Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17407
Revised: July 8, 2014
Accepted: September 5, 2014
Published online: December 14, 2014
Processing time: 227 Days and 11.4 Hours
AIM: To evaluate the effect of nitric oxide (NO) on the development and degree of liver failure in an animal model of acute hepatic failure (AHF).
METHODS: An experimental rat model of galactosamine-induced AHF was used. An inhibitor of NO synthase, nitroarginine methyl ester, or an NO donor, arginine, were administered at various doses prior to or after the induction of AHF.
RESULTS: All tested groups developed AHF. Following inhibition of the endogenous NO pathway, most liver parameters improved, regardless of the inhibitor dose before the induction of liver damage, and depending on the inhibitor dose after liver damage. Prophylactic administration of the inhibitor was more effective in improving liver function parameters than administration of the inhibitor after liver damage. An attempt to activate the endogenous NO pathway prior to the induction of liver damage did not change the observed liver function parameters. Stimulation of the endogenous NO pathway after liver damage, regardless of the NO donor dose used, improved most liver function parameters.
CONCLUSION: The endogenous NO pathway plays an important role in the development of experimental galactosamine-induced AHF.
Core tip: We investigated the role of the nitric oxide (NO) pathway in the pathogenesis of acute hepatic failure (AHF). The precise pathomechanism of AHF is poorly understood. In our study, most liver function parameters improved both before and after the induction of liver damage following inhibition of the NO pathway. Prophylactic administration of the inhibitor was more effective in improving liver function parameters. On the other hand, stimulation of the NO pathway after liver damage, regardless of the donor dose used, also improved most liver function parameters. Therefore, the NO pathway significantly influences the development of experimental galactosamine-induced AHF.
- Citation: Saracyn M, Brytan M, Zdanowski R, Ząbkowski T, Dyrla P, Patera J, Wojtuń S, Kozłowski W, Wańkowicz Z. Hepatoprotective effect of nitric oxide in experimental model of acute hepatic failure. World J Gastroenterol 2014; 20(46): 17407-17415
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17407.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17407
Acute hepatic failure (AHF) is a fulminant liver disease, which is frequently caused by toxic or infectious agents, and has a very poor prognosis[1]. According to the classical definition, AHF clinical symptoms are associated with rapidly progressing organ failure in patients with no previous signs of liver disease, and the development of encephalopathy within 4 wk of the onset of symptoms[2]. The clinical picture is dominated by jaundice, encephalopathy, hypoglycemia, hyperammonemia, metabolic acidosis, and complications that include renal failure, bacterial infections, hemostasis disorders, and finally multiple organ dysfunction syndrome[3]. More than 2500 new cases of AHF occur annually in the United States, and more than 1000 patients die from this disease[4]. Paracetamol poisoning is responsible for more than 40% of AHR-related deaths in this population. Other causes of AHF include seronegative hepatitis and infections with hepatotropic viruses[5]. The prognosis depends primarily on the causative agent, patient age, and dynamics of AHF. Although significant progress has been made in liver transplantation, AHF-related mortality remains high. Approximately 30% of patients die following AHF without the possibility of organ transplantation, and more than 25% of patients receive a liver transplant. Among those who receive a transplant, over 70% survive 1 year, and over 60% survive 5 years[6]. Knowledge of the pathogenesis of AHF is poor, despite its severe course, poor prognosis, and several decades of studies. Acute liver damage causes hemodynamic disorders, as portal pressure increases within a short time, splanchnic vessels dilate, and the opening of arteriovenous anastomoses causes increased portal venous flow, leading to hyperkinetic circulation[7]. Organ damage results in an increase in the level of many mediators of inflammation and the induction of bacterial endotoxins, leading to the development of sepsis and multiple organ dysfunction[8].
Nitric oxide (NO) has many biological effects on the nervous system, as well as on immune and cardiovascular systems[9,10]. Substrate availability and the presence of inhibitors of NO synthesis control the biological activity of NO. NO is obtained from the amino acid L-arginine (L-ARG) in a reaction catalyzed by NO synthase (NOS)[11]. Thus far, 3 different isoforms of this enzyme have been identified: neuronal NOS (nNOS) type I (constitutive) in the central and peripheral nervous system, inducible NOS (iNOS) type II (nonconstitutive) in the cells of the immune system, and endothelial NOS (eNOS) type III (constitutive) in the tunica intima of vascular walls[12,13]. The presence of both iNOS and eNOS isoforms of NOS has been confirmed in healthy liver[14]. Studies have shown that eNOS is uniformly distributed in hepatocytes in all parts of the lobule and in the hepatic arterial endothelium, sinusoids, hepatic veins, and bile duct epithelium. The inducible form is predominantly found in the hepatocytes of the periportal region of the lobule. In patients with AHF, eNOS has been detected within the cell nuclei of hepatocytes, particularly around areas of necrosis. However, iNOS has been evenly distributed in all parts of the liver in AHF, and this isoenzyme is more active. In vitro studies have shown that NO may be involved in processes inducing apoptosis, as well as in cell necrosis[15]. Both types of processes can lead to damage and the subsequent death of hepatocytes.
Some studies have confirmed the participation of NO in liver damage and necrosis, and liver failure in experimental models of acute hepatic ischemia or endotoxemia[16,17]. Other studies showed the opposite effect, with NO reducing the degree of damage and necrosis of hepatocytes during endotoxemia, decreasing the level of inflammatory mediators, and decreasing liver damage and mortality in AHF caused by acetaminophen or thioacetamide[18,19]. The aim of our study was to evaluate the impact of the regulation of the endogenous NO pathway on the development and degree of liver damage in a rat model of AHF induced by D-galactosamine.
The Local Bioethics Committee for experimental studies approved the present study. Ninety six randomly selected male Sprague-Dawley rats (body weight 200-250 g) were obtained from the Department of Experimental Animals of Polish Mother’s Memorial Hospital in Łódź. The animals were kept in standard group cages, fed a standard diet, and had free access to water and food. They were maintained under a natural day/night cycle of 12 h at a temperature 22 °C ± 2 °C and humidity of 45%-50%. The experiments were performed from 10.00 a.m. to 6.00 p.m. on natural moving animals in their waking time. The studies were carried out according to the guidelines of The Animal Scientific Procedures Act. During the course of the experiments, the rats were placed individually in glass metabolic cages, with free access to water and food.
To test the pathophysiological impact of NO in this model of AHF, the animals were given saline; galactosamine hydrochloride (Ga1N); an NOS inhibitor, N-nitro-L-arginine methyl ester (L-NAME); or an NO donor, L-ARG.
The rats were divided into the 12 groups, with 8 individuals in each group: Group 1 (Sham group): received 1 mL of 0.9% saline solution intraperitoneally (i.p.); Group 2: Received 1.1 g/kg body weight of Ga1N (Sigma Aldrich, Poland) i.p. as a 200 mg/mL solution in 0.9% saline; Group 3 (Control L-NAME group): Received 100 mg/kg of L-NAME (Sigma Aldrich, Poland) i.p.; Group 4: Received the same dose of L-NAME as Group 3 at 48 h and 24 h before Ga1N injection; Group 5: Received 200 mg/kg (double dose) of L-NAME 48 h and 24 h before the induction of liver injury; Group 6: Received 100 mg/kg of L-NAME 24 h and 48 h after Ga1N intoxication; Group 7: Received 200 mg/kg (double dose) of L-NAME 24 h and 48 h after the induction of liver injury; Group 8: Control L-ARG group: Received 150 mg/kg L-ARG (Sigma Aldrich, Poland) i.p.; Group 9: Received the same dose of L-ARG as Group 8 at 48 h and 24 h before Ga1N injection; Group 10: Received 300 mg/kg L-ARG (double dose) 48 h and 24 h before the induction of liver damage; Group 11: Received 150 mg/kg L-ARG 24 h and 48 h after Ga1N intoxication; Group 12: Received 300 mg/kg L-ARG (double dose) 24 h and 48 h after the induction of liver damage.
Urine samples were collected during 24 h from the 24th to 48th hour after saline or Ga1N injection and evaluated 48 h after saline or Ga1N injection. Blood samples (6 mL) were also collected 48 h after saline or Ga1N injection from the beating hearts of deeply anesthetized animals. Biochemical parameters, except ammonium, were determined in serum or urine using an autoanalyzer (Integra 700, Roche, United States). Bilirubin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and albumin reagents were purchased from Roche, Germany. An auto analyzer was used to determine the ammonium concentration in plasma with an EDTA-K3 anticoagulant. Urine osmolality was measured with an auto osmometer (Osmometer Automatic, Knauer, Germany).
After exsanguination, liver and kidney tissues were collected for histopathological examination. The liver and kidney sections were fixed in formalin, paraffin embedded, stained with hematoxylin and eosin and examined under a light microscope.
Statistical analysis was performed using the Student t-test and analysis of variance when multiple comparisons were required. Where appropriate, the Mann-Whitney U test was used to analyze nonparametric data. The limit of significance was taken as P < 0.05. All data are expressed as mean ± SE.
Table 1 shows the liver function parameters in the experimental animals, indicating the development of AHF. Compared with the sham group (Group 1), the administration of galactosamine at a dose of 1.1 g/kg body weight (Group 2) resulted in severe liver damage within 48 h, and the development of AHF, with a statistically significant increase in the plasma levels of bilirubin (P < 0.004), AST (P < 0.0001), ALT (P < 0.001), and ammonia (P < 0.005), and a decrease in the level of albumin (P < 0.001).
| Gr. (n) | Bils, mg/dL | ASTs, IU/L | ALTs, IU/L | Albumins, g/dL | Ammons, μmol/L |
| 1 (8) | |||||
| Sham | 0.40 ± 0.26 | 252.5 ± 149.8 | 56.2 ± 9.9 | 2.9 ± 0.1 | 52.8 ± 38.1 |
| 2 (8) | |||||
| Ga1N | 3.43 ± 1.35 | 1624.12 ± 692.92 | 2098.6 ± 886.1 | 2.6 ± 0.1 | 275.7 ± 73.7 |
| 3 (8) | |||||
| Sham L-NAME | 0.28 ± 0.17 | 221.37 ± 150.05 | 82.2 ± 16.2 | 2.98 ± 0.11 | 26.9 ± 7.8 |
| 4 (8) | |||||
| L-NAME/Ga1N | 3.27 ± 0.52 | 1309.25 ± 349.48 | 1343.75 ± 451.92 | 2.73 ± 0.14 | 210 ± 48.18 |
| 5 (8) | |||||
| 2xL-NAME/Ga1N | 2.67 ± 075 | 1682.25 ± 433.24 | 1590.12 ± 504.26 | 2.68 ± 0.15 | 193.87 ± 80.92 |
| 6 (8) | |||||
| Ga1N /L-NAME | 2.58 ± 0.46 | 1424.87 ± 422.99 | 1425 ± 475.61 | 2.76 ± 0.15 | 206.37 ± 51.11 |
| 7 (8) | |||||
| G Ga1N/2xL-NAME | 3.1 ± 0.75 | 1691.12 ± 370.80 | 1707.12 ± 448.87 | 2.7 ± 0.17 | 201.5 ± 42.33 |
| Gr. 2 vs Gr. 1 | P < 0.004 | P < 0.0001 | P < 0.001 | P < 0.001 | P < 0.005 |
| Gr. 3 vs Gr. 1 | P < 0.37 | P < 0.70 | P < 0.031 | P < 0.15 | P < 0.089 |
| Gr. 4 vs Gr. 2 | P < 0.77 | P < 0.30 | P < 0.064 | P < 0.17 | P < 0.068 |
| Gr. 5 vs Gr. 2 | P < 0.21 | P < 0.85 | P < 0.064 | P < 0.46 | P < 0.067 |
| Gr. 6 vs Gr. 2 | P < 0.13 | P < 0.52 | P < 0.098 | P < 0.12 | P < 0.060 |
| Gr. 7 vs Gr. 2 | P < 0.57 | P < 0.82 | P < 0.31 | P < 0.40 | P < 0.036 |
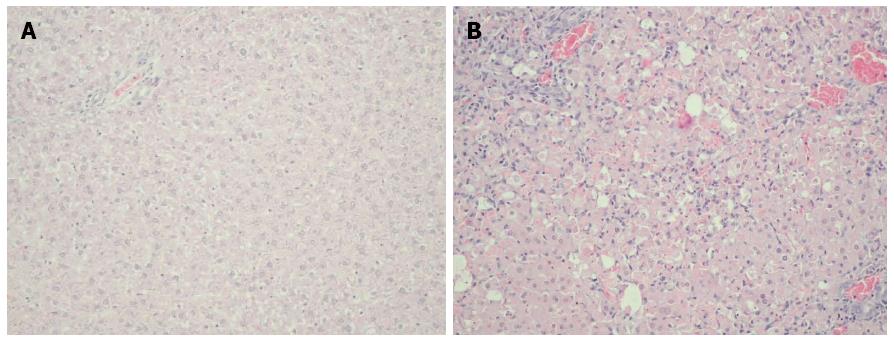
Histopathology of the liver of animals in the group dosed with Ga1N (Group 2) showed generalized and massive hepatocyte necrosis. No changes in histopathology were observed in the sham group (Group 1), which received only saline, compared with Group 2 (Figure 1).
The administration of the NO synthase inhibitor alone, without galactosamine poisoning, had no significant influence on liver biochemical parameters. No changes were observed in the concentration of bilirubin, AST, albumin, and ammonia in Group 3 compared with the sham group (Group 1) (Table 1). The administration of the lower dose of L-NAME before the administration of galactosamine decreased the level of ALT (P < 0.064) and ammonia (P < 0.068) at the border of statistical significance. The level of bilirubin and AST declined and the concentration of albumin increased in Group 4 compared with Group 2 but the changes were not statistically significant (Table 1). Compared with Group 4, the double dose of L-NAME (Group 5) did not produce any further improvement in liver function parameters, with the exception of an insignificant decrease in the levels of bilirubin and ammonia 4 (Table 1). The addition of L-NAME in Group 4 compared with the group that received only Ga1N (Group 2), did not significantly affect hepatocyte necrosis in the histopathological examination.
The inhibition of the endogenous NO pathway after the administration of galactosamine resulted in a similar effect. Although the administration of the lower dose of L-NAME after Ga1N intoxication decreased the levels of AST, ALT, and ammonia, and increased serum albumin in Group 6 compared with Group 2, the findings were not statistically significant. The level of bilirubin decreased, but this was also not statistically significant (Table 1) (Group 6 vs Group 2, respectively). The double dose of L-NAME (Group 7) did not result in any further improvement in liver function parameters compared with Group 2, with the exception of a significant decrease in the level of ammonia (P < 0.036) (Table 1).
L-NAME improved liver function parameters slightly more effectively when administered prior to than after Ga1N intoxication, both at the lower and higher doses of NO synthase inhibitor (Table 1) (Group 6 vs Group 4 and Group 7 vs Group 5, respectively). However, the opposite effect was observed in the case of bilirubin and ammonia with lower dose of L-NAME, although statistically significant differences still not observed between individual groups.
The administration of L-ARG alone (Group 8) did not result in significant changes in liver biochemical parameters compared with the sham group (Group 1) (Table 2). L-ARG did not significantly alter the concentration of bilirubin, ALT, and ammonia, and it only slightly decreased the level of albumin (P < 0.0007) and AST (P < 0.047) (Table 2). The administration of the lower dose of L-ARG in Group 9 prior to the induction of liver damage with Ga1N did not markedly affect liver function parameters, apart from significantly decreasing the level of ammonia (P < 0.032) (Table 2) compared with Group 2. Similarly, the administration of twice the dose of L-ARG did not considerably alter liver function parameters in Group 10, with the exception of ammonia, the concentration of which significantly declined in comparison with the sham group (Table 2). The histopathological examination of the liver revealed that the picture of hepatocyte necrosis was unchanged in Group 9 compared with Group 2.
| Gr. (n) | Bils, mg/dL | ASTs, IU/L | ALTs, IU/L | Albumins, mg/dL | Ammons,μmol/L |
| 1 (8) | |||||
| Sham | 0.40 ± 0.26 | 252.50 ± 149.8 | 56.2 ± 9.9 | 2.9 ± 0.1 | 52.8 ± 38.1 |
| 2 (8) | |||||
| Ga1N | 3.43 ± 1.35 | 1624.12 ± 692.92 | 2098.6 ± 886.1 | 2.6 ± 0.1 | 275.7 ± 73.7 |
| 8 (8) | |||||
| Sham L-ARG | 0.38 ± 0.21 | 126.37 ± 33.95 | 53.75 ± 7.37 | 2.67 ± 0.09 | 50.7 ± 46.2 |
| 9 (8) | |||||
| L-ARG/Ga1N | 2.96 ± 0.67 | 1484.87 ± 328.75 | 1546.37 ± 349.75 | 2.75 ± 0.1 | 199.12 ± 42.8 |
| 10 (8) | |||||
| 2xL-ARG/Ga1N | 2.92 ± 0.89 | 1611.75 ± 334.58 | 1603.75 ± 364.47 | 2.73 ± 0.06 | 193.87 ± 50.49 |
| 11 (8) | |||||
| Ga1N/L-ARG | 2.86 ± 0.94 | 1491.87 ± 421.49 | 1486.25 ± 450.83 | 2.42 ± 0.21 | 195.25 ± 41.69 |
| 12 (8) | |||||
| Ga1N/2xL-ARG | 2.96 ± 0.98 | 1446.25 ± 219.35 | 1435.62 ± 281.33 | 2.72 ± 0.11 | 180.5 ± 45.80 |
| gr. 2 vs gr. 1 | P < 0.004 | P < 0.0001 | P < 0.001 | P < 0.001 | P < 0.005 |
| gr. 8 vs gr. 1 | P < 0.11 | P < 0.047 | P < 0.60 | P < 0.0007 | P < 0.340 |
| gr. 9 vs gr. 2 | P < 0.41 | P < 0.63 | P < 0.14 | P < 0.09 | P < 0.032 |
| gr. 10 vs gr. 2 | P < 0.41 | P < 0.96 | P < 0.19 | P < 0.10 | P < 0.029 |
| gr. 11 vs gr. 2 | P < 0.37 | P < 0.67 | P < 0.12 | P < 0.06 | P < 0.024 |
| gr. 12 vs gr. 2 | P < 0.46 | P < 0.52 | P < 0.08 | P < 0.20 | P < 0.011 |
The stimulation of the endogenous NO pathway after the induction of Ga1N liver damage resulted in a slightly different effect. Following the administration of the lower dose of L-ARG after the application of Ga1N, levels of bilirubin, AST, and ALT decreased, although not significantly, and the ammonia concentration declined significantly in Group 11 compared with Group 2 (P < 0.024) (Table 2). The double dose of L-ARG reduced the level of AST more (though not significantly), the level of ALT (at the border of significance), and the ammonia concentration (P < 0.011) (Table 2) (Group 12 vs Group 2, respectively).
L-ARG was more effective in improving liver function parameters when administered after the induction of liver damage than prior to the induction of damage. It was particularly effective in reducing concentrations of AST, ALT, and ammonia when a higher dose of the NO donor was used. However, the differences between the groups were not significant (Table 2) (Group 11 vs Group 9 and Group 12 vs Group 10, respectively).
AHF in experimental models can be achieved by surgery, pharmacological methods, or infectious agents[20]. Surgery-induced AHF involves partial or complete hepatectomy, and partial or complete ligation of the hepatic artery, bile ducts, or portal vein[21]. Pharmacologically-induced AHF produces liver damage with various agents, such as acetaminophen, carbon tetrachloride, thioacetamide, concanavalin A, lipopolysaccharide, or galactosamine[22]. In the latter model, Ga1N administered i.p. to experimental animals, typically at a dose of about 1 g/kg body weight 24-48 h after poisoning, causes acute liver damage and the development of AHF[23]. Liver damage and failure manifest in encephalopathy, an increase in intracranial pressure, a dramatic rise in the concentration of bilirubin, transaminases, and ammonia, a decrease in the concentration of albumin, prolonged prothrombin time, and histopathologically generalized massive necrosis of hepatocytes.
In the present experiment, AHF developed in a typical way. Within 48 h after liver damage, the animals developed clinical and biochemical features of acute liver damage and liver failure, indicating impairment of detoxifying, secretory, and biosynthetic functions. A number of studies have described experimental AHF induced by Ga1N in various animal species[23-25]. Interestingly, despite many years of research, the exact mechanism of hepatocyte damage by Ga1N remains unclear. Furthermore, the known mechanism of transcriptional inhibition by Ga1N does not explain the rapid damage and the development of AHF. One study suggested that this process involves intestinal bacterial endotoxins, acute hepatitis, and tumor necrosis factor (TNF)-α, which seems to play a key role in mediating the damage to liver cells[26].
Previous studies have attempted to determine the role of endogenous NO in the development and course of AHF[27,28]. However, the exact mechanisms of the development of AHF, in which NO is involved, are not fully understood[29]. One study confirmed that the synthesis of endogenous NO in AHF is significantly increased[14]. However, other studies did not observe a similar effect, and one showed that the synthesis of endogenous NO may even be reduced[30,31].
In the current study, the inhibition of endogenous NO with both doses of NOS inhibitor improved liver function by decreasing the levels of bilirubin, AST, ALT, and ammonia, and increasing albumin, either before or after liver damage. The application of L-NAME prior to Ga1N administration rather than after intoxication with Ga1N was more effective in improving liver function parameters. This was observed with both doses of NOS inhibitor.
Endogenous NO may play a role in processes that result in damage and death of liver cells[15]. One experimental study reported increased activity, particularly of iNOS, and consequent increased synthesis of NO[14]. Irrespective of the etiology of AHF, previous studies found that iNOS was markedly more active than in control groups and that its activity was more pronounced in regions bordering the space of the portal vein and the hepatic triad structures[25,32-34]. Huang et al[35] also detected increased eNOS activity in liver preparations of rats with thioacetamide-induced AHF. The same study found that the level of mRNA, as well as the expression of eNOS protein, was elevated and that both were positively correlated with the degree of liver damage and the severity of clinical symptoms of AHF. Therefore, it seems that inhibition of the NO pathway can have a beneficial effect.
Our study confirmed this hypothesis. Likewise, Rahman et al[34] showed hepatoprotective effects of the prophylactic administration of a selective inhibitor of iNOS, aminoguanidine, in an experimental animal model of AHF induced by an intraperitoneal injection of thioacetamide. In their study, aminoguanidine administered for 5 d prior to thioacetamide-induced liver damage reduced mortality of animals and improved clinical and biochemical markers of liver failure. In our study, a less selective inhibitor of NOS, L-NAME, which can inhibit both iNOS and eNOS, also improved liver function parameters, both before and after galactosamine-induced liver damage. However, there are also data suggesting an adverse effect of endogenous NO blockade on the course and outcome of experimental AHF. According to a study by Chu et al[36] performed in rats with thioacetamide-induced AHF, L-NAME administered intragastrically for 2 d before and 3 d after liver damage increased the mortality of animals and potentiated neurological and clinical symptoms of AHF. In the same study, inhibition of the NO pathway also increased the levels of plasma endotoxins and TNF-α, and the increase was positively correlated with the severity of liver damage and clinical signs of AHF. In a similar experimental model, Chu et al[19] showed that the selective blockade of iNOS compared with simultaneous inhibition of both isoforms of NOS had beneficial effects on the mortality of experimental animals and all markers of liver failure. According to these authors, eNOS played an important role in the development of AHF and its complications, as inhibition of iNOS alone had no effect on markers of liver damage and failure or on mortality of the experimental animals.
On the other hand, the activation of endogenous NO in our study did not produce substantially different results. The administration of either a lower or higher dose of L-ARG prior to the induction of liver damage by Ga1N did not significantly affect liver function parameters. However, administration of both doses of L-ARG after the application of Ga1N clearly decreased the degree of liver failure and reduced levels of bilirubin, AST, ALT, and ammonia. L-ARG administered after the induction of liver damage was also more effective in improving liver function parameters then when applied prior to Ga1N, in particular, at the higher dose of the NO donor.
L-ARG is the only known substance that provides nitrogen for the synthesis of endogenous NO by NOS[37]. Moreover, the concentration of L-ARG appears to be an important factor controlling the speed and efficiency of NO formation[38]. Experimental and clinical data on the level of L-ARG in AHF are ambiguous, with increased and decreased concentrations and no change reported[39-41].
