Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17426
Revised: April 9, 2014
Accepted: July 22, 2014
Published online: December 14, 2014
Processing time: 292 Days and 0.1 Hours
AIM: To assess the anti-cancer effect of lobaplatin on human gastric cancer cells, and to explore the underlying molecular mechanisms.
METHODS: The human gastric cancer cell lines MKN-28, AGS and MKN-45 were used. The cytotoxicity of lobaplatin was detected using an MTS cell proliferation assay. Flow cytometry was used to detect cell apoptosis using Annexin V-FITC Apoptosis Detection Kit. The expression of apoptosis-regulated genes was examined at the protein level using Western blot.
RESULTS: Lobaplatin inhibited the proliferation of human gastric cancer cells and induced apoptosis, which may be associated with the up-regulation of Bax expression, poly(ADP-ribose) polymerase (PARP) cleavage, p53 expression and the reduction of Bcl-2 expression.
CONCLUSION: The cytotoxicity of lobaplatin may be due to its ability of inducing apoptosis of gastric cancer cells, which would support the potential use of lobaplatin for the therapy of gastric cancer.
Core tip: Gastric cancer is one of the common malignancies and the main cause of death. Although cisplatin had become a primary therapeutic drug in advanced gastric cancer, drug resistance was the leading cause of treatment failure. To find an effective treatment is particularly urgent and important. Lobaplatin has been investigated in patients with advanced solid tumors, yet it has not been comprehensively studied in gastric cancer cells. In this study, we found the cytotoxicity and the apoptosis promoting effect of lobaplatin, which can provide a new basis for its clinical application in gastric cancer.
- Citation: Yin CY, Lin XL, Tian L, Ye M, Yang XY, Xiao XY. Lobaplatin inhibits growth of gastric cancer cells by inducing apoptosis. World J Gastroenterol 2014; 20(46): 17426-17433
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17426.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17426
Gastric cancer is one of the common malignancies and the main cause of death in the world. Chemotherapy is a primary method in the therapy of advanced gastric cancer. An increased understanding of the carcinogenesis processes and the response scheme of gastric cancer can contribute to progress in the treatment of gastric cancer. Research of anticancer drugs has been conducted due to the success of cisplatin. Forty years after the discovery of the biological activity of cisplatin for the first time, oxaliplatin and carboplatin as routine medications are widely used in clinical practice today, while nedaplatin, heptaplatin and lobaplatin have only been authorized respectively in Japan, South Korea and China[1].
Lobaplatin is a representative of the third generation platinum drugs. It has been investigated in patients with advanced solid tumors, including relapsed ovarian cancer, canine appendicular osteosarcoma and hepatocellular carcinoma[2-5]. However, there are few reports about lobaplatin in the treatment of gastric cancer, and its mechanism of action has not been clearly understood[6]. The experiments in this study were performed to enhance our understanding of the pharmacological effects of lobaplatin in human gastric cancer. In this study, we found that lobaplatin was most cytotoxic in human gastric cancer cells with poor differentiation state and that lobaplatin induced apoptosis of human gastric cancer cells.
Lobaplatin was purchased from Hainan Chang’an International Pharmaceutical Co., Ltd (Hainan, China). The antibodies against poly(ADP-ribose) polymerase (PARP), p53, Bcl-2 (100), Bax (N-20), β-actin, and the secondary antibodies (horseradish peroxidase–linked anti-rabbit immunoglobulin G, anti-mouse immunoglobulin G, and anti-goat immunoglobulin G) were purchased from Santa Cruz Biotechnology (CA, United States). The other materials were sourced as follows: Dulbecco’s modified Eagle’s medium (DMEM), RPMI 1640, penicillin and streptomycin (×100), fetal bovine serum (FBS), trypsin-EDTA and dimethyl-sulfoxide (Gibco); CellTiter 96 AQueous One Solution Cell Proliferation Assay (MTS) (Promega, United States); Tween 20 (Promega); Annexin V-FITC Apoptosis Detection Kit (BioVision); Protein Assay Kit (Bio-Rad); ECL Plus Western Blotting Detection System (Pierce). All of the other reagents used are widely available commercially.
The human gastric cancer cell lines MKN-45 (poorly differentiated) and AGS (moderately differentiated) were cultured in RPMI 1640 supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, and maintained in an incubator with a humidified atmosphere of 5% CO2 at 37 °C. The MKN-28 (well-differentiated) cell line was cultured in DMEM supplemented with 10% FBS, 100 U/mL penicillin, and 100 μg/mL streptomycin, and maintained in an incubator with a humidified atmosphere of 5% CO2 at 37 °C.
Cell proliferation assay was carried out according to the protocol for the MTS assay supplied by the manufacturer (Promega). In brief, 24 h after seeding, cells were exposed to lobaplatin of different concentrations (0, 1, 5, 10 and 25 μg/mL). After incubation for 24, 48 or 72 h, 10 μL of the MTS solution was added to each well, and then cultures were continued to incubate for 2 h at 37 °C. The absorbance was read at 450 nm on a microplate reader (Tecan Infinite F200, Switzerland), and the inhibitory rate was calculated as [1 - (ODtreated/ODcontrol) × 100%].
Lobaplatin-induced apoptosis in AGS, MKN-28 and MKN-45 cells was determined by flow cytometry using the Annexin V-FITC Apoptosis Detection Kit. Simple steps were as follows. 3 × 105 cells were plated and treated for 24 h with lobaplatin (0, 1, 5, and 10 μg/mL in MKN-45 and AGS, and 0, 25, 50, and 100 μg/mL in MKN-28). And then, cells were harvested, washed twice in PBS, and incubated with Annexin V and propidium iodide for 10 min at room temperature in the dark. Finally, the gastric cancer cells were analyzed with the FACSAria Flow Cytometer.
Briefly, 3 × 105 cells were cultured in 6-well plates and processed with lobaplatin of different concentrations (0, 2.5, 5, and 10 μg/mL in MKN-45 and AGS, and 0, 10, 25, and 50 μg/mL in MKN-28) for 24 h. The cultured cells were trypsinized, washed with PBS, and then re-suspended in lysis buffer at 4 °C. The lysates were denatured in loading buffer at 99 °C for 10 min. Proteins were transferred onto a PVDF membrane after being separated using SDS-PAGE gel electrophoresis. The membrane was incubated with the primary antibody at 4 °C overnight. After being blocked in blocking buffer with 5% nonfat milk [20 mmol/L TBS (pH 7.5) containing 0.1% Tween 20], the membrane was incubated at 37 °C with the appropriate horseradish peroxidase-conjugated secondary antibody. The reactive bands were visualized using the ECL Plus Western Blotting Detection System. The level of β-actin was used as a loading control in each sample.
Numerical data are expressed as mean ± SD. The statistical significance was determined using a two-tailed Student’s t-test between different groups. A P-value < 0.05 was defined as statistical significance.
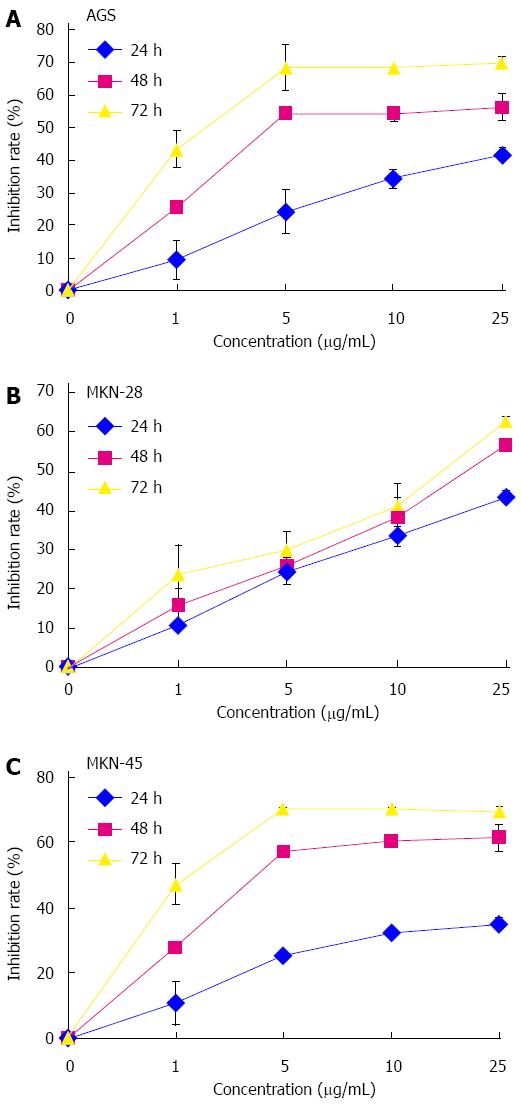
Antitumor activity of lobaplatin was evaluated by cell proliferation MTS assay after 24, 48, and 72 h of treatment (Figure 1) in AGS, MKN-28, and MKN-45 cells. Lobaplatin inhibited the proliferation of these three human gastric cancer cell lines in a dose-dependent manner, and the IC50 values were 6.11 ± 1.44 μg/mL (AGS), 16.10 ± 0.81 μg/mL (MKN-28), and 1.78 ± 0.16 μg/mL (MKN-45), respectively. As revealed by the IC50 values, the cytotoxicity of lobaplatin varied in different cell lines. Moreover, lobaplatin was less cytotoxic to normal human gastric epithelial GES-1 cells (IC50 value 56.17 ± 1.57 μg/mL) than it was to cancer cells.
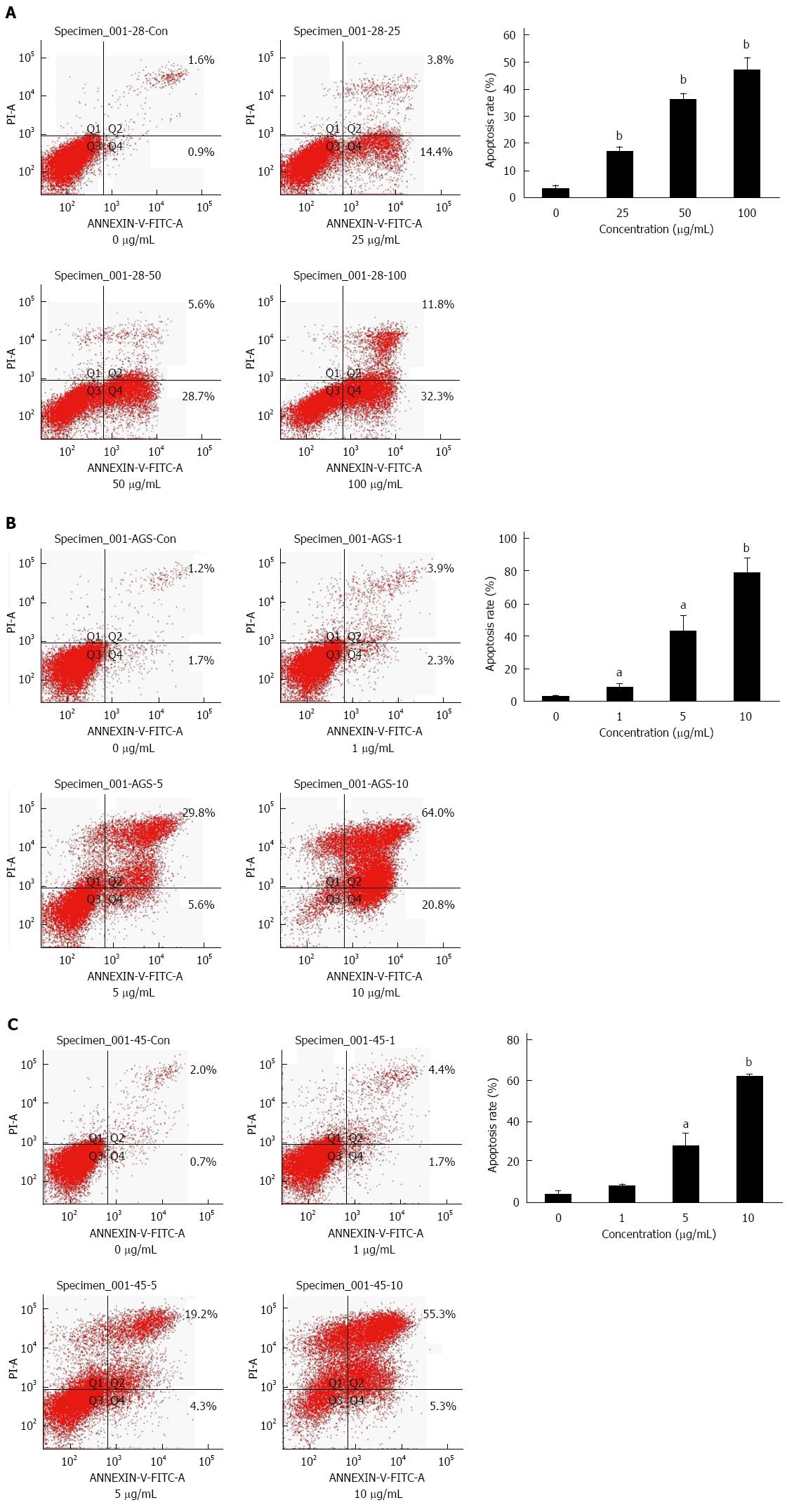
To assess whether lobaplatin-induced growth inhibition in gastric cancer cells was related to cell apoptosis, AGS, MKN-28, and MKN-45 cells were treated with lobaplatin as described above, and cell apoptotic was evaluated by flow cytometry with the Annexin V-FITC Apoptosis Detection Kit. After exposure to lobaplatin for 24 h, the percentage of apoptotic MKN-28 cells significantly increased in a dose-dependent manner [2.3% in 0 μg/mL, 15.6% in 25 μg/mL (P < 0.01), 27.6% in 50 μg/mL (P < 0.01), and 35.4% in 100 μg/mL (P < 0.01)] (Figure 2A). When the AGS and MKN-45 cells were exposed to lobaplatin for 24 h, similar effects were obtained (Figure 2B, C). These results suggest that lobaplatin can effectively induce apoptosis in three gastric cancer cell lines despite their different differentiation states.
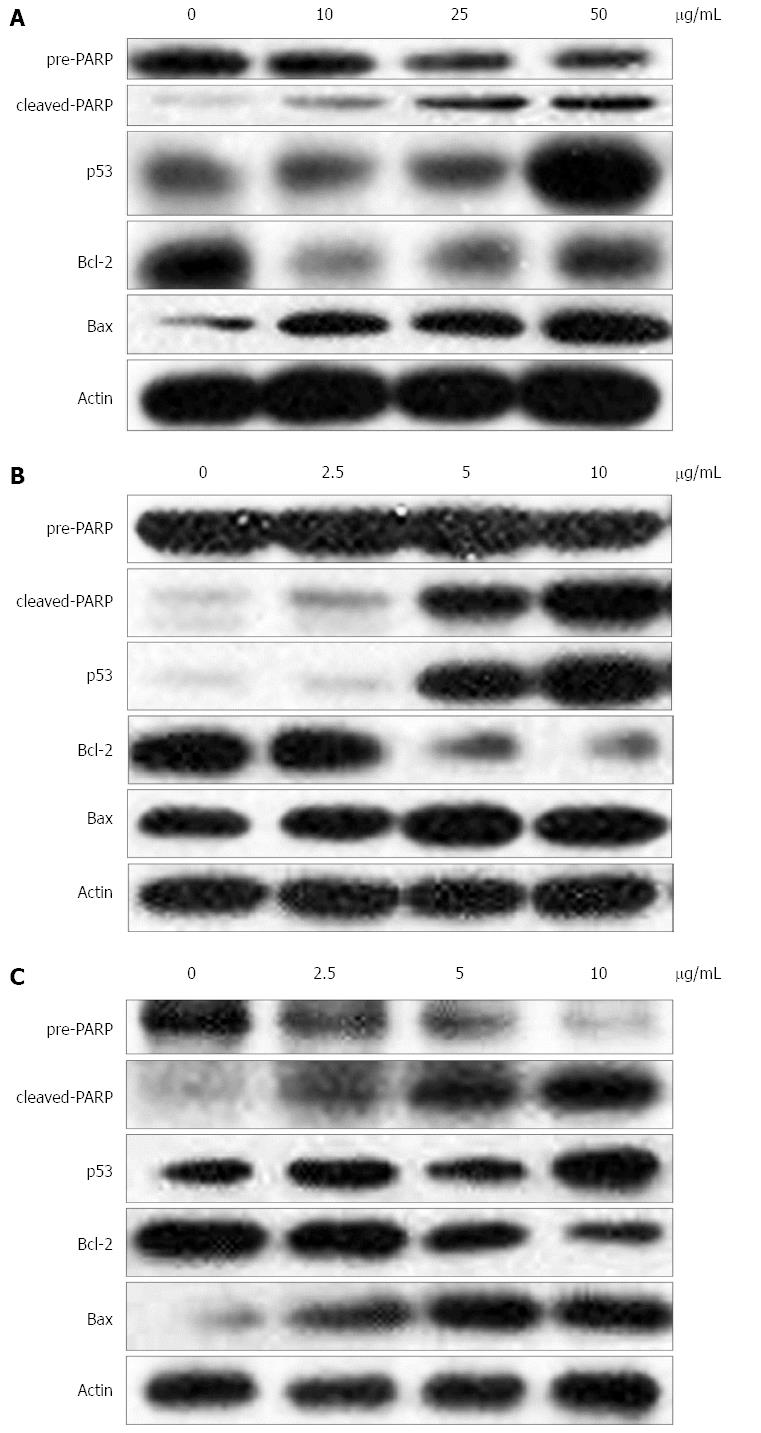
Bcl-2 family proteins are critical regulatory factors in response to apoptosis. The expression changes of Bax and Bcl-2 were analyzed in gastric cancer cells after exposure to lobaplatin. The results showed an increase in the expression of Bax and a dose-dependent reduction in the levels of Bcl-2 protein compared with the control cells (Figure 3).
To clarify the mechanism by which the apoptotic pathway is activated by lobaplatin, its effects on the activation of apoptosis associated proteins PARP and p53 were assessed. A dose-dependent increase in the expression of p53 and cleavage of PARP was found under the treatment by lobaplatin in three gastric cancer cells (Figure 3). This result indicates that lobaplatin might activate p53-dependent canonical mitochondrial apoptotic pathways in gastric cancer cells.
Gastric cancer is one of the common malignancies and the second leading cause of cancer-related death[7]. At present, surgery is the conventional strategy for the treatment of gastric cancer. However, more than half of the patients have been in the advanced stages when diagnosed and cannot undergo a surgical operation. Therefore, chemotherapy is currently the main treatment for advanced gastric cancer. However, the overall response rate is generally less than 50% in gastric cancer and concurrent with high incidence of adverse effects[8]. Therefore, it is increasingly concerned about new chemotherapy drugs to treat gastric cancer.
Platinum drugs have been widely used to treat a variety of malignant tumors. Clinically three common platinum drugs (cisplatin, carboplatin and oxaliplatin) are marketed for different malignancies. The new platinum compounds, lobaplatin, nedaplatin and heptaplatin, have gained official approval for anti-cancer purposes (regionally limited)[9]. Cisplatin is the first generation platinum drug, and has become a major compound in the treatment of solid tumors, such as bronchial carcinoma, ovarian cancer, germ cell cancer, and bladder cancer, but its clinical application is limited by its side effects including gastrointestinal, renal, neurological toxicities and ototoxicity[10]. Lobaplatin [D-19466; 1,2-diamino-methyl-cyclobutane-platinum (II)- lactate] is a representative one of the third-generation platinum drugs, containing a 1,2-bis(aminomethyl) cyclobutane stable ligand with lactic acid as the leaving group. Its anti-tumor activity is primarily due to the conformation of DNA-drug adducts, mainly as AG and GG intra-strand cross-links. It had been reported that lobaplatin effected the expression of the c-Myc gene, which is involved in cell proliferation, apoptosis and oncogenesis[11]. Phase II clinical trials of lobaplatin have also been completed in the United States, Australia, European Union, Brazil and South Africa for the treatment of various cancers, including lung, breast, ovarian and esophageal cancers, as well as CML[12]. However, this drug has not yet been used to treat gastric cancer. Therefore, this study investigated the effects of lobaplatin in three gastric cancer cell lines and explored the underlying molecular mechanisms.
We found that lobaplatin inhibited the survival of different human gastric cancer cells in a dose-dependent manner, showing an in vitro anti-tumor effect. Interestingly, we found that lobaplatin was more effective on the less-differentiated MKN-45 cells (IC50 value 1.78 ± 0.16 μg/mL) than the moderately differentiated AGS cells (IC50 value 6.11 ± 1.44 μg/mL), while it presented less effect on the MKN-28 cells (IC50 value 16.10 ± 0.81 μg/mL) which are well-differentiated. Lobaplatin presented almost no toxicity to the normal human gastric epithelial cell line GES-1 (IC50 value 56.17 ± 1.57 μg/mL), which indicates that lobaplatin shows a therapeutic effect that is specific to human gastric cancer cells. This result is in agreement with previous findings in other solid tumors, such as hepatocellular carcinoma and ovarian cancer[13,14].
Lobaplatin has been reported to induce apoptosis of cancer cells in the past few years. Zhang et al[15] reported that lobaplatin inhibited tumor cells by affecting the expression of the c-Myc gene, which is involved in cell proliferation, apoptosis and oncogenesis, but the exact mechanism is not fully understood. Our research found that lobaplatin could induce gastric cancer cell apoptosis in multiple cell lines (AGS, MKN-28 and MKN-45) with different differentiation states. The results indicated a decrease in Bcl-2 expression and an increase in Bax expression in three gastric cancer cell lines. The change in the ratio of Bax to Bcl-2 promotes the release of cytochrome C from the mitochondria to the cytosol. Then cytochrome C can bind to APAF-1 in the cytosol, and lead to the activation of PARP and caspase 3[16]. Our study further indicated that lobaplatin activated PARP cleavage in a dose-dependent manner. These results showed that PARP played an important role in lobaplatin induced apoptosis in gastric cancer cells with different differentiation. The induction of a p53-independent apoptotic mechanism by lobaplatin has potential anti-tumor effects on gastric cancer. These results are similar to the results of our initial research showing that Licochalcone A induced apoptosis of gastric cancer cells[17]. Because of the pro-apoptotic effects of lobaplatin in gastric cancer cells with different differentiation degrees, we speculate that lobaplatin is especially useful for the treatment of poorly differentiated gastric cancer.
The tumor suppressor p53 is an essential regulator that plays an important role in the antitumor effect of platinum agents. The p53 protein can prevent cancer development, including cell cycle stagnation and the induction of apoptosis by a series of mechanisms. It has been reported that lobaplatin arrested cell cycle progression of hepatocellular carcinoma cells in the G1 and G2/M phases in a time-dependent manner[5,18,19]. To further investigate the mechanism, p53 expression were further studied before and after lobaplatin treatment in multiple gastric cancer cell lines. The results show that p53 levels increased in a dose-dependent manner after exposure to lobaplatin, indicating that the p53-dependent apoptotic pathway is activated by lobaplatin. Whether lobaplatin influences the cell cycle in gastric cancer merits further research.
In conclusion, lobaplatin inhibited the growth of gastric cancer cell mainly by inducing apoptosis via the caspase-dependent mitochondrial pathway. Therefore, lobaplatin is a promising candidate against gastric cancer. It warrants additional investigation and clinical evaluation.
Gastric cancer is one of the leading causes of death worldwide. Cisplatin was an important drug in the treatment of advanced gastric cancer, but drug resistance reduced the actual effect. Lobaplatin has been investigated in patients with advanced solid tumors, yet it has not been comprehensively studied in gastric cancer cells.
Lobaplatin is a new representative compound of third-generation platinum drugs. It appears to overcome tumor resistance to cisplatin and carboplatin. And it has been investigated in some solid tumors, but rarely in gastric cancer. In the study of the inhibitory effect of lobaplatin on gastric cancer cells, we found that lobaplatin was most cytotoxic in human gastric cancer cells with poor differentiation state and that lobaplatin induced the apoptosis of gastric cancer cells.
Studies have reported that lobaplatin can inhibit the proliferation of some solid tumors, such as primary hepatic carcinoma, cholangiocarcinoma, non-small-cell lung cancer and relapsed ovarian cancer. In this study, we investigated the anti-tumor effect of lobaplatin in gastric cancer cells. We used both cancer cells and normal epithelial cell lines to perform the experiments. We found lobaplatin caused cytotoxicity and induced apoptosis via the caspase-dependent mitochondrial pathway in gastric cancer cells. Lobaplatin is a promising anti-cancer candidate drug for the treatment of gastric cancer.
The study results suggest that lobaplatin is a potential chemotherapy agent that can provide a theoretical basis for the clinical treatment of gastric cancer.
Apoptosis, also called programmed cell death, is generally characterized by distinct morphological characteristics and energy-dependent biochemical mechanisms. Apoptosis is considered a vital component of various processes including normal cell turnover, hormone-dependent atrophy, embryonic development, etc. Inappropriate apoptosis is a factor in many human diseases including neurodegenerative diseases, autoimmune disorders and many types of cancer. Anti-cancer drugs can lead to characteristic tumor cell changes (morphology, signaling proteins) and death.
The purpose of the present study was to determine possible effects of lobaplatin on growth, viability and apoptosis of various gastric cancer cells. It is an interesting work in which the effects of the drug on gastric cancer cells in various degrees of differentiation were compared.
P- Reviewer: Berg T S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Ma S
| 1. | Galanski M, Jakupec MA, Keppler BK. Update of the preclinical situation of anticancer platinum complexes: novel design strategies and innovative analytical approaches. Curr Med Chem. 2005;12:2075-2094. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 543] [Cited by in RCA: 575] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 2. | Kirpensteijn J, Teske E, Kik M, Klenner T, Rutteman GR. Lobaplatin as an adjuvant chemotherapy to surgery in canine appendicular osteosarcoma: a phase II evaluation. Anticancer Res. 2002;22:2765-2770. [PubMed] |
| 3. | McKeage MJ. Lobaplatin: a new antitumour platinum drug. Expert Opin Investig Drugs. 2001;10:119-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 122] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 4. | Welink J, Boven E, Vermorken JB, Gall HE, van der Vijgh WJ. Pharmacokinetics and pharmacodynamics of lobaplatin (D-19466) in patients with advanced solid tumors, including patients with impaired renal of liver function. Clin Cancer Res. 1999;5:2349-2358. [PubMed] |
| 5. | Wu Q, Qin SK, Teng FM, Chen CJ, Wang R. Lobaplatin arrests cell cycle progression in human hepatocellular carcinoma cells. J Hematol Oncol. 2010;3:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Peng Z, Xu S, Li H, Sun C, Fu M, Gao M. Advanced gastric cancer with brain metastasis effectively treated by arterial infusion chemotherapy: A case report. Oncol Lett. 2014;7:449-451. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Weis VG, Goldenring JR. Current understanding of SPEM and its standing in the preneoplastic process. Gastric Cancer. 2009;12:189-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 107] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Farhat FS, Kattan J, Ghosn MG. Role of capecitabine and irinotecan combination therapy in advanced or metastatic gastric cancer. Expert Rev Anticancer Ther. 2010;10:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Berger G, Gasper R, Lamoral-Theys D, Wellner A, Gelbcke M, Gust R, Nève J, Kiss R, Goormaghtigh E, Dufrasne F. Fourier transform infrared (FTIR) spectroscopy to monitor the cellular impact of newly synthesized platinum derivatives. Int J Oncol. 2010;37:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Zhang J, Wang L, Xing Z, Liu D, Sun J, Li X, Zhang Y. Status of bi- and multi-nuclear platinum anticancer drug development. Anticancer Agents Med Chem. 2010;10:272-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Wheate NJ, Walker S, Craig GE, Oun R. The status of platinum anticancer drugs in the clinic and in clinical trials. Dalton Trans. 2010;39:8113-8127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1203] [Cited by in RCA: 1263] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 13. | Harstrick A, Bokemeyer C, Scharnofkse M, Hapke G, Reile D, Schmoll HJ. Preclinical activity of a new platinum analogue, lobaplatin, in cisplatin-sensitive and -resistant human testicular, ovarian, and gastric carcinoma cell lines. Cancer Chemother Pharmacol. 1993;33:43-47. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 14. | Zhou B, Shan H, Zhu KS, Jiang ZB, Guan SH, Meng XC, Zeng XC. Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol. 2010;21:333-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 15. | Zhang J, Liu D, Li Y, Sun J, Wang L, Zang A. Status of non-classical mononuclear platinum anticancer drug development. Mini Rev Med Chem. 2009;9:1357-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Del Poeta G, Bruno A, Del Principe MI, Venditti A, Maurillo L, Buccisano F, Stasi R, Neri B, Luciano F, Siniscalchi A. Deregulation of the mitochondrial apoptotic machinery and development of molecular targeted drugs in acute myeloid leukemia. Curr Cancer Drug Targets. 2008;8:207-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 17. | Xiao XY, Hao M, Yang XY, Ba Q, Li M, Ni SJ, Wang LS, Du X. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett. 2011;302:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 128] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 18. | Avery-Kiejda KA, Bowden NA, Croft AJ, Scurr LL, Kairupan CF, Ashton KA, Talseth-Palmer BA, Rizos H, Zhang XD, Scott RJ. P53 in human melanoma fails to regulate target genes associated with apoptosis and the cell cycle and may contribute to proliferation. BMC Cancer. 2011;11:203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 19. | Zheng T, Meng X, Wang J, Chen X, Yin D, Liang Y, Song X, Pan S, Jiang H, Liu L. PTEN- and p53-mediated apoptosis and cell cycle arrest by FTY720 in gastric cancer cells and nude mice. J Cell Biochem. 2010;111:218-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 70] [Article Influence: 5.0] [Reference Citation Analysis (0)] |