Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17552
Revised: February 10, 2014
Accepted: June 2, 2014
Published online: December 14, 2014
Processing time: 373 Days and 16.7 Hours
AIM: To prospectively investigate the detection rate of laterally spreading tumors (LSTs) of the colorectum by computed tomography (CT) colonography (CTC).
METHODS: Patients with LSTs measuring ≥ 20 mm detected during colonoscopy were prospectively enrolled in the study. All patients underwent colonoscopy and subsequent CTC on the same day. CTC was performed using multi-detector CT without contrast in the prone and supine positions. Two radiologists blinded to the existence of LSTs read the virtual endoscopic images as well as 2-D images. LSTs were classified into granular and non-granular types based on colonoscopic appearance.
RESULTS: Forty-seven pathologically proven LSTs were evaluated prospectively. Histology included adenomas in 19, mucosal cancers in 19 and T1 cancers in 9. The mean diameter of the LSTs was 35.1 mm. Twenty-eight (60%) LSTs were correctly identified by CTC, and the configuration was similar to the colonoscopic appearance in most cases. Detection rate for the granular type was significantly higher than that for the non-granular type (71% vs 31%, P = 0.013). Detection rate of adenomas was significantly lower than mucosal cancers (32% vs 79%, P = 0.008) and T1 cancers (32% vs 78%, P = 0.042).
CONCLUSION: The detection rate of LSTs by CTC, particularly the non-granular type was not acceptable. Practitioners should be aware of the relatively low detection rate when using CTC.
Core tip: Laterally spreading tumors (LSTs) are a major target for colon screening. Nevertheless, it is still unknown what percentage of LSTs can be identified with computed tomography (CT) colonography (CTC). It has been reported that CTC may miss flat neoplastic lesions regardless of their size. It is a fascinating clinical question whether non-granular type LSTs, which have a very flat appearance on optical colonoscopy, can be identified with CTC. This study demonstrated that the detection rate of LSTs by CTC, particularly the non-granular type was not acceptable. Practitioners should be aware of the relatively low detection rate when using CTC.
- Citation: Togashi K, Utano K, Kijima S, Sato Y, Horie H, Sunada K, Lefor AT, Sugimoto H, Yasuda Y. Laterally spreading tumors: Limitations of computed tomography colonography. World J Gastroenterol 2014; 20(46): 17552-17557
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17552.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17552
Laterally spreading tumors (LSTs) in the large intestine are defined as an epithelial neoplasm measuring 1cm or greater with a low vertical axis that extends laterally along the luminal wall[1-4]. Histologically, more than 30% of LSTs contain high-grade dysplasia or invasive cancer[1,3]. Therefore, LSTs represent advanced lesions[5], and constitute a major target for colon screening. LSTs are divided into two subtypes based on their endoscopic morphology[1,2]. The LST polypoid type is referred to as the LST granular type (LST-G), whereas the LST non-polypoid type is referred to as the LST non-granular type (LST-NG). LST-NG have a biologically aggressive nature with a submucosal invasion rate higher than that of LST-G[1,3,4].
CT colonography (CTC) is an alternative to optical colonoscopy in a colon screening program[6]. Polyps measuring 1 cm or greater are identifiable by CTC with a greater than 90% detection rate[7,8]. However, it has been reported that flat adenomas can be easily missed with CTC[9]. Since the majority of LSTs show a flat appearance regardless of their diameter, it is still unknown what percentage of LSTs can be identified with CTC, thus being a key question to be addressed in a clinical trial. The aim of this study is to prospectively investigate the detection rate of LSTs by CTC.
Prior to commencement, the study was approved by the Institutional Review Board, and was registered at UMIN Clinical Trial Registry (ID: UMIN000002755). In April 2008 and February 2010, patients with LSTs measuring approximately 2 cm or greater on colonoscopy at Jichi Medical University Hospital were prospectively enrolled. The definition of LSTs is according to previous reports[1-4]. The morphology of LSTs was classified into LST-G and LST-NG based upon colonoscopic appearance. Written informed consent was obtained after colonoscopy if the lesion was suspicious for a LST measuring 2 cm or greater based on colonoscopic findings. Since accurate measurement of the diameter could not be made during colonoscopy, the final study group includes patients with lesions smaller than 2 cm as shown on the resected specimen. Lesions mimicking LSTs based on colonoscopic findings but invading to the muscularis propria or beyond were excluded from analysis.
Patients underwent bowel preparation with 2 litters of polyethylene glycol lavage solution (Niflec®, Ajinomoto Pharma, Tokyo, Japan) or 1.8 L of magnesium citrate solution (Magcorol P®, Horii Pharma, Tokyo, Japan). Prior to CTC, patients underwent conventional colonoscopy after administration of 10 mg of scopolamine butyl bromide or glucagon, but without sedation. Instead of electric cleansing with fecal tagging, the mucosal surface was cleansed by washing, particularly in the area where the lesions were located, and the luminal liquid aspirated as much as possible during colonoscopy. Biopsy specimens were not taken from the lesions. CTC was performed by 40-row or 64-row multi-detector CT immediately after colonoscopy. All patients were placed in the left lateral decubitus position, and an enema tube inserted into the anus. Room air was gently insufflated into the colon until the patient had abdominal distension. A standard scout image was obtained to assess colonic distension. The patient did not receive any contrast medium. CTC scans were obtained by MDCT (Sensation 40 or Definition; SIEMENS, Forchheim, Germany) with the patient in the prone and supine positions. The CT technique involved the use of 40 mm or 64 mm × 0.6 mm collimation, a slice width of 1.25 mm, a reconstruction interval of 1.0 mm, pitch of 0.9 and scanner settings of 120 kVp and 200 mAs.
Prior to the study, two radiologists (SK and YS) with at least 3 years’ experience in abdominal imaging received instruction by an expert radiologist (KU) in reading CTC images for two days and consequently read at least 100 CTC cases. Completely blinded to the location, number, size and configuration of the LSTs in the study group, examiners initially evaluated virtual colonoscopic images and then 2-D images (primary three-dimensional search method) using a workstation (ZAIO station NG1®, ZAIO Software, Tokyo, Japan) to screen for LSTs. If two examiners made a different judgement, they discussed the results. One of the authors (KU), an experienced radiologist who was not involved in initial lesion detection, matched the lesions found on CTC and colonoscopy on the basis of an established algorithm that incorporated the location of the lesion (within one colonic segment) and its size (within 50% of its reference standard measure)[10,11].
Patient age and size of the LSTs are expressed as mean ± SD. Detection rate is expressed as a percentage (95% confidence interval). The χ2 test or Fischer’s exact test was used to analyze the detection rate by morphology, anatomical location, size category and histology. All P values are two-tailed. P values less than 0.05 were considered to indicate statistical significance. All statistical analyses were performed with the use of Intercooled Stata 8.0® for Windows (Stata Corp., TX, United States).
Two patients refused participation in the study. Eight patients were excluded from analysis because two patients did not undergo resection and six patients proved to have a cancer invading to the muscularis propria or beyond in the resected specimen. A total of 44 patients (14 women, 30 men; age 66.6 ± 8.9 years, range 42-82 years) were studied prospectively. Bowel preparation was excellent for all patients. Three patients had synchronous double LSTs. Therefore, the total number of LSTs analyzed was 47. Six LSTs for which the maximum diameter was less than 20 mm in the resected specimens (15 mm in 2, 18 mm in 2 and 19 mm in 2) were included in the analysis. Table 1 shows the morphology, anatomical location, size category and histology of the LSTs. The mean diameter of the LSTs was 35.1 ± 21.0 mm and ranged from 15 mm to 100 mm.
| Morphology | Granular type | 34 (72) |
| Non-granular type | 13 (28) | |
| Anatomical location | Proximal colon | 18 (38) |
| Distal colon | 19 (40) | |
| Rectum | 10 (21) | |
| Size | 15-29 mm | 24 (51) |
| 30-39 mm | 11 (23) | |
| 40 mm or greater | 12 (26) | |
| Histology | Adenoma | 19 (40) |
| Mucosal cancer (Tis) | 19 (40) | |
| Invasive cancer (T1) | 9 (19) |
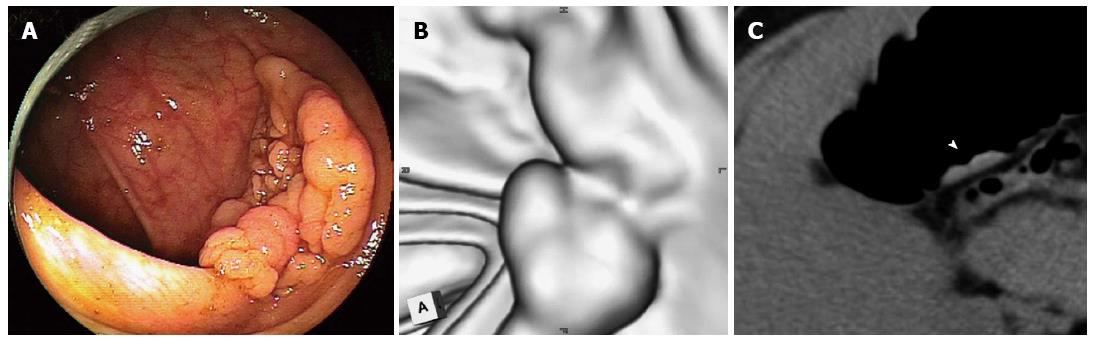
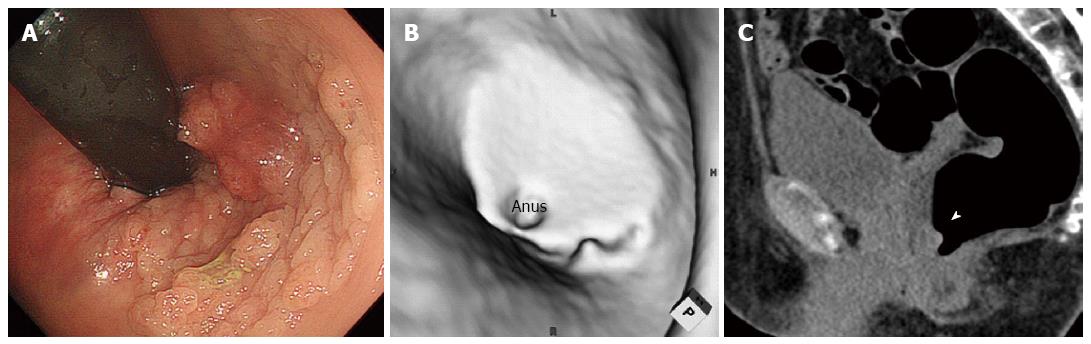
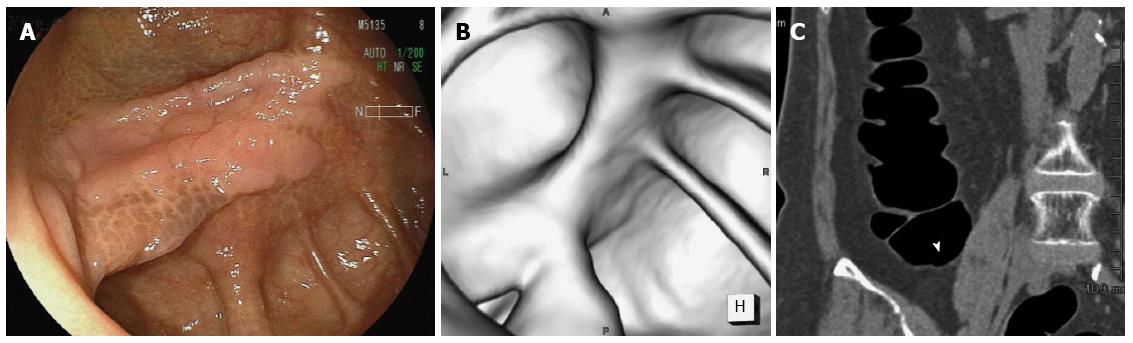
Twenty-eight (60%) of the LSTs were correctly identified by CTC, and the configuration was similar to the colonoscopic appearance in most cases. One example is shown in Figure 1. A 36 mm lesion located in the transverse colon reveals a multi-nodular configuration on optical colonoscopy (Figure 1A), which is classified as a LST-G. The virtual colonoscopic image resembles the optical colonoscopic image although grooves on the surface are not clearly depicted on virtual colonoscopy (Figure 1B). Multiplanar reconstruction imaging depicted the lesion clearly (arrow head, Figure 1C). Histologically, the lesion is an adenoma. Another example is shown in Figure 2. A 58 mm lesion is located close to the anus on optical colonoscopy (Figure 2A). On virtual colonoscopy, however, only a part of the lesion can be identified despite its large size (Figure 2B). On axial imaging (Figure 2C), a part of the lesion was visible (arrow head), similar to the virtual colonoscopic image. Accordingly, the lesion was judged as “unable to detected by CTC”. Histologically, the lesion is an adenoma. Another example is shown in Figure 3. On optical colonoscopy, this 30mm lesion is located at the bottom of the cecum. The demarcation line of the lesion is clearly depicted from the hue (Figure 3A). On virtual colonoscopy, in contrast, the lesion is unable to be detected because it is completely flat. Multiplanar reconstruction imaging was unable to depict the lesion, although the arrow-head indicates a possible location (Figure 3C). Histologically, the lesion is an adenoma.
Table 2 shows detection rates by morphology, anatomical location, size category (15-29 mm, 30-39 mm, 40 mm or greater) and histology. The detection rate for a LST-G was significantly higher than that for a LST-NG (71% vs 31%, P = 0.020). Correctly identified LSTs had a tendency toward a larger size, but there was no significant difference between the “30-39 mm” and “40 mm or greater” groups (P = 0.089). The detection rate for adenomas was significantly lower than that for mucosal cancers (32% vs 79%. P = 0.008) and T1 cancers (32% vs 78%, P = 0.042).
| n | Detection rate (95%CI) | P value | ||
| Overall | 47 | 60% (44-74) | ||
| Morphology | Granular type | 34 | 71% (53-85) | 0.013 |
| Non-granular type | 13 | 31% (9-61) | ||
| Location | Proximal colon | 18 | 44% (22-69) | 0.250 |
| Distal colon | 19 | 68% (43-87) | ||
| Rectum | 10 | 70% (35-93) | ||
| Size | 15-29 mm | 24 | 54% (33-74) | 0.134 |
| 30-39 mm | 11 | 45% (17-77) | ||
| 40 mm or greater | 12 | 83% (52-98) | ||
| Histology | Adenoma | 19 | 32% (13-57) | 0.006 |
| Mucosal cancer (Tis) | 19 | 79% (54-94) | ||
| Invasive cancer (T1) | 9 | 78% (40-97) |
Advanced lesions[5] defined as adenomas or cancers measuring 1 cm or greater, containing villous components or high-grade dysplasia on histological examination are the main targets for colon screening. According to the size criterion, LSTs are advanced lesions. In this study, LSTs measuring 20 mm or greater on colonoscopy were selected for the study group. However, the detection rate of LSTs was unsatisfactorily low to apply this technique for colon screening. In particular, the detection rate of LST-NG was approximately 30%, suggesting that LST-NG could not be detected using CTC.
Given that a flat morphology was defined as a broad-based lesion with a height of less than one half of its width[12], LST-NGs as well as LST-Gs are included as flat lesions. Flat morphology is considered one of the main causes for missed lesions evaluated by multi-detector row CTC[9]. For an expert radiologist, the detection rate of flat lesions is equivalent to that for polypoid lesions[12], but the positive predictive value for a flat lesion is lower than that for other types of colorectal lesions[13]. This implies that it is still difficult to screen for flat lesions even if the lesions are larger than 1 cm in diameter. Some authors have insisted that lesions with a height of 1 mm or less are not seen on CTC[14]. From this viewpoint, LST-NGs representing flat lesions with a very low axis cannot be identified by CTC. As shown in Figure 3, the hue rather than a slight difference in mucosal height is the key finding to identify the lesion by optical colonoscopy. This is strongly associated with a low detection rate by CTC for flat lesions.