In our study, NO activation following the induction of liver damage improved liver function parameters. In addition, this effect was dependent on the L-ARG dose. Our observations are consistent with those of Fiorucci et al[18], who reported that NO released in the liver following the administration of a specific ester of ursodeoxycholic acid, resulted in a hepatoprotective effect. The same study showed that in acetaminophen-induced AHF in mice, an external supply of NO reduced mortality, the level of ALT, and apoptosis and necrosis of hepatocytes in histopathological analysis. NO also reduced hepatic expression of all inflammatory mediators tested, such as TNF-α and interferon-γ, as well as mediators of apoptosis, Fas/FasL and caspases 3 and 9. In addition, the NO donor prevented changes in mitochondrial membrane polarization and the transfer of mitochondrial enzymes into the cytosol, thereby inhibiting mechanisms leading to damage and apoptosis of liver cells. Although we used a different model of AHF and different experimental animals, we observed a similar beneficial effect of the activation of the NO pathway on biochemical markers of acute liver damage and failure. In another study of AHF in rats induced by Ga1N, Kono et al[42] demonstrated that administration of a low dose of lipopolysaccharides 24 h before liver damage decreased mortality from 19% to 4% and substantially reduced all studied biochemical markers of liver damage. This effect was abolished by the NO synthase inhibitor, thus proving that the hepatoprotective effect of lipopolysaccharides is associated with activation of endogenous NO synthesis. Huang et al[43,44] showed that pravastatin and simvastatin potentiate endogenous production of NO and have hepatoprotective effects in experimental AHF induced by thioacetamide. Both simvastatin and pravastatin administered for 2 d before and 3 d after liver damage reduced animal mortality, clinical signs of encephalopathy, and the level of bilirubin and transaminases. Therefore, activation of the endogenous NO pathway not only improves biochemical markers of liver damage but also clinical symptoms of liver function and animal survival in the course of experimental AHF.
The experimental nature of the present study is a limitation. Differences in the natural development and course of liver diseases in humans and animals mean that the findings are not directly applicable to the human population. The number of animals in each group may seem small. However, according to current opinion, it is sufficient to obtain reliable results in experimental studies. Although using greater numbers of animals might aid the statistical validity of the results, it may raise bioethical dilemmas. The importance of this study is that it provides an assessment of the impact of endogenous NO on a galactosamine-induced model of AHF. To the best of our knowledge, this is the first such study in the literature. Other studies cited in this paper investigated this issue in other experimental models of AHF. We have confirmed that NO plays an important role in the development of hepatic failure in galactosamine-induced AHF, providing further evidence that NO plays a significant role in the pathogenesis of liver diseases in various experimental models.
Our study showed that in the course of experimental galactosamine-induced AHF, inhibition of the endogenous NO pathway prior to the induction of liver damage, regardless of the dose of inhibitor used, improved some liver function parameters, but it did not affect hepatocyte necrosis. Inhibition of the endogenous NO pathway after the induction of liver damage improved most liver function parameters, and this effect was correlated with the inhibitor dose. Prophylactic administration of the NO synthase inhibitor before liver injury was more effective in improving parameters of liver function when compared with administration after liver damage.
In contrast, an attempt to activate the endogenous NO pathway prior to the induction of liver damage, regardless of the dose of the NO donor, did not change liver function parameters or hepatocyte necrosis. Stimulation of the endogenous NO pathway after liver damage improved liver function parameters, irrespective of the donor dose.
We conclude that the NO pathway has a significant influence on the development and degree of experimental galactosamine-induced AHF.
Acute hepatic failure (AHF), also known as fulminant liver failure, is liver disease that develops rapidly. Toxic (mainly paracetamol) or infectious agents are the most common cause. AHF has a very poor prognosis. Approximately 30% of patients die following AHF, and more than 25% of patients undergo a liver transplant, of which about 70% survive 1 year. Knowledge of the pathogenesis of the disease is still limited, despite decades of research. The endogenous nitric oxide (NO) pathway may be one of the pathomechanisms potentially involved in AHF.
NO plays a wide variety of functions, including mediation of inflammatory reactions and potent vasodilation. Some studies have shown that NO may be involved in processes leading to damage and death of liver cells, and thus the development of AHF. However, other studies showed exactly the opposite effect, with NO decreasing the degree of damage and necrosis of liver cells, reducing the level of inflammatory mediators, and decreasing liver damage and mortality in the course of AHF.
Inhibition of the endogenous NO pathway both before and after liver injury improved most of the studied liver function parameters. Prophylactic administration of the inhibitor was more effective in improving liver function parameters than administration after liver damage. Activation of the endogenous NO pathway prior to liver damage had no effect on the observed liver function parameters, and stimulation of the endogenous NO pathway after liver damage, regardless of the donor dose used, improved most liver function parameters. Hence, the endogenous NO pathway plays an important role in the development of AHF.
The results of this experimental work point to potential directions for future research on new forms of AHF therapy in humans. It seems that the first step should include studies of endogenous NO synthesis inhibitors.
AHF is a syndrome of clinical symptoms associated with rapidly progressing liver failure in patients with no preceding signs of liver disease. NO is responsible for a variety of biological effects in the central and peripheral nervous system, the immune system, and the cardiovascular system. For example, it acts as a neuromediator, mediator of inflammation, and a potent substance dilating blood vessels.
This manuscript points to evaluate “hepatoprotective effect of handling of the nitric oxide pathway in a rodent model of acute hepatic failure induced by galactosamine”.
P- Reviewer: Boscá L, Santoro N, Trifan A S- Editor: Ma YJ L- Editor: Cant MR E- Editor: Ma S
| 1. | Bernal W, Wendon J. Acute liver failure. N Engl J Med. 2013;369:2525-2534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 736] [Cited by in RCA: 846] [Article Influence: 70.5] [Reference Citation Analysis (2)] |
| 2. | Whitehouse T, Wendon J. Acute liver failure. Best Pract Res Clin Gastroenterol. 2013;27:757-769. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 3. | Wlodzimirow KA, Eslami S, Abu-Hanna A, Nieuwoudt M, Chamuleau RA. Systematic review: acute liver failure - one disease, more than 40 definitions. Aliment Pharmacol Ther. 2012;35:1245-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 88] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 4. | Wlodzimirow KA, Eslami S, Chamuleau RA, Nieuwoudt M, Abu-Hanna A. Prediction of poor outcome in patients with acute liver failure-systematic review of prediction models. PLoS One. 2012;7:e50952. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Lee WM. Recent developments in acute liver failure. Best Pract Res Clin Gastroenterol. 2012;26:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 70] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Alqahtani SA. Update in liver transplantation. Curr Opin Gastroenterol. 2012;28:230-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Javlé P, Yates J, Kynaston HG, Parsons KF, Jenkins SA. Hepatosplanchnic haemodynamics and renal blood flow and function in rats with liver failure. Gut. 1998;43:272-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Lee WM. Acute liver failure. Semin Respir Crit Care Med. 2012;33:36-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 187] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 9. | Loscalzo J. The identification of nitric oxide as endothelium-derived relaxing factor. Circ Res. 2013;113:100-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | Hu LS, George J, Wang JH. Current concepts on the role of nitric oxide in portal hypertension. World J Gastroenterol. 2013;19:1707-1717. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 49] [Cited by in RCA: 51] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Klinger JR, Abman SH, Gladwin MT. Nitric oxide deficiency and endothelial dysfunction in pulmonary arterial hypertension. Am J Respir Crit Care Med. 2013;188:639-646. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 12. | Huang H, Silverman RB. Recent advances toward improving the bioavailability of neuronal nitric oxide synthase inhibitors. Curr Top Med Chem. 2013;13:803-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 13. | Carnicer R, Crabtree MJ, Sivakumaran V, Casadei B, Kass DA. Nitric oxide synthases in heart failure. Antioxid Redox Signal. 2013;18:1078-1099. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | McNaughton L, Puttagunta L, Martinez-Cuesta MA, Kneteman N, Mayers I, Moqbel R, Hamid Q, Radomski MW. Distribution of nitric oxide synthase in normal and cirrhotic human liver. Proc Natl Acad Sci USA. 2002;99:17161-17166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 141] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 15. | Wang JH, Redmond HP, Wu QD, Bouchier-Hayes D. Nitric oxide mediates hepatocyte injury. Am J Physiol. 1998;275:G1117-G1126. [PubMed] |
| 16. | Jiang W, Desjardins P, Butterworth RF. Minocycline attenuates oxidative/nitrosative stress and cerebral complications of acute liver failure in rats. Neurochem Int. 2009;55:601-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Laskin DL, Rodriguez del Valle M, Heck DE, Hwang SM, Ohnishi ST, Durham SK, Goller NL, Laskin JD. Hepatic nitric oxide production following acute endotoxemia in rats is mediated by increased inducible nitric oxide synthase gene expression. Hepatology. 1995;22:223-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 18. | Fiorucci S, Antonelli E, Distrutti E, Mencarelli A, Farneti S, Del Soldato P, Morelli A. Liver delivery of NO by NCX-1000 protects against acute liver failure and mitochondrial dysfunction induced by APAP in mice. Br J Pharmacol. 2004;143:33-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Chu CJ, Chang CC, Wang TF, Lee FY, Chang FY, Chen YC, Chan CC, Huang HC, Wang SS, Lee SD. Detrimental effects of nitric oxide inhibition on hepatic encephalopathy in rats with thioacetamide-induced fulminant hepatic failure: role of nitric oxide synthase isoforms. J Gastroenterol Hepatol. 2006;21:1194-1199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 20. | Rahman TM, Hodgson HJ. Animal models of acute hepatic failure. Int J Exp Pathol. 2000;81:145-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 131] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 21. | Panis Y, McMullan DM, Emond JC. Progressive necrosis after hepatectomy and the pathophysiology of liver failure after massive resection. Surgery. 1997;121:142-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 146] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Kelly JH, Koussayer T, He DE, Chong MG, Shang TA, Whisennand HH, Sussman NL. An improved model of acetaminophen-induced fulminant hepatic failure in dogs. Hepatology. 1992;15:329-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Sielaff TD, Hu MY, Rollins MD, Bloomer JR, Amiot B, Hu WS, Cerra FB. An anesthetized model of lethal canine galactosamine fulminant hepatic failure. Hepatology. 1995;21:796-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Keppler D, Lesch R, Reutter W, Decker K. Experimental hepatitis induced by D-galactosamine. Exp Mol Pathol. 1968;9:279-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 359] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 25. | Blitzer BL, Waggoner JG, Jones EA, Gralnick HR, Towne D, Butler J, Weise V, Kopin IJ, Walters I, Teychenne PF. A model of fulminant hepatic failure in the rabbit. Gastroenterology. 1978;74:664-671. [PubMed] |
| 26. | Leist M, Gantner F, Künstle G, Bohlinger I, Tiegs G, Bluethmann H, Wendel A. The 55-kD tumor necrosis factor receptor and CD95 independently signal murine hepatocyte apoptosis and subsequent liver failure. Mol Med. 1996;2:109-124. [PubMed] |
| 27. | Brenner T, Fleming TH, Rosenhagen C, Krauser U, Mieth M, Bruckner T, Martin E, Nawroth PP, Weigand MA, Bierhaus A. L-arginine and asymmetric dimethylarginine are early predictors for survival in septic patients with acute liver failure. Mediators Inflamm. 2012;2012:210454. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 28. | Hazam RK, Deka M, Kar P. Role of nitric oxide synthase genes in hepatitis E virus infection. J Viral Hepat. 2014;21:671-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 29. | Sharma V, Ten Have GA, Ytrebo L, Sen S, Rose CF, Dalton RN, Turner C, Revhaug A, van-Eijk HM, Deutz NE. Nitric oxide and L-arginine metabolism in a devascularized porcine model of acute liver failure. Am J Physiol Gastrointest Liver Physiol. 2012;303:G435-G441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | Wu YL, Lian LH, Jiang YZ, Nan JX. Hepatoprotective effects of salidroside on fulminant hepatic failure induced by D-galactosamine and lipopolysaccharide in mice. J Pharm Pharmacol. 2009;61:1375-1382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 31. | Ytrebø LM, Sen S, Rose C, Davies NA, Nedredal GI, Fuskevaag OM, Ten Have GA, Prinzen FW, Williams R, Deutz NE. Systemic and regional hemodynamics in pigs with acute liver failure and the effect of albumin dialysis. Scand J Gastroenterol. 2006;41:1350-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 32. | Wang W, Sun L, Deng Y, Tang J. Synergistic effects of antibodies against high-mobility group box 1 and tumor necrosis factor-α antibodies on D-(+)-galactosamine hydrochloride/lipopolysaccharide-induced acute liver failure. FEBS J. 2013;280:1409-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Jiang W, Gao M, Sun S, Bi A, Xin Y, Han X, Wang L, Yin Z, Luo L. Protective effect of L-theanine on carbon tetrachloride-induced acute liver injury in mice. Biochem Biophys Res Commun. 2012;422:344-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 34. | Rahman TM, Hodgson HJ. The effects of early and late administration of inhibitors of inducible nitric oxide synthase in a thioacetamide-induced model of acute hepatic failure in the rat. J Hepatol. 2003;38:583-590. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 35. | Huang HC, Wang SS, Chan CY, Chen YC, Lee FY, Chang FY, Chu CJ, Lin HC, Lu RH, Lee SD. Role of hepatic nitric oxide synthases in rats with thioacetamide-induced acute liver failure and encephalopathy. J Chin Med Assoc. 2007;70:16-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 36. | Chu CJ, Wang SS, Lee FY, Chang FY, Lin HC, Hou MC, Chan CC, Wu SL, Chen CT, Huang HC. Detrimental effects of nitric oxide inhibition on hepatic encephalopathy in rats with thioacetamide-induced fulminant hepatic failure. Eur J Clin Invest. 2001;31:156-163. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Hoang HH, Padgham SV, Meininger CJ. L-arginine, tetrahydrobiopterin, nitric oxide and diabetes. Curr Opin Clin Nutr Metab Care. 2013;16:76-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 38. | Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593-615. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 970] [Cited by in RCA: 1606] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 39. | Cardounel AJ, Cui H, Samouilov A, Johnson W, Kearns P, Tsai AL, Berka V, Zweier JL. Evidence for the pathophysiological role of endogenous methylarginines in regulation of endothelial NO production and vascular function. J Biol Chem. 2007;282:879-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 184] [Cited by in RCA: 187] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 40. | Yagnik GP, Takahashi Y, Tsoulfas G, Reid K, Murase N, Geller DA. Blockade of the L-arginine/NO synthase pathway worsens hepatic apoptosis and liver transplant preservation injury. Hepatology. 2002;36:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 62] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 41. | Tietge UJ, Bahr MJ, Manns MP, Böker KH. Plasma amino acids in cirrhosis and after liver transplantation: influence of liver function, hepatic hemodynamics and circulating hormones. Clin Transplant. 2002;16:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 42. | Kono T, Kotani H, Asama T, Mamiya N, Ohara K, Yoneda M, Iwamoto J, Kasai S. Protective effect of pretreatment with low-dose lipopolysaccharide on D-galactosamine-induced acute liver failure. Int J Colorectal Dis. 2002;17:98-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 43. | Huang HC, Wang SS, Lee FY, Chan CY, Chang FY, Lin HC, Chu CJ, Chen YC, Lee SD. Simvastatin for rats with thioacetamide-induced liver failure and encephalopathy. J Gastroenterol Hepatol. 2008;23:e236-e242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 44. | Huang HC, Chang CC, Wang SS, Chan CY, Lee FY, Chuang CL, Hsin IF, Teng TH, Lin HC, Lee SD. Pravastatin for thioacetamide-induced hepatic failure and encephalopathy. Eur J Clin Invest. 2012;42:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |