentgenol. 2007;189:799-806. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 279] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 35. | Venkatesh SK, Wang G, Teo LL, Ang BW. Magnetic resonance elastography of liver in healthy Asians: normal liver stiffness quantification and reproducibility assessment. J Magn Reson Imaging. 2014;39:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |