Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17541
Revised: March 27, 2014
Accepted: June 2, 2014
Published online: December 14, 2014
Processing time: 371 Days and 0.3 Hours
AIM: To investigate the significance of downregulation of liver fatty acid-binding protein (L-FABP) expression in hepatocellular carcinoma (HCC).
METHODS: Tissue microarrays of 146 cases of HCC were used to perform immunohistochemical staining for L-FABP. For each L-FABP-negative HCC, further immunohistochemical staining was performed using a representative whole-tissue section to confirm the downregulation of L-FABP expression and to assess the intratumoral heterogeneity of the staining pattern. Clinical data were retrieved from the clinical files, and histological slides were reviewed. Immunohistochemical staining for cytokeratin (CK) 7, CK 19, β-catenin, glutamine synthetase (GS), and serum amyloid A were also performed on the tissue microarrays. Clinicopathological features of the L-FABP-negative and L-FABP-positive HCC cases were compared. Furthermore, L-FABP and GS gene expression in HCC and cholangiocarcinoma cell lines were analyzed using real-time reverse transcription polymerase chain reaction. Mutation analysis of HNF1A [encoding hepatocyte nuclear factor 1 (HNF1)α] was performed for L-FABP-negative HCC cases.
RESULTS: Sixteen (10.9%) of the 146 cases of HCC stained negative for L-FABP. When we examined the correlation between the downregulation pattern of L-FABP and tumor size, most cases of smaller HCC (≤ 2 cm in diameter) exhibited focal downregulation, while most cases of larger HCC (> 2 cm in diameter) exhibited diffuse downregulation. The correlation was statistically significant (P = 0.036). When the HCC was smaller, the L-FABP-negative area often corresponded to a “nodule-in-nodule” appearance. Among the small HCC cases, tumor differentiation was significantly lower, and the frequency of intratumoral inflammation was significantly lower in L-FABP-negative cases than in L-FABP-positive cases (P = 0.032 and P = 0.009, respectively). The frequency of positivity for β-catenin and GS staining was significantly higher in L-FABP-negative cases of small HCC than in L-FABP-positive cases of small HCC (P = 0.009 and P = 0.000, respectively). Among six HCC cell lines examined, four showed higher expression of L-FABP, and the remaining two cell lines showed lower or no expression of L-FABP. Two of the 16 L-FABP-negative HCC cases possessed a mutation in exon 4 of HNF1A.
CONCLUSION: In smaller HCC, L-FABP downregulation probably occurs because of phenotypic changes during tumor progression. Moreover, this downregulation correlated with tumor differentiation and intratumoral inflammation.
Core tip: The significance of the downregulation of liver fatty acid-binding protein (L-FABP) expression in hepatocellular carcinoma (HCC) is largely unknown. In the present study, we performed immunohistochemical staining for L-FABP in 146 cases of HCC. We found that, in smaller HCC, L-FABP downregulation occurs, probably because of phenotypic changes during tumor progression. Moreover, L-FABP downregulation correlated with tumor differentiation and intratumor inflammation.
- Citation: Inoue M, Takahashi Y, Fujii T, Kitagawa M, Fukusato T. Significance of downregulation of liver fatty acid-binding protein in hepatocellular carcinoma. World J Gastroenterol 2014; 20(46): 17541-17551
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17541.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17541
Liver cancer is the fifth most common cancer in men worldwide and the seventh most common cancer in women. Furthermore, it has a high rate of mortality[1-4]. Hepatocellular carcinoma (HCC) accounts for approximately 90% of primary liver cancers[5]. Most cases of HCC are associated with liver cirrhosis related to chronic infection with hepatitis B virus (HBV) or hepatitis C virus (HCV). HCC is predominant among men, with the mean age of patients who are diagnosed with HCC being 55-65 years[1].
Hepatocellular adenoma (HCA) is a benign liver neoplasm comprising hepatocytes. The incidence of HCA is much lower than that of HCC, and 85% of all cases of HCA occur in young women[6]. The major risk factor for HCA is exposure to estrogenic or androgenic steroids, and most young women with HCA use oral contraceptives. Although rare, HCA may show malignant transformation to HCC[7-9]. HCA is a heterogeneous entity, and it is subclassified into four groups according to the genotype and phenotype: hepatocyte nuclear factor 1 (HNF1)α-inactivated HCA (H-HCA), β-catenin-activated HCA (β-HCA), inflammatory HCA (IHCA) and unclassified HCA[6,10].
Fatty acid-binding proteins (FABPs) bind and sequester potentially toxic long-chain fatty acids in the cytosol so that they may be rapidly removed via oxidative or storage organelles[11-13]. Mammals have a large family of FABPs. Liver-FABP (L-FABP or FABP1) is the first of the FABPs to be described so far. It is expressed in very high levels in liver, intestine and kidneys[11]. FABP1 is positively regulated by HNF1α. Downregulation of L-FABP expression is a characteristic feature of H-HCA cases[6,14]. Therefore, the absence of L-FABP expression in immunohistochemistry is an excellent diagnostic clue for H-HCA. This subtype of HCA has several unique clinicopathological features, including marked and diffuse steatosis, and absence of significant inflammation or nuclear atypia[6,14].
While the downregulation of L-FABP expression is critically important in the diagnostic classification of HCA, and is correlated with various clinicopathological features, the significance of downregulation of L-LABP expression in HCC is largely unknown. In the present study, we performed immunohistochemical staining of L-FABP in 146 cases of HCC, and investigated the clinicopathological characteristics of HCC in terms of its potential correlation with the downregulation of L-FABP expression.
One hundred and thirty six HCC cases were retrieved from the pathology archives of Toranomon Hospital, and 10 HCC cases were retrieved from the pathology archives of Teikyo University Hospital from 2003 to 2010. Clinical data, including age, sex, HBV or HCV infection, and Child-Pugh classification, were retrieved from the clinical files.
All cases of HCC were surgically resected. If multiple nodules were present in a patient, a single representative lesion was evaluated. Paraffin tissue sections were stained with hematoxylin-eosin, Masson, and reticulin. Histological slides were reviewed by two hepatopathologists (M.I. and T.F.). The results of the evaluation of the two pathologists did not differ considerably. If there were any minor differences of opinion, a final decision was made after discussion. For each tumor, the following variables were systematically recorded: tumor size, differentiation, pseudo-glandular formations, nuclear grade, tumor stage, presence of fatty change, inflammation, fibrosis and cholestasis. Tumor differentiation was evaluated according to WHO classification[15]. Nuclear grade was evaluated as grades 1-4 according to Armed Forces Institute of Pathology grading system[16] and classified as low-grade (grade 1 or 2) or high-grade (grade 3 or 4). Tumor stage was evaluated according to the General Rules for the Clinical and Pathological Study of Primary Liver Cancer in Japan[17]. Fatty change was defined by the presence of fat droplets in more than 10% of tumor cells. Inflammation was defined by the presence of focal or diffuse inflammatory infiltrate at 100× magnification. Fibrosis was defined by the presence of fibrous septa. Cholestasis was defined by the presence of bile pigment in tumor cells or dilated canaliculi. In addition, the non-tumorous liver tissue was evaluated and classified as normal liver, chronic hepatitis or liver cirrhosis.
For immunohistochemical staining, tissue microarrays of 146 cases of HCC were prepared using 3-mm tissue cores. Formalin-fixed paraffin-embedded tissue sections, cut at 3-μm thickness, were deparaffinized with xylene and rehydrated with graded ethanol. Immunohistochemistry was performed using Dako Autostaniner Link 48 (Dakocytomation, Glostrup, Denmark) and the following primary antibodies: L-FABP (polyclonal; Abcam, Cambridge, United Kingdom; 1:50 dilution), cytokeratin (CK) 7 (clone: OV-TL12/30; Dakocytomation; 1:60 dilution), CK 19 (clone: RCK108; Progen Biotechnik GmbH, Heidelberg, Germany; 1:60 dilution), β-catenin (clone: 14; BD biosciences, Franklin Lakes, NJ, United States; 1:100 dilution), glutamine synthetase (GS) (clone: GS-6; Merk Millipore, Billerica, MA, United States; 1:500 dilution) and serum amyloid A (SAA) (clone: 115; Abcam; 1:350 dilution). Both CK 7 and CK 19 are markers of cholangiocytes, β-catenin and GS are markers of β-HCA, and SAA is a marker of IHCA. After pretreatment, by heating in a water bath with citrate buffer (pH 6.0) for L-FABP, CK 19, β-catenin, GS, and SAA, and with ethylenediaminetetraacetic acid buffer (pH 9.0) for CK 7 at 98 °C for 40 min, endogenous peroxidase was quenched with 3% hydrogen peroxide in distilled water for 5 min. The slides were incubated with primary antibodies for 30 min at room temperature, and the sections were then stained by a detection method using EnVisonTM FLEX (Dakocytomation), according to the manufacturer’s protocol, and counterstained with hematoxylin.
After staining for L-FABP, the results were considered as positive if more than 5% of tumor cells stained positive. For each L-FABP-negative case of HCC, further immunohistochemical staining for L-FABP was performed using a representative whole-tissue section to confirm the downregulation of L-FABP expression and to assess the intratumoral downregulation pattern. In the evaluation of immunohistochemistry using whole-tissue sections, the staining was considered negative if the L-FABP labeling index was less than 5% in some tumor areas. In such cases, the downregulation pattern was considered as diffuse if the L-FABP labeling index was less than 5% in the whole tumor tissue, and it was considered as focal if the L-FABP labeling index was more than 5% in distinctive areas within the tumor. For CK 7 and CK 19, the staining was considered positive if more than 5% of tumor cells stained positive. For β-catenin, the staining was considered positive if the nuclei of the tumor cells stained positive, irrespective of the number of positive cells. For GS, the staining was considered positive if strong and diffuse expression was observed in the tumor tissue. For SAA, the staining was considered positive if more than 30% of tumor cells stained positive.
Clinicopathological features were compared between the L-FABP-negative and L-FABP-positive cases. We found that the immunohistochemical staining pattern for L-FABP tended to be different between smaller and larger HCCs; hence, we divided the cases into small (≤ 2 cm in diameter) and large (> 2 cm in diameter) HCC. In this study, we classified HCCs into small and large tumors using the above-mentioned standard, because tumor stage of HCC is classified using the same standard in the General Rules for the Clinical and Pathological Study of Primary Liver Cancer in Japan[17]. Actually, staging systems using the same standard have been reported to better reflect patients’ prognosis[18-20]. The ethics committee of Toranomon Hospital approved the overall design of the study.
To confirm downregulation of L-FABP expression in HCC in the in vitro system, we analyzed mRNA expression levels of the gene in six human HCC and two human cholangiocarcinoma cell lines by real-time reverse transcription polymerase chain reaction (real-time RT-PCR). We also analyzed mRNA expression levels of the GS gene using the same cell lines to confirm the correlation between L-FABP and GS expression. Six human HCC cell lines (HepG2, HuH7, PLC-PRF-5, Li-7, HLF, and HuH6) and two cholangiocarcinoma cell lines (HuCCT1 and RBE) were cultured, as previously described[21]. Total RNA was extracted from the eight cell lines using RNeasy Plus Mini kit (Qiagen, Valencia, CA, United States) and reverse transcribed using the QuantiTect Reverse Transcription kit (Qiagen). An Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany) assessed the quality and quantity of the RNA samples. An ABI 7300 real-time PCR system and the Power SYBR Green PCR Master Mix kit (Life Technologies, Carlsbad, CA, United States) performed the quantitative real-time PCR. The primers for complementary DNA amplification of L-FABP, GS, and TATA box-binding protein (TBP) genes were as follows: L-FABP, (forward) GCTGGGTCCAAAGTGATCCA and (reverse) TGTCACCTTCCAACTGAACCA; GS, (forward) GGTACTGGAGAAGGACTGCG and (reverse) CCATCGAAATTCCACTCAGGC; TBP, (forward) ACCACGGCACTGATTTTCAGTT and (reverse) GCATATTTTCTTGCTGCCAGTCT. TBP was used as an internal control. All the samples were assayed in triplicate. Absolute quantification of the copy number of each gene was performed using a standard curve constructed with serially diluted control plasmids obtained by TA cloning from the PCR products of normal liver tissue. The expression level of TBP mRNA normalized the mRNA expression level of each gene.
Mutation analysis of HNF1A: We performed mutation analysis of HNF1A for all L-FABP-negative HCC tissues. After deparaffinization and rehydration, DNA extraction was performed using QIAamp DNA FFPE Tissue Kit (Qiagen), following the manufacturer’s protocol. In HCC cases with focal downregulation of L-FABP, DNA was extracted separately from the L-FABP-positive and L-FABP-negative areas. A mutation was detected in both L-FABP-negative and L-FABP-positive areas in one HCC case, and the non-tumorous liver tissue was also examined in that case. We chose to study exons 3 and 4 of HNF1A because more than half of the mutations are reported to occur in these exons in HCAs[10,22]. The primers used for the mutation analysis were as follows: exon 3, (forward) TCTGTGCCTGCAGAGTTCAC and (reverse) CACTAGCGTCTCTCGCTCCT; exon 4, (forward) AGGTGCGTGTCTACAACTGG and (reverse) CCTTGTCCCCACATACCACT. After PCR was performed using a Veriti Thermal Cycler (Life Technologies), the amplicons of these genes were directly sequenced using a 3130xl Genetic Analyzer (Life Technologies) to determine mutations.
Statistical analysis was performed for the 146 HCC cases. Patients’ age and tumor size are presented as mean ± SD. Student’s t-test was performed to assess the significance of differences for these variables. The Mann-Whitney U test was used to compare tumor differentiation, tumor stage, background liver tissue, and Child-Pugh classification. Other frequency and categorical data were compared using Fisher’s exact test. P values of < 0.05 were considered statistically significant.
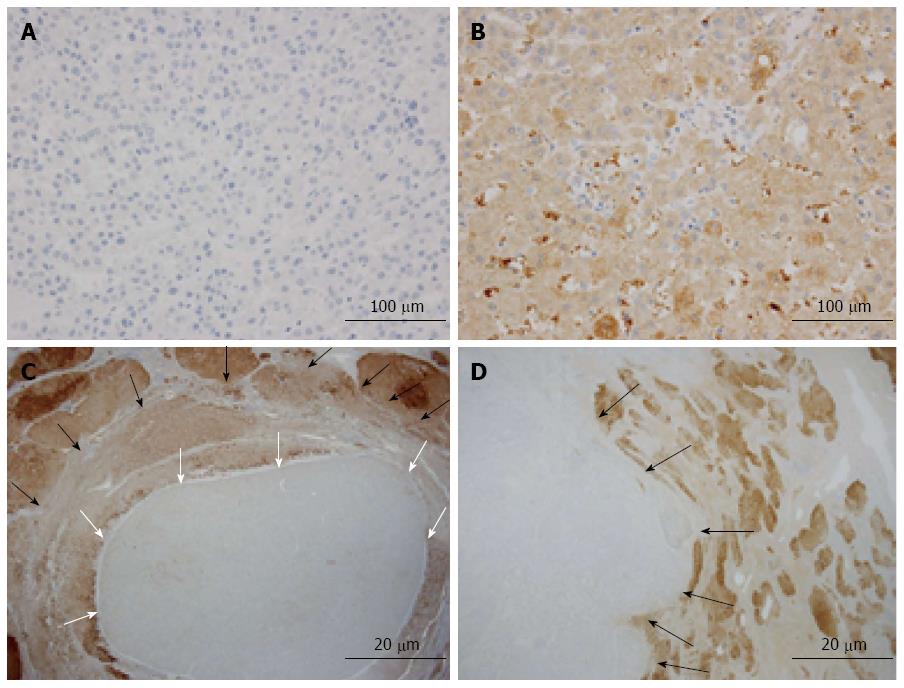
We evaluated the clinicopathological characteristics of 146 cases of HCC (Table 1). Sixteen (10.9%) of the 146 cases tested negative for L-FABP after immunohistochemical staining of tissue microarrays (Figure 1A, B). For each L-FABP-negative HCC, further immunohistochemical staining for L-FABP was performed using a representative whole-tissue section to confirm the downregulation of L-FABP expression and to assess the intratumoral heterogeneity of the staining pattern. As a result, the absence of L-FABP expression was confirmed using the whole-tissue section for all 16 cases. Among the above-mentioned 16 L-FABP-negative cases, ten cases exhibited focal downregulation (Figure 1C), and six cases exhibited diffuse downregulation (Figure 1D). Non-tumorous hepatocytes were positive for L-FABP in all cases.
| Characteristics | n = 146 |
| Age (yr) | 64.2 ± 10.1 |
| 36-60 | 49 (33.6) |
| 61-70 | 51 (34.9) |
| 71-82 | 46 (31.5) |
| Gender | |
| Male | 105 (72.0) |
| Female | 41 (28.0) |
| Viral infection | |
| HBV | 32 (22.0) |
| HCV | 98 (67.1) |
| NonB nonC | 16 (10.9) |
| Child-Pugh classification | |
| A | 139 (95.2) |
| B | 7 (4.8) |
| C | 0 (0) |
| Background liver tissue | |
| Normal | 4 (2.8) |
| Chronic hepatitis | 56 (39.7) |
| Liver cirrhosis | 81 (57.5) |
| Tumor size (mm) | 27.3 ± 25.4 |
| 8-10 | 7 (4.8) |
| 11-20 | 78 (53.4) |
| 21-30 | 29 (19.9) |
| 31-250 | 32 (21.9) |
| Differentiation | |
| Well | 33 (22.6) |
| Moderate | 93 (63.7) |
| Poor | 20 (13.7) |
| Pseudoglandular pattern | |
| Absent | 97 (66.4) |
| Present | 49 (33.6) |
| Nuclear grade | |
| Low | 116 (79.5) |
| High | 30 (20.5) |
| Fatty change | |
| Absent | 121 (82.9) |
| Present | 25 (17.1) |
| Inflammation | |
| Absent | 75 (51.4) |
| Present | 71 (48.6) |
| Fibrosis | |
| Absent | 104 (71.2) |
| Present | 42 (28.8) |
| Cholestasis | |
| Absent | 95 (65.0) |
| Present | 51 (35.0) |
| Tumor stage | |
| 1 | 65 (44.5) |
| 2 | 61 (41.8) |
| 3 | 16 (11.0) |
| 4 | 4 (2.7) |
| L-FABP immunostaining | |
| Negative | 16 (10.9) |
| Positive | 130 (89.1) |
| CK 7 immunostaining | |
| Negative | 68 (46.6) |
| Positive | 78 (53.4) |
| CK 19 immunostaining | |
| Negative | 136 (93.2) |
| Positive | 10 (6.8) |
| β-catenin immunostaining | |
| Negative | 101 (69.2) |
| Positive | 45 (30.8) |
| GS immunostaining | |
| Negative | 110 (75.3) |
| Positive | 36 (24.7) |
| SAA immunostaining | |
| Negative | 136 (93.2) |
| Positive | 10 (6.8) |
When the correlation between the downregulation pattern of L-FABP expression and tumor size was examined, among the above-mentioned 16 L-FABP-negative cases, most small HCC cases (≤ 2 cm in diameter) exhibited focal downregulation, and most large HCC cases (> 2 cm in diameter) exhibited diffuse downregulation. The correlation was statistically significant (P = 0.036) (Table 2). In small HCCs, the L-FABP-negative area often corresponded to a “nodule-in-nodule” appearance. The downregulation pattern of L-FABP expression tended to be different between small and large HCC; therefore, we divided the cases into small and large HCC in subsequent analyses.
Table 3 shows the relationship between the expression of L-FABP and clinical features. Among patients with small HCC, those who were negative for L-FABP were significantly older than those who were positive for L-FABP (71.3 ± 7.6 years vs 63.4 ± 9.3 years, P = 0.009). Conversely, in cases of large HCC, L-FABP-negative patients were significantly younger than L-FABP-positive patients (54.6 ± 10.8 years vs 64.8 ± 10.7 years, P = 0.044). No significant correlations were observed between the expression of L-FABP and sex, viral infection, or Child-Pugh classification.
| Characteristics | Tumor size ≤2 cm | P value | Tumor size > 2 cm | P value | Total | P value | |||
| L-FABP negative n = 11 | L-FABP positive n = 74 | L-FABP negative n = 5 | L-FABP positive n = 56 | L-FABP negative n = 16 | L-FABP positive n = 130 | ||||
| Age | 71.3 ± 7.6 | 63.4 ± 9.3 | 0.009b | 54.6 ± 10.8 | 64.8 ± 10.7 | 0.044a | 66.0 ± 11.5 | 64.0 ± 9.9 | 0.462 |
| Gender | 0.729 | 0.320 | 0.771 | ||||||
| Male | 7 (63.6) | 52 (70.3) | 5 (100) | 41 (73.2) | 12 (75.0) | 93 (71.5) | |||
| Female | 4 (36.4) | 22 (29.7) | 0 (0) | 15 (26.8) | 4 (25.0) | 37 (28.5) | |||
| Viral infection | |||||||||
| HBV | 3 (27.3) | 16 (21.6) | 0.465 | 2 (40.0) | 11 (19.6) | 0.286 | 5 (31.3) | 27 (20.8) | 0.253 |
| HCV | 7 (63.6) | 50 (67.6) | 0.732 | 3 (60.0) | 38 (67.9) | 0.806 | 10 (62.5) | 88 (67.7) | 0.761 |
| NonB nonC | 1 (9.1) | 8 (10.8) | 0.671 | 0 (0) | 7 (12.5) | 0.531 | 1 (6.2) | 15 (11.5) | 0.451 |
| Child-Pugh classification | 0.499 | 0.540 | 0.343 | ||||||
| A | 11 (100) | 71 (95.9) | 5 (100) | 52 (92.9) | 16 (100) | 123 (94.6) | |||
| B | 0 (0) | 3 (4.1) | 0 (0) | 4 (7.1) | 0 (0) | 7 (5.4) | |||
| C | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
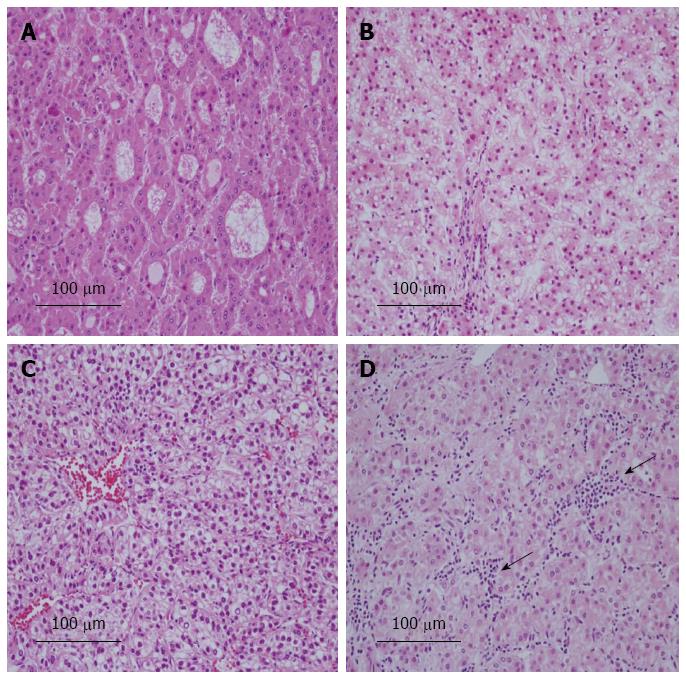
Table 4 shows the relationship between the expression of L-FABP and pathological features. Tumor differentiation was significantly poorer in L-FABP-negative small HCCs than in L-FABP-positive small HCCs (P = 0.032) (Figure 2A, B). All L-FABP-negative HCCs were moderately or poorly differentiated, irrespective of the tumor size. Furthermore, in small HCCs, the frequency of intratumoral inflammation was significantly lower in L-FABP-negative cases than in L-FABP-positive cases (P = 0.009) (Figure 2C, D). In large HCCs, no significant correlations were observed between the expression of L-FABP and the pathological features examined.
| Characteristics | Tumor size ≤2 cm | P value | Tumor size > 2 cm | P value | Total | P value | |||
| L-FABP negative n = 11 | L-FABP positive n = 74 | L-FABP negative n = 5 | L-FABP positive n = 56 | L-FABP negative n = 16 | L-FABP positive n = 130 | ||||
| Tumor size (mm) | 16.0 ± 2.1 | 15.8 ± 3.3 | 0.566 | 45.4 ± 29.5 | 43.3 ± 33.6 | 0.931 | 25.2 ± 20.8 | 27.6 ± 26.0 | 0.652 |
| Differentiation | 0.032a | 0.766 | 0.110 | ||||||
| Well | 0 (0.0) | 26 (35.1) | 0 (0.0) | 7 (12.5) | 0 (0.0) | 33 (25.4) | |||
| Moderate | 10 (90.9) | 43 (58.1) | 4 (80.0) | 36 (64.3) | 14 (87.5) | 79 (60.8) | |||
| Poor | 1 (9.1) | 5 (6.8) | 1 (20.0) | 13 (23.2) | 2 (12.5) | 18 (13.8) | |||
| Pseudoglandular pattern | 0.478 | 0.218 | 0.461 | ||||||
| Absent | 8 (72.7) | 49 (66.2) | 2 (40.0) | 38 (67.9) | 10 (62.5) | 87 (66.9) | |||
| Present | 3 (27.3) | 25 (33.8) | 3 (60.0) | 18 (32.1) | 6 (37.5) | 43 (33.1) | |||
| Nuclear grade | 0.299 | 0.641 | 0.424 | ||||||
| Low | 8 (72.7) | 62 (83.8) | 4 (80.0) | 42 (75.0) | 12 (75.0) | 104 (80.0) | |||
| High | 3 (27.3) | 12 (16.2) | 1 (20.0) | 14 (25.0) | 4 (25.0) | 26 (20.0) | |||
| Fatty change | 0.659 | 0.436 | 0.458 | ||||||
| Absent | 9 (81.8) | 60 (81.1) | 5 (100.0) | 47 (83.9) | 14 (87.5) | 107 (82.3) | |||
| Present | 2 (18.2) | 14 (18.9) | 0 (0.0) | 9 (16.1) | 2 (12.5) | 23 (17.7) | |||
| Inflammation | 0.009b | 0.660 | 0.039a | ||||||
| Absent | 9 (81.8) | 29 (39.2) | 3 (60.0) | 34 (60.7) | 12 (75.0) | 63 (48.5) | |||
| Present | 2 (18.2) | 45 (60.8) | 2 (40.0) | 22 (39.3) | 4 (25.0) | 67 (51.5) | |||
| Fibrosis | 0.160 | 0.466 | 0.105 | ||||||
| Absent | 10 (90.9) | 53 (71.6) | 4 (80.0) | 37 (66.1) | 14 (87.5) | 90 (69.2) | |||
| Present | 1 (9.1) | 21 (28.4) | 1 (20.0) | 19 (33.9) | 2 (12.5) | 40 (30.8) | |||
| Cholestasis | 0.224 | 0.369 | 0.120 | ||||||
| Absent | 9 (81.8) | 48 (64.9) | 4 (80.0) | 34 (60.7) | 13 (81.3) | 82 (63.1) | |||
| Present | 2 (18.2) | 26 (35.1) | 1 (20.0) | 22 (39.3) | 3 (18.7) | 48 (36.9) | |||
| Tumor stage | 0.833 | 0.789 | 0.646 | ||||||
| 1 | 8 (72.7) | 56 (75.7) | 0 (0.0) | 1 (1.8) | 8 (50.0) | 57 (43.8) | |||
| 2 | 3 (27.3) | 18 (24.3) | 3 (60.0) | 37 (66.1) | 6 (37.5) | 55 (42.3) | |||
| 3 | 0 (0.0) | 0 (0.0) | 2 (40.0) | 14 (25.0) | 2 (12.5) | 14 (10.8) | |||
| 4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (7.1) | 0 (0.0) | 4 (3.1) | |||
| Background liver tissue | 0.649 | 0.394 | 0.301 | ||||||
| Normal | 0 (0.0) | 0 (0.0) | 0 (0.0) | 4 (7.7) | 0 (0.0) | 4 (3.2) | |||
| CH | 3 (27.3) | 25 (34.2) | 2 (40.0) | 26 (50.0) | 5 (31.3) | 51 (40.8) | |||
| LC | 8 (72.7) | 48 (65.8) | 3 (60.0) | 22 (42.3) | 11 (68.7) | 70 (56.0) | |||
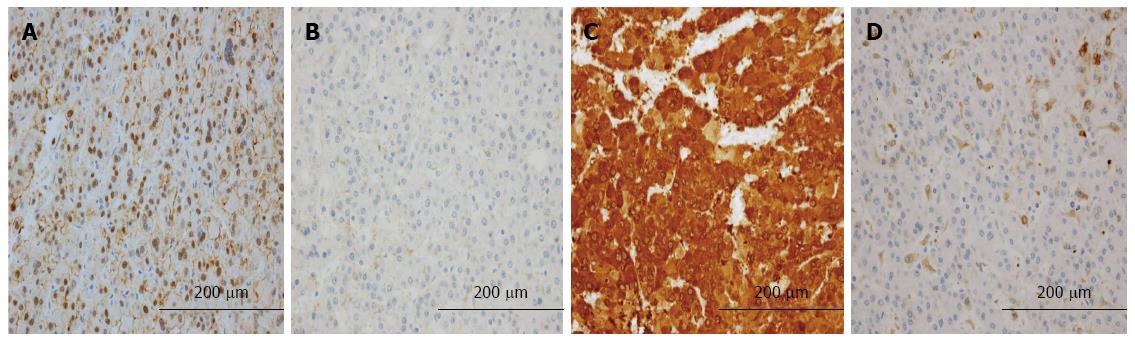
Table 5 shows the relationship between the expression of L-FABP and the expression of other immunohistochemical markers. The frequency of positivity for β-catenin (Figure 3A, B) and GS (Figure 3C, D) was significantly higher in L-FABP-negative small HCCs than in L-FABP-positive small HCCs (P = 0.009 and P = 0.000, respectively). In large HCCs, no significant correlations were observed between the expression of L-FABP and the expression of other immunohistochemical markers.
| Immunostaining | Tumor size ≤2 cm | P value | Tumor size > 2 cm | P value | Total | P value | |||
| L-FABP negative n = 11 | L-FABP positive n = 74 | L-FABP negative n = 5 | L-FABP positive n = 56 | L-FABP negative n = 16 | L-FABP positive n = 130 | ||||
| CK 7 | 0.430 | 0.515 | 0.512 | ||||||
| Negative | 4 (36.4) | 33 (44.6) | 3 (60.0) | 28 (50.0) | 7 (43.8) | 61 (46.9) | |||
| Positive | 7 (63.6) | 41 (55.4) | 2 (40.0) | 28 (50.0) | 9 (56.2) | 69 (53.1) | |||
| CK 19 | 0.423 | 0.703 | 0.301 | ||||||
| Negative | 11 (100) | 68 (91.9) | 5 (100) | 52 (92.9) | 16 (100) | 120 (92.3) | |||
| Positive | 0 (0) | 6 (8.1) | 0 (0) | 4 (7.1) | 0 (0) | 10 (7.7) | |||
| β-catenin | 0.009b | 0.433 | 0.073 | ||||||
| Negative | 4 (36.4) | 57 (77.0) | 4 (80.0) | 36 (64.3) | 8 (50.0) | 93 (71.5) | |||
| Positive | 7 (63.6) | 17 (23.0) | 1 (20.0) | 20 (35.7) | 8 (50.0) | 37 (28.5) | |||
| GS | 0.000b | 0.393 | 0.001b | ||||||
| Negative | 3 (27.3) | 62 (83.8) | 3 (60.0) | 42 (75.0) | 6 (37.5) | 104 (80.0) | |||
| Positive | 8 (72.7) | 12 (16.2) | 2 (40.0) | 14 (25.0) | 10 (62.5) | 26 (20.0) | |||
| SAA | 0.268 | 0.918 | 0.301 | ||||||
| Negative | 11 (100) | 65 (87.8) | 5 (100) | 55 (98.2) | 16 (100) | 120 (92.3) | |||
| Positive | 0 (0) | 9 (12.2) | 0 (0) | 1 (1.8) | 0 (0) | 10 (7.7) | |||
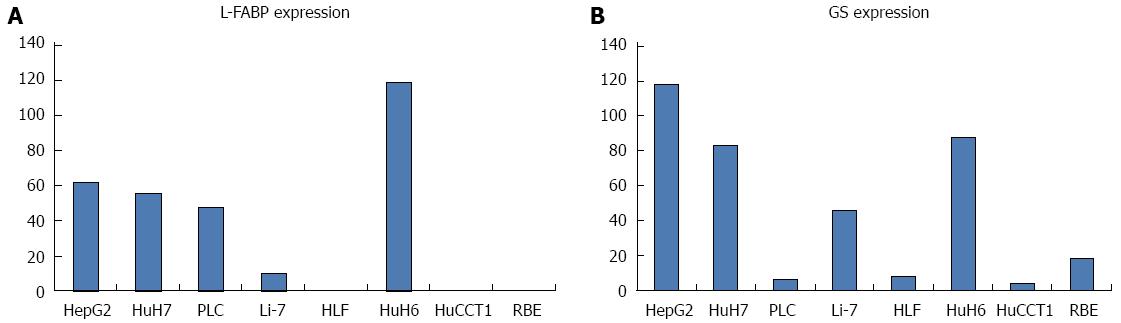
Figure 4A, B show the expression levels of L-FABP and GS mRNA in each cell line, as determined by real-time RT-PCR. Among the six HCC cell lines examined, HepG2, HuH7, PLC, and HuH6 showed higher expression of L-FABP. However, Li-7 and HLF showed lower expression of L-FABP; in particular, L-FABP expression was almost undetectable in HLF. Expression levels of L-FABP were very low or undetectable in the two CC cell lines examined (HuCCT1 and RBE). No obvious correlation was observed between L-FABP and GS expression in HCC cell lines.
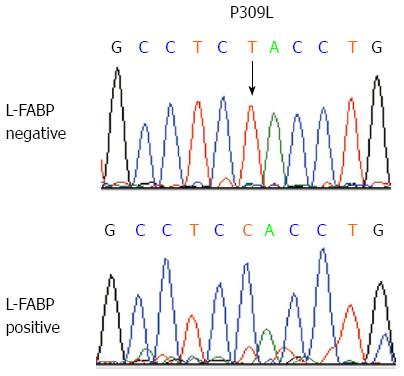
Mutation of HNF1A in L-FABP-negative HCCs: We performed mutation analysis of exons 3 and 4 of HNF1A for the 16 L-FABP-negative HCC cases and found that two cases had mutations. Both of the cases showed focal downregulation of L-FABP. One case had a missense mutation (926C>T, P309L) only in the L-FABP-negative area (Figure 5). The other case had a frame-shift mutation (872_873delC, P291fs) in both the L-FABP-negative and L-FABP-positive tumor areas. In this case, the L-FABP labeling index in the L-FABP-positive tumor area was relatively low (approximately 10%). Mutations were not observed in the non-tumorous liver tissue in this case. The remaining 14 tumors had no mutations.
In the present study, we performed immunohistochemical staining for L-FABP using 146 cases of HCC. In small (≤ 2 cm in diameter) HCCs, focal downregulation of L-FABP expression was usually noted, and it was significantly correlated with the patient age, tumor differentiation, and the frequency of intratumor inflammation, as well as the expression of β-catenin and GS.
In recent years, the molecular and clinicopathological study of HCA has progressed, and HCA has been divided into four subtypes: H-HCA, β-HCA, IHCA, and unclassified HCA[6,10]. Immunohistochemical staining for L-FABP, β-catenin, GS, and SAA is critical for the pathological diagnosis of these four subtypes. The absence of L-FABP expression is characteristic of H-HCA. Strong and diffuse expression of GS (a target of β-catenin), which is associated with aberrant cytoplasmic and nuclear expression of β-catenin, is characteristic of β-HCA. IHCA is characterized by the increased expression of SAA. Each HCA subtype has unique clinicopathological features. H-HCA shows typically prominent and diffuse steatosis, as well as the absence of substantial inflammation or nuclear atypia. In β-HCA, which has an increased risk of malignant transformation, steatosis and inflammation are usually absent, and nuclear atypia and a pseudo-glandular growth patterns are frequently observed. IHCA typically exhibits focal or diffuse inflammation and sinusoidal dilatation. However, the significance of these immunohistochemical markers, especially L-FABP in HCC, is largely unknown.
In the present study, 10.9% of the cases of HCC exhibited downregulation of L-FABP expression, suggesting that downregulation of the protein is not specific to HCA. In small HCCs, we often noted focal downregulation of L-FABP expression, and this often corresponded to the “nodule-in-nodule” appearance. Conversely, in large HCCs, we often noted diffuse downregulation of L-FABP expression in the tumor tissue. In general, the tumor tissue of H-HCA is almost completely negative for L-FABP[6]. Therefore, most small HCCs with downregulation of L-FABP expression do not represent malignant transformation of H-HCA, but rather represent phenotypic changes in tumor progression. The fact that differentiation of L-FABP-negative cases was significantly lower than that of L-FABP-positive cases in the present study reinforces this hypothesis. In large HCCs, although the regions with such phenotypic changes may grow to demonstrate diffuse distribution, several cases may represent malignant transformation of H-HCA. In general, HCAs exhibiting malignant transformation are large, and the diameters of such tumors are usually greater than 5 cm[23]. In addition, individuals with HCA are much younger than those with HCC[6]. In the present study, among those with large HCC, L-FABP-negative patients were significantly younger than L-FABP-positive patients. This distribution is probably because several L-FABP-negative cases of large HCC represent malignant transformation of H-HCA. However, no obvious component of HCA was confirmed in any of the cases upon pathological review. The reason behind the age-related differences, where L-FABP-negative patients were significantly older than L-FABP-positive patients for small HCCs, is difficult to answer, and further studies are needed to address this issue.
In small HCCs, the frequency of β-catenin and GS immunostaining were significantly higher in L-FABP-negative cases than in L-FABP-positive cases. In a previous examination of 96 liver tumors with a confirmed or possible diagnosis of HCA, no tumors were mutated in both HNF1α and β-catenin[10]. Accordingly, mutations of HNF1A (the gene encoding HNF1α) and β-catenin genes may be directly related to tumorigenesis in HCAs; however, mutations of these two genes might occur during tumor progression in HCCs. In the present study, the frequency of intratumoral inflammation in L-FABP-negative cases was significantly lower than that in L-FABP-positive cases of small HCC. This histopathological feature coincides with that of H-HCA and β-HCA. In general, steatosis is prominent, and nuclear atypia of tumor cells are inconspicuous in H-HCA[6,14]. However, in the present study, L-FABP-negativity was not significantly associated with steatosis and nuclear atypia in HCC. In general, nuclear atypia is prominent in β-HCA[6,14], and steatosis is inconspicuous in cases of HCC with a higher expression of GS[24]. Therefore, with regard to steatosis and nuclear atypia, the influence of HNF1α and L-FABP inactivation might have been counteracted by β-catenin and GS activation in small HCCs. In large HCCs, no obvious differences in pathological features were observed between L-FABP-negative and L-FABP-positive cases. This may be because various genetic mutations might have accumulated during tumor growth, thereby masking the influence of HNF1α and L-FABP inactivation.
In the real-time RT-PCR analysis using cell lines, many human HCC cell lines showed higher expression of L-FABP, but a small number of HCC cell lines showed lower or no expression of the gene. This result coincides with the results of immunohistochemical analysis using HCC tissues. In the immunohistochemical analysis using HCC tissues, significant correlation between L-FABP and GS expression was observed only in small tumors, and this correlation was not observed in the analysis of cell lines.
L-FABP is positively regulated by HNF1α. In the present study, we performed mutation analysis of exons 3 and 4 of HNF1A for 16 L-FABP-negative HCC cases, and found mutations in only two cases. In a previous study, 44 of 96 (46%) HCA cases possessed mutations in HNF1A, and those mutated cases showed phenotypes consistent with H-HCA[10]. In another study, 10 of 16 (63%) HCA cases possessed mutations in HNF1A[22]. More than half of the mutations were observed in exons 3 and 4 in those studies. Therefore, the frequency of mutations in exons 3 and 4 of HNF1A in L-FABP-negative HCCs in the present study was lower than that in HCAs. Many of the L-FABP-negative HCCs may possess inactivating mutations of HNF1A in sites other than in exons 3 and 4, or mechanisms other than mutations in HNF1A may be associated with the downregulation of L-FABP in HCC. In the present study, a mutation of HNF1A was observed not only in the L-FABP-negative area, but also in the L-FABP-positive area in one HCC. In this case, the labeling index of L-FABP in the L-FABP-positive tumor area was relatively low (approximately 10%). It is conceivable that admixed L-FABP-negative tumor cells had the mutation in HNF1A. This was a somatic mutation because no mutations were observed in the non-tumorous liver tissue.
To date, only one study has reported immunohistochemical staining for L-FABP using a series of HCC cases[25]. In that study, approximately 52% of the cases of HCC were positive for L-FABP. Accordingly, the frequency of downregulation of L-FABP expression was higher than in the present study. However, the results of both studies cannot be directly compared, because different antibodies and staining methods were used. In addition, in the previous study, one representative tissue section was immunostained for each case, and the detailed criteria for L-FABP positivity were not described. In the present study, we used tissue microarrays to examine the frequency of L-FABP-positive or L-FABP–negative cases because it was difficult to perform immunohistochemical staining using whole-tissue sections for as many as 146 HCC cases. We evaluated the results of immunohistochemical staining for L-FABP as positive if more than 5% of tumor cells were stained positive. However, as shown in Table 2, more than half of L-FABP-negative cases showed only focal downregulation when evaluated by whole-tissue sections. Therefore, the possibility of sampling bias may not be negligible and further studies are needed to examine more accurately the frequency of L-FABP positivity in HCC. The previous study did not observe different L-FABP downregulation patterns between cases of small and large HCC. In addition, the differences in the clinicopathological features of L-FABP-negative and L-FABP-positive cases of HCC were not examined in detail.
The present study has limitations. The rate of L-FABP-negative HCC was as low as 10.9%, and there were only 16 L-FABP-negative HCC cases. Thus, the conclusions of the present study with regard to clinicopathological characteristics of L-FABP-negative HCCs may not be sufficiently convincing. Further studies using a larger number of cases should be performed in the future.
In summary, approximately 10% of the cases of HCC exhibited downregulation of L-FABP expression. In most of the small HCCs, downregulation of L-FABP expression may have resulted from phenotypic changes during tumor progression. In addition, in small HCCs, the downregulation of L-FABP expression was significantly associated with poor differentiation and lack of inflammation, as well as higher expression of β-catenin and GS.
We are grateful to Ms. Yurie Soejima, Ms. Arisa Kumagai, and Mr. Masato Watanabe for providing expert technical assistance.
Although the downregulation of liver fatty acid-binding protein (L-FABP) expression is critically important in the diagnostic classification of hepatocellular adenoma (HCA) and it is correlated with various clinicopathological features, the significance of the downregulation of L-FABP expression in hepatocellular carcinoma (HCC) is largely unknown.
In recent years, HCA has been divided into four subtypes, and immunohistochemical staining for L-FABP, β-catenin, glutamine synthetase, and serum amyloid A is critical for the pathological diagnosis of these four subtypes. Although the significance of these immunohistocehmical markers in HCA has been studied in detail, the significance of them, especially L-FABP in HCC, has not been studied in detail.
In the present study, the authors performed immunohistochemical staining for L-FABP in 146 cases of HCC. The authors found that, in small HCCs, L-FABP downregulation probably occurs because of phenotypic changes during tumor progression; furthermore, it was correlated with tumor differentiation and intratumoral inflammation.
By examining the significance of L-FABP downregulation in HCC, this study determined an aspect of the clinicopathological characteristics of HCC. This may lead to more accurate pathological diagnosis of HCC.
FABPs bind/sequester potentially toxic long-chain fatty acids in the cytosol so that they may be rapidly removed via oxidative or storage organelles. Mammals have a large family of FABPs, and L-FABP (or FABP1) was the first FABP described. It is expressed in very high levels in the liver, intestine and kidneys. FABP1 is positively regulated by HNF1α.
In this paper, the authors studied the significance of L-FABP expression in HCC using immunohistochemical staining. This study found that most of the small HCCs (≤ 2 cm in diameter) exhibited focal downregulation and most of the large HCCs (> 2 cm in diameter) exhibited diffuse downregulation. In small HCCs, L-FABP downregulation probably occurs because of phenotypic changes during tumor progression. Moreover, L-FABP downregulation was correlated with tumor differentiation and intratumoral inflammation. These topics are important for understanding tumor differentiation and intratumoral inflammation.
P- Reviewer: Balzan smp, Du Z, Grassi G, Hashimoto N, Kondo Y, Tsai JF S- Editor: Ma YJ L- Editor: Stewart GJ E- Editor: Ma S
| 1. | El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264-1273.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2183] [Cited by in RCA: 2507] [Article Influence: 192.8] [Reference Citation Analysis (2)] |
| 2. | Kim do Y, Han KH. Epidemiology and surveillance of hepatocellular carcinoma. Liver Cancer. 2012;1:2-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 3. | Cabibbo G, Craxì A. Epidemiology, risk factors and surveillance of hepatocellular carcinoma. Eur Rev Med Pharmacol Sci. 2010;14:352-355. [PubMed] |
| 4. | Yang JD, Roberts LR. Epidemiology and management of hepatocellular carcinoma. Infect Dis Clin North Am. 2010;24:899-919, viii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 152] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 5. | El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557-2576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3846] [Cited by in RCA: 4264] [Article Influence: 236.9] [Reference Citation Analysis (2)] |
| 6. | Bioulac-Sage P, Balabaud C, Wanless I. Focal nodular hyperplasia and hepatocellular adenoma. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer 2010; 198-204. |
| 7. | Micchelli ST, Vivekanandan P, Boitnott JK, Pawlik TM, Choti MA, Torbenson M. Malignant transformation of hepatic adenomas. Mod Pathol. 2008;21:491-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 72] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Bioulac-Sage P, Laumonier H, Couchy G, Le Bail B, Sa Cunha A, Rullier A, Laurent C, Blanc JF, Cubel G, Trillaud H. Hepatocellular adenoma management and phenotypic classification: the Bordeaux experience. Hepatology. 2009;50:481-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 349] [Cited by in RCA: 296] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 9. | Dokmak S, Paradis V, Vilgrain V, Sauvanet A, Farges O, Valla D, Bedossa P, Belghiti J. A single-center surgical experience of 122 patients with single and multiple hepatocellular adenomas. Gastroenterology. 2009;137:1698-1705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 259] [Article Influence: 16.2] [Reference Citation Analysis (1)] |
| 10. | Zucman-Rossi J, Jeannot E, Nhieu JT, Scoazec JY, Guettier C, Rebouissou S, Bacq Y, Leteurtre E, Paradis V, Michalak S. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43:515-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 606] [Cited by in RCA: 535] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 11. | Atshaves BP, Martin GG, Hostetler HA, McIntosh AL, Kier AB, Schroeder F. Liver fatty acid-binding protein and obesity. J Nutr Biochem. 2010;21:1015-1032. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 12. | Storch J, Thumser AE. Tissue-specific functions in the fatty acid-binding protein family. J Biol Chem. 2010;285:32679-32683. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 13. | Smathers RL, Petersen DR. The human fatty acid-binding protein family: evolutionary divergences and functions. Hum Genomics. 2011;5:170-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 261] [Cited by in RCA: 348] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 14. | Bioulac-Sage P, Balabaud C, Zucman-Rossi J. Subtype classification of hepatocellular adenoma. Dig Surg. 2010;27:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Theise ND, Curado MP, Franceschi S, Hytiroglou P, Kudo M, Park YN, Sakamoto M, Torbenson M, Wee A. Hepatocellular carcinoma. WHO classification of tumours of the digestive system. 4th ed. Lyon: International Agency for Research on Cancer 2010; 205-216. |
| 16. | Hepatocellular carcinoma. In: Ishak KG, Goodman ZD, Stocker JT. Tumors of the liver and intrahepatic bile ducts, 3rd series. Washington D.C. : Armed Forces Institute of Pathology 2001; 199-230. |
| 17. | Liver Cancer Study Group of Japan. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer. 5th ed. Tokyo: Kanehara 2009; . |
| 18. | Kudo M, Chung H, Haji S, Osaki Y, Oka H, Seki T, Kasugai H, Sasaki Y, Matsunaga T. Validation of a new prognostic staging system for hepatocellular carcinoma: the JIS score compared with the CLIP score. Hepatology. 2004;40:1396-1405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 267] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 19. | Tateishi R, Yoshida H, Shiina S, Imamura H, Hasegawa K, Teratani T, Obi S, Sato S, Koike Y, Fujishima T. Proposal of a new prognostic model for hepatocellular carcinoma: an analysis of 403 patients. Gut. 2005;54:419-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 203] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 20. | Ikai I, Takayasu K, Omata M, Okita K, Nakanuma Y, Matsuyama Y, Makuuchi M, Kojiro M, Ichida T, Arii S. A modified Japan Integrated Stage score for prognostic assessment in patients with hepatocellular carcinoma. J Gastroenterol. 2006;41:884-892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 21. | Kumagai A, Fukushima J, Takikawa H, Fukuda T, Fukusato T. Enhanced expression of farnesoid X receptor in human hepatocellular carcinoma. Hepatol Res. 2013;43:959-969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 22. | Bluteau O, Jeannot E, Bioulac-Sage P, Marqués JM, Blanc JF, Bui H, Beaudoin JC, Franco D, Balabaud C, Laurent-Puig P. Bi-allelic inactivation of TCF1 in hepatic adenomas. Nat Genet. 2002;32:312-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 245] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 23. | Farges O, Ferreira N, Dokmak S, Belghiti J, Bedossa P, Paradis V. Changing trends in malignant transformation of hepatocellular adenoma. Gut. 2011;60:85-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 204] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 24. | Dal Bello B, Rosa L, Campanini N, Tinelli C, Torello Viera F, D’Ambrosio G, Rossi S, Silini EM. Glutamine synthetase immunostaining correlates with pathologic features of hepatocellular carcinoma and better survival after radiofrequency thermal ablation. Clin Cancer Res. 2010;16:2157-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 3.9] [Reference Citation Analysis (0)] |