In present study, the detection rate for both mucosal cancers and T1 cancers is nearly 80%, whereas that for adenomas is approximately 30%. A previous report using a computer-aided diagnosis system showed that the detection rate for flat T1 cancers is 83.3%[15] and that for stage Tis or T1 adenocarcinomas is 90%[16]. These facts demonstrate that most invasive LSTs can be detected by CTC and validate CTC as an alternative to optical colonoscopy in a colon screening program.
A possible limitation of this study is the experience level of the radiologists who read the CTC images. Two novice readers received systematic education for two days and consequently read at least 100 CTC cases. According to the latest report, novice CTC readers obtained sensitivity equal to that of experienced readers after practicing an average of 164 CTC studies[17]. These data suggest that novice readers may not reach the same proficiency as an experienced reader. It is also acknowledged that there is no appropriate control group for this study. Consequently, sensitivity as well as specificity cannot be calculated based on the study data.
Bowel preparation was excellent for all patients based on the appearance during optical colonoscopy carried out before the CTC procedure where actual cleansing was used in this study instead of electric cleansing with fecal tagging. Therefore, the detection rate to identify LSTs may actually be higher than that in the standard setting of CTC. Nevertheless, the detection rate of LSTs by CTC, particularly LST-NGs, was not acceptable even with advanced lesions. Practitioners should be aware of the relatively low detection rate when using CTC to screen for these lesions.
Laterally spreading tumors (LSTs) are advanced lesions of the colon, thus being a major target for screening. Computed tomography colonography (CTC) is an alternative to optical colonoscopy. Polyps measuring 1 cm or greater are identified by CTC with a greater than 90% detection rate. However, it has been reported that flat adenomas can be easily missed with CTC. Since the majority of LSTs show a flat appearance, it is still unknown what percentage of LSTs can be identified with CTC.
This is the first report to demonstrate that the detection rate of LSTs by CTC is relatively low. In particular, the detection rate of the non-granular type, which has an aggressive nature, is not acceptable in clinical practice.
Previous studies have reported a low detection rate of CTC for flat lesions, but no study has focused on large flat lesions measuring 2 cm or greater. This study demonstrates that CTC can miss flat neoplastic lesions regardless of their size.
Practitioners should be aware of the relatively low detection rate of LSTs by CTC, particularly LST-NGs, when using CTC to screen for these lesions. To use CTC widely for colon screening, it is imperative to develop a new method of CTC to detect such flat lesions as LSTs.
LSTs are defined as epithelial neoplasms measuring 1 cm or greater with a low vertical axis that extends laterally along the luminal wall. Based on their endoscopic morphology, LSTs are divided into two subtypes, including LST granular type (LST-G) and LST non-granular type (LST-NG). CTC is an imaging procedure that uses a CT scanner to produce two- and three-dimensional images of the large intestine. Virtual colonoscopy, as well as an air enema image, is obtained in the three-dimensional imaging.
The authors of this manuscript demonstrate that the detection rate of laterally spreading tumors using CTC is relatively low in prospective method. Overall, this study is interesting and valuable. In my opinion, this is priority publishing for the journal.
P- Reviewer: Nagata K, Sali L S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
| 1. | Kudo S, Kashida H, Tamura T, Kogure E, Imai Y, Yamano H, Hart AR. Colonoscopic diagnosis and management of nonpolypoid early colorectal cancer. World J Surg. 2000;24:1081-1090. [PubMed] |
| 2. | Hiraoka S, Kato J, Tatsukawa M, Harada K, Fujita H, Morikawa T, Shiraha H, Shiratori Y. Laterally spreading type of colorectal adenoma exhibits a unique methylation phenotype and K-ras mutations. Gastroenterology. 2006;131:379-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 3. | Uraoka T, Saito Y, Matsuda T, Ikehara H, Gotoda T, Saito D, Fujii T. Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut. 2006;55:1592-1597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 307] [Article Influence: 16.2] [Reference Citation Analysis (0)] |
| 4. | Kaku E, Oda Y, Murakami Y, Goto H, Tanaka T, Hasuda K, Yasunaga M, Ito K, Sakurai K, Fujimori T. Proportion of flat- and depressed-type and laterally spreading tumor among advanced colorectal neoplasia. Clin Gastroenterol Hepatol. 2011;9:503-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Winawer SJ, Zauber AG. The advanced adenoma as the primary target of screening. Gastrointest Endosc Clin N Am. 2002;12:1-9, v. [PubMed] |
| 6. | Levin B, Lieberman DA, McFarland B, Andrews KS, Brooks D, Bond J, Dash C, Giardiello FM, Glick S, Johnson D. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. Gastroenterology. 2008;134:1570-1595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1423] [Cited by in RCA: 1457] [Article Influence: 85.7] [Reference Citation Analysis (0)] |
| 7. | Pickhardt PJ, Choi JR, Hwang I, Butler JA, Puckett ML, Hildebrandt HA, Wong RK, Nugent PA, Mysliwiec PA, Schindler WR. Computed tomographic virtual colonoscopy to screen for colorectal neoplasia in asymptomatic adults. N Engl J Med. 2003;349:2191-2200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1495] [Cited by in RCA: 1282] [Article Influence: 58.3] [Reference Citation Analysis (0)] |
| 8. | Johnson CD, Chen MH, Toledano AY, Heiken JP, Dachman A, Kuo MD, Menias CO, Siewert B, Cheema JI, Obregon RG. Accuracy of CT colonography for detection of large adenomas and cancers. N Engl J Med. 2008;359:1207-1217. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 848] [Cited by in RCA: 702] [Article Influence: 41.3] [Reference Citation Analysis (0)] |
| 9. | Arnesen RB, Adamsen S, Svendsen LB, Raaschou HO, von Benzon E, Hansen OH. Missed lesions and false-positive findings on computed-tomographic colonography: a controlled prospective analysis. Endoscopy. 2005;37:937-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Park SH, Ha HK, Kim MJ, Kim KW, Kim AY, Yang DH, Lee MG, Kim PN, Shin YM, Yang SK. False-negative results at multi-detector row CT colonography: multivariate analysis of causes for missed lesions. Radiology. 2005;235:495-502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Johnson CD, Harmsen WS, Wilson LA, Maccarty RL, Welch TJ, Ilstrup DM, Ahlquist DA. Prospective blinded evaluation of computed tomographic colonography for screen detection of colorectal polyps. Gastroenterology. 2003;125:311-319. [PubMed] |
| 12. | Pickhardt PJ, Nugent PA, Choi JR, Schindler WR. Flat colorectal lesions in asymptomatic adults: implications for screening with CT virtual colonoscopy. AJR Am J Roentgenol. 2004;183:1343-1347. [PubMed] |
| 13. | Pickhardt PJ, Wise SM, Kim DH. Positive predictive value for polyps detected at screening CT colonography. Eur Radiol. 2010;20:1651-1656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Park SH, Ha HK, Kim AY, Kim KW, Lee MG, Kim PN, Shin YM, Byeon JS, Yang SK, Kim JH. Flat polyps of the colon: detection with 16-MDCT colonography--preliminary results. AJR Am J Roentgenol. 2006;186:1611-1617. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 15. | Taylor SA, Iinuma G, Saito Y, Zhang J, Halligan S. CT colonography: computer-aided detection of morphologically flat T1 colonic carcinoma. Eur Radiol. 2008;18:1666-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Park SH, Kim SY, Lee SS, Bogoni L, Kim AY, Yang SK, Myung SJ, Byeon JS, Ye BD, Ha HK. Sensitivity of CT colonography for nonpolypoid colorectal lesions interpreted by human readers and with computer-aided detection. AJR Am J Roentgenol. 2009;193:70-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Liedenbaum MH, Bipat S, Bossuyt PM, Dwarkasing RS, de Haan MC, Jansen RJ, Kauffman D, van der Leij C, de Lijster MS, Lute CC. Evaluation of a standardized CT colonography training program for novice readers. Radiology. 2011;258:477-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |