Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17399
Revised: March 14, 2014
Accepted: July 15, 2014
Published online: December 14, 2014
Processing time: 409 Days and 18.6 Hours
AIM: To evaluate a hybrid bioartificial liver support system (HBALSS) in cynomolgus monkeys with acute liver failure.
METHODS: To establish a model of acute liver failure, 0.3 g/kg of D-galactosamine was injected intravenously into cynomolgus monkeys. Chinese human liver cells were introduced into a perfusion bioreactor to carry out hybrid bioartificial liver support treatment. Forty-eight hours after the injection, one group of cynomolgus monkeys received HBALSS care, and a second experimental group received no treatment. Clinical manifestations of all animals, survival time, liver and kidney functions and serum biochemistry changes were recorded. Simultaneous detection of the number, viability and function of hepatocytes in the hybrid bioartificial liver were also performed.
RESULTS: Forty-eight hours after the injection of D-galactosamine, serum biochemistry levels were significantly increased, whereas albumin levels and the Fischer index were significantly reduced compared to baseline (all Ps < 0.05). Of the ten monkeys in the HBALSS treatment group, five survived, with an average duration of survival of 128 ± 3 h. All cynomolgus monkeys in the control group died, with a duration of survival of 112 ± 2 h. Survival time was significantly longer with HBALSS treatment (P < 0.05). Moreover, the number, viability and function of hepatocytes were maintained at a high level with HBALSS.
CONCLUSION: The novel hybrid bioartificial liver plays a significant role in liver support by significantly reducing serum biochemistry levels and extending animal survival time.
Core tip: In this study, the authors evaluated the safety, efficacy, and clinical feasibility of a hybrid bioartificial liver support system (HBALSS) with Chinese human liver cells for the treatment of acute liver failure in monkeys. The bioartificial liver significantly reduced serum biochemistry levels and extended animal survival time. Furthermore, the number, viability and function of hepatocytes in the HBALSS were maintained at a high level during treatment. The results demonstrate that the novel HBALSS has the potential to be a safe, reliable bridge treatment for acute liver failure patients awaiting donor-organ availability.
- Citation: Zhang Z, Zhao YC, Cheng Y, Jian GD, Pan MX, Gao Y. Hybrid bioartificial liver support in cynomolgus monkeys with D-galactosamine-induced acute liver failure. World J Gastroenterol 2014; 20(46): 17399-17406
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17399.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17399
Acute liver failure (ALF) is a critical clinical condition with intensive, rapid progression and an ominous prognosis. Although there has been progress in comprehensive medical treatment in recent years, the mortality rate of ALF remains high at approximately 80%[1-3]. Liver transplantation is currently the only effective treatment for ALF[4-8]. However, due to a variety of reasons, a limited number of liver transplantations are being carried out[9,10]. To bridge the time between ALF and suitable organ availability, patients are placed on a bioartificial liver support system.
Over the past 20 years, hepatocytes from a variety of sources have been intensely studied, though not fully, clinically tested. Hepatocytes can be obtained from adult or fetal animals, liver cell lines, and liver stem cells, however, each of these sources provides a disadvantage. Ideally in liver transplantation, human hepatocytes are the perfect candidate cell types, but sources for adult hepatocytes are scarce and limited. Additionally, when in vitro, adult hepatocytes rapidly lose metabolic capacity, therefore, it is impossible to obtain a large number of hepatocytes. The ethical issues which entail the use of fetal hepatocytes for clinical applications are complex and difficult to deal with[11].
Research in hepatic stem cells has determined the various sources within the adult body and its potentials in regeneration and differentiation, but these discoveries are not sufficient yet to support its clinical application. Although C3A human liver cells were used in previous phase I-II clinical trials, it still needs further evaluation[12]. Additional new liver cell lines have been used for research, though their clinical effect needs further investigation[13]. Therefore, it is important to find a well-differentiated human liver cell line with vigorous growth, does not induce tumorigenicity, and maintains adequate liver cell function for the support of a bioartificial liver during clinical treatment.
In this study, Chinese human liver cells (CL-1) were used to seed the development of a novel human cell hybrid bioartificial liver. CL-1 cells derived from histologically normal liver tissue are well differentiated and display a high degree of metabolic function[14]. CL-1 cells were grown in a microgravity culture, seeded in a perfusion bioreactor, and used to treat cynomolgus monkeys with artificially induced ALF. Because their physical structure so closely resembles that of humans, cynomolgus monkeys were chosen as the animal model for development of a hybrid bioartificial liver support system (HBALSS)[15]. Moreover, heterogeneous rejection is relatively low. The experimental results achieved in this study will further validate the safety and effectiveness of the system while exploring its feasibility in clinical applications.
Hepatocytes were grown using a three-dimensional human cell/tissue culture system, known as the rotary cell culture system (RCCS; Synthecon Inc., Houston, TX, United States), which simulates microgravity in three-dimensional culture microcarriers. The CL-1 cells (HL-7702 [L-02]) were purchased from the Cell Bank in the Chinese Academy of Sciences. The cells were amplified in tissue culture flasks (Corning Inc., Corning, NY, United States). Microcarriers (100 mg; GE Healthcare, Little Chalfont, United Kingdom) were mixed with 2 × 106 CL-1 cells at 37 °C, 5% CO2 for 12 h with 25 mL of high glucose DMEM medium (Gibco of Thermo Fisher Scientific Inc., Waltham, MA, United States) containing 10% fetal bovine serum (Gibco). Then, another 25 mL of high glucose DMEM medium containing 10% fetal bovine serum, 30 mmol/L HEPES (Sigma-Aldrich, St. Louis, MO, United States) and no growth factor was added. The rotation speed of the microgravity culture system was maintained at 13.5-15.5 r/min. The 25 mL of the medium was replaced every 24 h.
Fifteen cynomolgus male monkeys (ordinary level) from seven to eight years-old, weighing 6.5-7.0 kg, were purchased from Guangdong Entomological Institute, South China Primate Research and Development Center (Certificate of Conformity SCXK [Guangdong] 2009-2010 complying with the GB 14925-94 proposal for all experimental monkeys). All animals were housed individually in a controlled unit in a mechanically ventilated confinement facility under standard field conditions. Monkeys were fed three times a day and water was provided ad libitum. The project was approved by the Institutional Review Board of the Second Affiliated Hospital of Southern Medical University, Guangzhou, China (No. ZJYY-2011-GDEK-001).
Because of humane considerations, the Committee requested all experiments should be performed with anesthesia to minimize suffering. Experiments on animals were carried out in compliance with Guidance to Treat Animals, promulgated by the Ministry of Science and Technology in 2006. Anesthesia was induced with ketamine (15 mg/kg) and atropine (0.5-1.0 mg/kg), and then maintained intravenously with propofol (≤ 8.5 mg/kg per hour), fentanyl (≤ 0.015 mg/kg per hour), and atracurium (≤ 0.7 mg/kg per hour) depending on the depth of anesthesia. The humane endpoint was set in keeping with the general well-being or behavior of the monkeys.
The human cell HBALSS was jointly developed by the Institute for Regenerative Medicine, Southern Medical University and the Chinese Academy of Sciences, Beijing Daiboruike Technology Development Co., Ltd. The abiotic part consists of non-biologic membrane plasma separator, blood perfusion, and three peristaltic pump components. The biologic part contains a perfusion bioreactor with 4.0 × 109 of CL-1 cells seeded in microcarriers. It also consists of a membrane oxygenator, membrane plasma separator, a 37 °C temperature water bath, and three double-headed peristaltic pumps. Both the abiotic and biologic parts are connected through the three abiotic valves to form the closed-loop piping.
D-galactosamine (D-gal) was dissolved in a 5% glucose solution to make a concentration of 1.0 g/mL. The pH value was then adjusted to 6.8 using 1 mol/L sodium hydroxide and was filter sterilized. The cynomolgus monkeys were injected with 0.5 mL/kg of ketamine and sumianxin II (im). Two hours later, D-gal (0.3 g/kg) was injected within 10 min via the external jugular vein. Cynomolgus monkeys appeared normal after treatment. A humane endpoint evaluation was used in our experiments, and death was used as the indicator.
Forty-eight hours after the injection of D-gal, all cynomolgus monkeys were randomly divided into two groups. In the HBALSS treatment group (n = 10), cynomolgus monkeys were anesthetized and treated with HBALSS for 6 h. In the ALF control group (n = 5), cynomolgus monkeys were anesthetized only for monitoring and plasma perfusion.
Forty-eight hours after the establishment of ALF, plasma perfusion treatment was provided to monkeys in the ALF control group. Monkeys with liver failure were placed under anesthesia with ketamine and sumianxin II, and the femoral veins were connected with a double-lumen hemodialysis catheter to establish cycle paths, which was then separately connected to the arterial and venous ports of a DX-10-type blood purification machine (Beijing Daiboruike Technology Development Co., Ltd., Beijing, China). Unfractionated heparin was given as a first dose of 180 IU/kg and maintenance doses of 30 IU/kg per hour. The blood purifier was filled with hydroxyethyl starch sodium chloride and the rate of blood flow was 15-20 mL/min. After blood plasma entered the membrane separator, it would then enter the blood purifier to remove bilirubin.
CL-1 cells were cultured in RCCS and placed in the perfusion bioreactor, which was connected with the membrane oxygenator, membrane plasma separator, and blood purifier tube via three-way valves. Two hours after plasma perfusion treatment, the three-way valve was switched on. Then the plasma in the blood purifier flowed into the biologic part at a flow rate of 5 mL/min and circulated at a speed of 30 mL/min in the bioartificial liver. The plasma then reacts with CL-1 cells and performs the function of biosynthesis and metabolism for 4 h.
During HBALSS treatment, the vital signs of cynomolgus monkeys, including the ability to stand, walk, changes in consciousness, eating habits, vision, hearing, and tactile response to stimuli, convulsions and tremors, were observed and recorded. Any symptoms and their duration were recorded. Bleeding, allergies, high fever and other serious adverse reactions were recorded. Serum levels of aspartate aminotransferase (AST), albumin (ALB), total bilirubin, total bile acid, urea nitrogen, creatinine, and ammonia were examined at baseline, before treatment (at 48 h) and after treatment (54 h). Then the Fischer index was calculated. The duration of survival of cynomolgus monkeys was recorded and surviving animals were sacrificed with a lethal injection of pentobarbital and KCl (iv). After the death of the cynomolgus monkeys, a detailed autopsy was performed and the animal’s organs were immediately fixed with formalin. Hematoxylin and eosin staining was carried out to assess organ necrosis and inflammatory cell infiltration under microscopic examination.
Viability and function of human CL-1 hepatocytes in the bioartificial liver were determined before and after HBALSS treatment. Trypan blue dye exclusion was used to determine the number of viable CL-1 cells. CL-1 liver cell effector function was evaluated by determining the levels of alanine transaminase (ALT), AST, lactate dehydrogenase, urea, and ALB in the culture medium with a Cobas C311 Chemistry Analyzer (Roche, Basel, Germany), according to the manufacturer’s instructions.
Significance between groups was compared using Student’s t-tests, and survival rates between the groups were analyzed using a χ2 test. Survival time was analyzed using Kaplan-Meier survival analysis and the Log-rank test. All data analyses were performed using SPSS version 13.0 statistical software (SPSS Inc., Chicago, IL, United States), with P < 0.05 indicating significance.
There were no significant changes in the vital signs of animals in each group before and after D-gal administration. Twelve hours after administration, food intake began to decrease, while serum transaminases began to increase with the progression of ALF. The levels of bilirubin, bile acids, ammonia, and lactic acid gradually increased and platelets decreased. Prothrombin time was gradually extended. Serum biochemistry results from 48 h before and after D-gal administration are shown in Table 1. Levels of ammonia, ALT, total bilirubin, total bile acid, blood urea nitrogen, and creatinine were significantly higher than compared previously (Ps < 0.05), whereas albumin levels and the Fischer index were significantly reduced (Ps < 0.05). These indicate that the liver failure model was established successfully.
| Indicators | 48 h before administration | 48 h after administration | P |
| ALT (U/L) | 28.3 ± 4.7 | 494.2 ± 31.9 | 4.09 × 10-18 |
| Ammonia (μmol/L) | 28.9 ± 3.0 | 149.5 ± 12.3 | 3.14 × 10-16 |
| TBiL (μmol/L) | 1.2 ± 0.2 | 16.7 ± 2.1 | 1.06 × 10-13 |
| TBA (U/L) | 6.0 ± 1.4 | 51.1 ± 7.3 | 2.46 × 10-13 |
| BUN (μmol/L) | 3.7 ± 0.9 | 6.2 ± 0.9 | 2.96 × 10-6 |
| Cr (μmol/L) | 76.7 ± 5.7 | 133.5 ± 6.2 | 1.66 × 10-12 |
| ALB (g/L) | 41.3 ± 1.6 | 37.3 ± 0.6 | 5.86 × 10-8 |
| Fischer index | 3.2 ± 0.2 | 2.2 ± 0.1 | 1.49 × 10-11 |
As shown in Table 2, when HBALSS was not connected to the animal, the number and viability of CL-1 cells decreased. The total number of CL-1 cells did not decrease significantly over 48 h, compared with previous readings (0 h), and cell viability after 24 h was not significantly decreased. In addition, cell function, as measured by ALT, AST, lactate dehydrogenase, urea, and ALB, remained unaffected at 48 h, suggesting that the HBALSS meets the requirements needed for a hybrid artificial liver (Table 3).
| Indicators | 0 h | 24 h | 48 h | 72 h |
| Hepatocytes (n× 109) | 4.1 ± 0.3 | 3.9 ± 0.4 | 3.8 ± 0.3 | 3.0 ± 0.1 |
| P | - | 0.3675 | 0.2254 | 0.0239 |
| A value | 0.985 ± 0.105 | 0.919 ± 0.048 | 0.751 ± 0.024 | 0.530 ± 0.053 |
| P | - | 0.3900 | 0.0610 | 0.0173 |
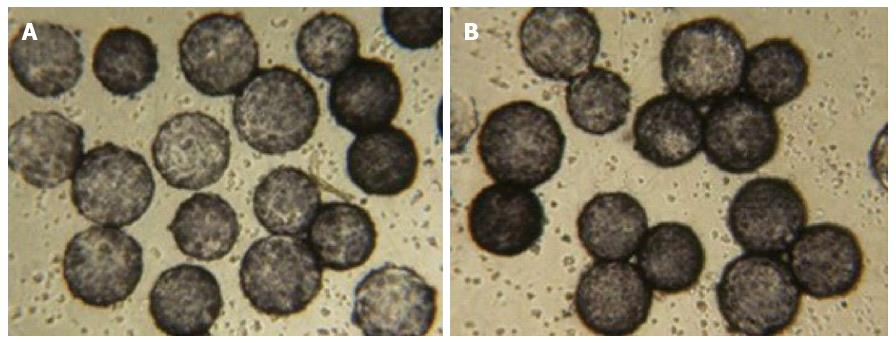
As shown in Figure 1, before HBALSS treatment, CL-1 cells were seeded in RCCS and the viability was determined at 95%-99%. After HBALSS treatment, trypan blue dye testing showed that the viability of cells was maintained at 85%-89%. Throughout the course of treatment, there was no detachment of cells from the microcarriers. The CL-1 cells in microcarriers were maintained at a high level of cell viability, which meets the requirement for the development of a hybrid artificial liver.
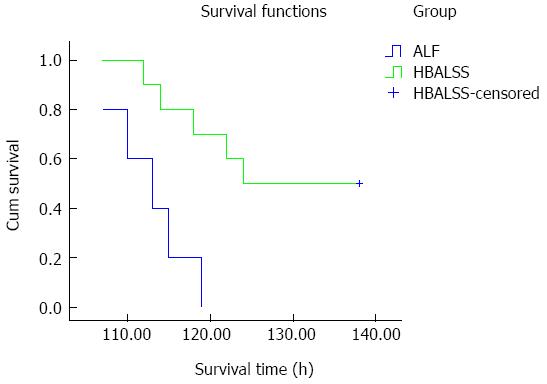
All ten cynomolgus monkeys with ALF survived during the entire course of the HBALSS treatment. There was no liquid leakage in the piping system and no obvious bleeding, allergies, high fever, or other serious adverse reactions found in the monkeys. Although transient fluctuations in heart rate and blood pressure were observed when the vessel was being connected to the system, heart rate, oxygen saturation, respiration, and general arterial blood pressure remained stable (Table 4). Although all 10 of the monkeys survived during HBALSS treatment, five died after treatment. Their survival duration was significantly longer than in the ALF control group (P < 0.01) (Table 5, Figure 2).
| Time (h) | Heart rate (per min) | P | Blood oxygen (%) | P | Respiratory rate (per min) | P | Artery blood pressure (mmHg) | P |
| 0 | 90.5 ± 3.8 | - | 99.7 ± 0.5 | - | 15.3 ± 1.2 | - | 114.6 ± 4.1 | - |
| 1 | 94.1 ± 3.4 | 0.000959 | 99.6 ± 0.5 | 0.343436 | 13.9 ± 1.4 | 0.000528 | 117.9 ± 4.8 | 0.000093 |
| 2 | 92.7 ± 3.8 | 0.005743 | 99.1 ± 0.7 | 0.005121 | 15.4 ± 2.0 | 0.797625 | 116.9 ± 6.4 | 0.102480 |
| 3 | 98.1 ± 3.1 | 0.000002 | 99.4 ± 0.5 | 0.193422 | 16.8 ± 1.9 | 0.006689 | 110.4 ± 4.5 | 0.003146 |
| 4 | 91.9 ± 3.8 | 0.271246 | 99.0 ± 0.8 | 0.009535 | 15.2 ± 1.4 | 0.780352 | 110.5 ± 4.8 | 0.002654 |
| 5 | 89.6 ± 3.5 | 0.330509 | 99.6 ± 0.5 | 0.678310 | 15.5 ± 2.0 | 0.678310 | 108.8 ± 5.9 | 0.002654 |
| 6 | 91.2 ± 4.6 | 0.381557 | 99.7 ± 0.5 | 1 | 16.4 ± 1.6 | 0.003241 | 113.7 ± 4.7 | 0.261975 |
| Average | 92.6 ± 4.4 | - | 99.4 ± 0.6 | - | 15.5 ± 1.8 | - | 113.3 ± 5.8 | - |
| Group | Survived | Survival time | 95%CI |
| (n) | (h) | ||
| Control (n = 5) | 0 | 112.8 ± 2.06 | 108.76-116.84 |
| Experimental (n = 10) | 5 | 128 ± 3.32 | 121.49-134.51 |
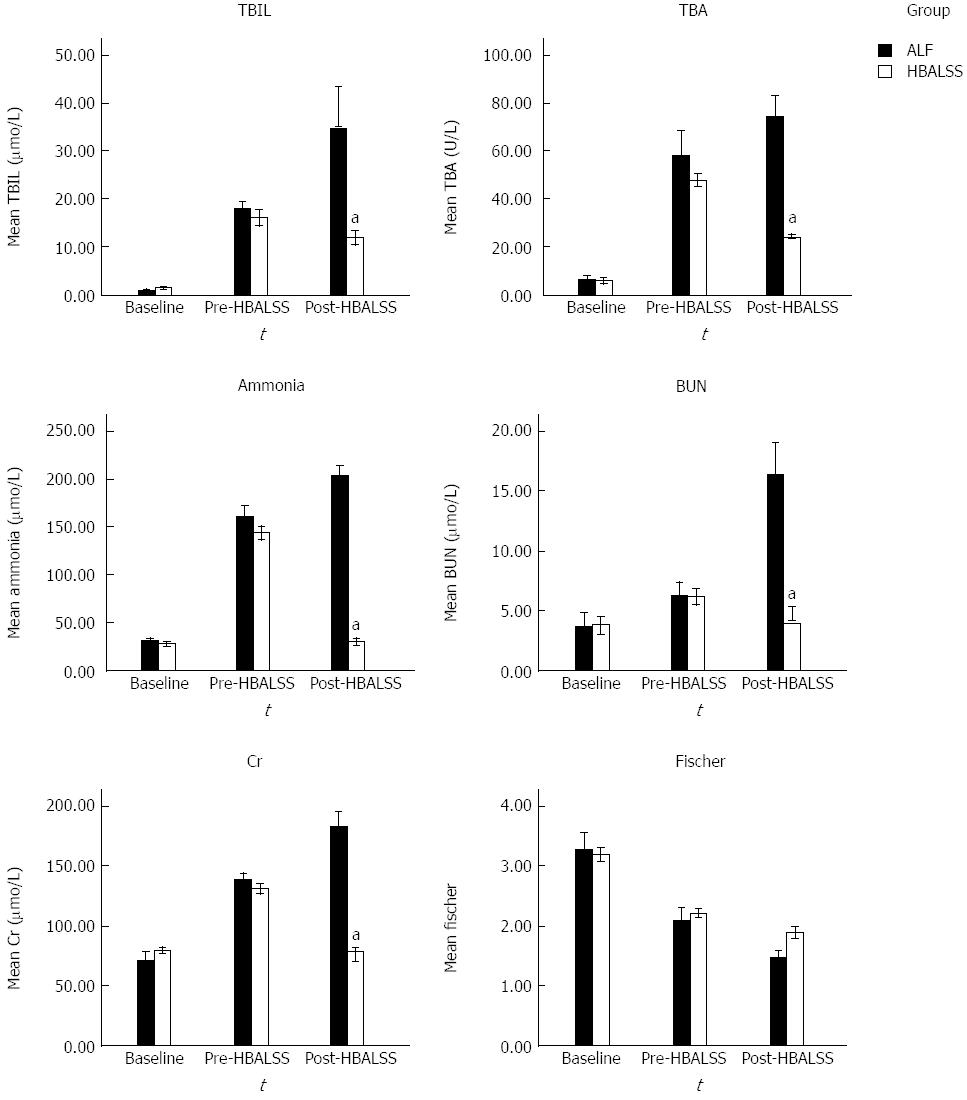
As shown in Figure 3, the levels of ALT, total bilirubin, total bile acid, urea nitrogen, creatinine, and ammonia increased in the ALF control group, but levels of ALB and the Fischer index decreased. However, in the HBALSS treatment group, the baseline levels were normal, and before treatment, the levels of ALT, total bilirubin, total bile acid, urea nitrogen, creatinine and ammonia increased, but there was a decrease in the level of ALB and in the Fischer index. After treatment, only the Fischer index continued to decrease.
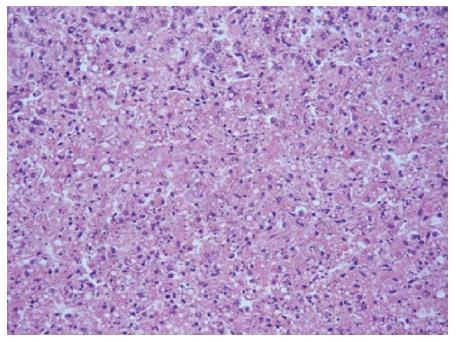
There was no bloody ascites observed in any of the dead monkeys, but the livers were slightly enlarged, soft, blunt in the edges, and the liver capsules were smooth. In the ALF control group, portions of the liver surfaces showed mottled coloring, and there was sporadic bleeding. Cross sectioning of the livers showed blood congestion and yellow milky-like necrosis substances. The remaining livers were red or brownish gray in color and the edges were free of bile.
Hepatocytes showed diffuse swelling and were sinusoidal. These hepatocytes appeared narrow due to pressure. Their cytoplasm appeared loose, and there were signs of vacuolar degeneration, nuclear fragmentation, and dissolution. Part of the portal areas showed small amounts of neutrophil and lymphocyte infiltration (Figure 4).
A novel human cell hybrid bioartificial liver, designed to provide a bridge therapy for ALF patients awaiting a compatible organ, was developed in this study. This system is comprised of human hepatic CL-1 cells grown in microgravity culture and seeded in a perfusion bioreactor. For the pre-clinical evaluation of this HBALSS, a stable, potentially reversible, and well reproducible large-animal model of ALF was devised with cynomolgus monkeys. The cynomolgus monkey is the closest evolutionary primate to humans, making it the most suitable animal model for the preclinical evaluation of artificial livers. Studies have shown that ALF induced by D-gal is highly reproducible and potentially reversible[16,17]. Moreover, the histology of livers used in this model is very similar to hepatocytes infected with hepatitis virus or drug-induced ALF in humans[18]. Therefore, as described by Terblanche et al[19], this is an ideal animal model of ALF.
To verify the safety of HBALSS used in this study, we monitored adverse reactions and vital signs of these monkeys throughout the treatment period. All monkeys tolerated the treatment well, with no serious complications, such as bleeding, clotting, allergies, or high fever.
To optimize treatment efficacy, we used human hepatic CL-1 cells, which displayed a high level of differentiation and metabolic function in our previous studies[14,20]. There are many different human liver cell lines available, all with varied differentiation and metabolic properties[21-25]. Although CL-1 cells are derived from histologically normal liver tissue, they display a certain degree of tumorigenicity, though less so than in other liver cell lines obtained from tumors. The DNA index of CL-1 cells is 1.43, indicating that they are well-differentiated, but not normal diploid cells. These cells are immortalized, and thus able to be maintained in culture, providing a distinct advantage to this cell line. Moreover, we previously showed that nude mice inoculated subcutaneously with CL-1 cell debris, failed to develop tumors[20].
The new bioartificial liver examined in this study employs a double-barrier system, which uses a second sub-plasma technology that effectively prevents the leakage of hepatocytes from the bioreactor into the patient. The survival time of the animals is critical for evaluating the efficacy of the HBALSS[26-28]. Flendrig et al[29] reported that Academic Medical Center bioartificial liver treatment for swine acute hepatic failure, using 1.4 × 1010 porcine hepatocytes, significantly reduced blood ammonia levels and prolonged survival. In this study, none of the monkeys died during the treatment of HBALSS, while the survival rate after treatment was 50%. However, untreated monkeys with ALF died at 120 h, demonstrating that HBALSS treatment effectively prolongs the survival time of animals.
ALF is mainly due to metabolic disorders resulting from abrupt, massive necrosis of hepatocytes. Thus, an effective and alternative artificial liver supporting system must be able to replace the liver function for detoxification, metabolism, and synthesis. The bioartificial liver supporting system for ALF patients is mainly dependent on the biologic functionality of donor hepatocytes. Here, 4 × 109 human hepatocytes were seeded into the reactor; the same cell number, viability, and function were confirmed 24 h after seeding, thus meeting the requirements for an artificial liver. Preliminary results suggested that the animals could not tolerate more than 6 h of treatment, which is why only 6 h of hybrid bioartificial liver treatment was carried out. HBALSS reduced the levels of liver enzymes, such as ALT, bilirubin and total bile acid in the ALF animal, suggesting that the bioreactor hepatocytes play an active role in detoxification. Further, HBALSS treatment resulted in significant increases in ALB levels, likely resulting from both enhanced production by the animal’s liver and secretion by bioreactor hepatocytes. Serum ALB was filled in the bioreactor and pipes before the experiment, also contributing to the observed elevation in levels. Therefore, HBALSS could improve the animal’s clinical levels and extend the survival time, confirming the effectiveness of using the hybrid bioartificial liver.
Studies have shown that the accumulation of ammonia and aromatic amino acids is closely related to hepatic encephalopathy[30-32]. Blood ammonia levels and the Fischer index are two key indicators used for HBALSS evaluation. HBALSS treatment reduced ammonia and aromatic amino acid levels in the ALF animal, improving the Fischer index. Further, rapid reduction of blood ammonia and elevated Fischer indices indicated that the hepatocytes in the bioreactor could remove ammonia and had a role in balancing amino acids.
In summary, the perfusion bioreactor seeded with CL-1 cells cultured in RCCS, was effective as a new human cell hybrid bioartificial liver. This HBALSS significantly reduced the concentrations of serum AST, total bilirubin, total bile acid, urea nitrogen, and creatinine, as well as improved the Fischer index and survival time. The results in this animal model of ALF are encouraging and will need to be explored further in phase I clinical trials to assess its potential as a safe, reliable bridge treatment for ALF patients awaiting donor-organ availability.
Acute liver failure (ALF) is a critical clinical condition with intensive, rapid progression and an ominous prognosis. The mortality rate of ALF remains high at approximately 80%. Liver transplantation is currently the only effective treatment for ALF. However, due to various reasons, a limited number of liver transplantations are performed.
To bridge the time between ALF and suitable organ availability, patients are placed on a bioartificial liver support system. Bioartificial systems use liver cells as a biologic component to partially take over the synthetic, regulatory and detoxifying functions of the liver. The research hotspot is how to improve cell viability and function in the bioreactor.
In this study, the perfusion bioreactor with Chinese human liver cells was effective as a new human cell hybrid bioartificial liver. This hybrid bioartificial liver support system (HBALSS) was found to significantly reduce the concentrations of serum biochemistry levels and extend animal survival time. Furthermore, the number, viability, and function of hepatocytes in the HBALSS were maintained at a high level during treatment. These results demonstrate that the novel HBALSS has the potential to be a safe, reliable bridge treatment for ALF patients.
The experimental results achieved in this study will further validate the safety and effectiveness of the system while exploring its feasibility in clinical applications. The results in this animal model will need to be explored further in clinical trials to assess its potential as a safe, reliable bridge treatment for ALF patients.
HBALSS is an experimental extracorporeal device that uses living cell lines to provide detoxification and synthesis support to the failing liver. It is usually used as a bridge treatment for ALF patients waiting for liver transplantation.
This is an experimental study to evaluate the safety and efficacy of HBALSS in a cynomolgus monkey model of ALF and assess its feasibility in clinical application. The authors demonstrate successful establishment of the animal model of ALF, and show that the novel hybrid bioartificial liver can significantly reduce serum biochemistry levels and extend survival time, displaying its role in liver support.
P- Reviewer: Hilmi IA, Hashimoto N, Waisberg J S- Editor: Gou SX L- Editor: AmEditor E- Editor: Liu XM
| 2. | Shakil AO, Kramer D, Mazariegos GV, Fung JJ, Rakela J. Acute liver failure: clinical features, outcome analysis, and applicability of prognostic criteria. Liver Transpl. 2000;6:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 3. | Chamuleau RA, Deurholt T, Hoekstra R. Which are the right cells to be used in a bioartificial liver? Metab Brain Dis. 2005;20:327-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 61] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Esquivel CO, Keeffe EB, Garcia G, Imperial JC, Millan MT, Monge H, So SK. Resection versus transplantation for hepatocellular carcinoma. J Gastroenterol Hepatol. 1999;14 Suppl:S37-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | van Hoek B, de Boer J, Boudjema K, Williams R, Corsmit O, Terpstra OT. Auxiliary versus orthotopic liver transplantation for acute liver failure. EURALT Study Group. European Auxiliary Liver Transplant Registry. J Hepatol. 1999;30:699-705. [PubMed] |
| 6. | Ostapowicz G, Lee WM. Acute hepatic failure: a Western perspective. J Gastroenterol Hepatol. 2000;15:480-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 7. | Lee KK, Kim DG, Moon IS, Lee MD, Park JH. Liver transplantation versus liver resection for the treatment of hepatocellular carcinoma. J Surg Oncol. 2010;101:47-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 8. | Chan SC. Liver transplantation for fulminant hepatic failure: as early as necessary and as late as possible. J Gastroenterol Hepatol. 2011;26:3-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 9. | Caraceni P, Van Thiel DH. Acute liver failure. Lancet. 1995;345:163-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 75] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Bernal W, Wendon J. Liver transplantation in adults with acute liver failure. J Hepatol. 2004;40:192-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 65] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 11. | Poyck PP, van Wijk AC, van der Hoeven TV, de Waart DR, Chamuleau RA, van Gulik TM, Oude Elferink RP, Hoekstra R. Evaluation of a new immortalized human fetal liver cell line (cBAL111) for application in bioartificial liver. J Hepatol. 2008;48:266-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Filippi C, Keatch SA, Rangar D, Nelson LJ, Hayes PC, Plevris JN. Improvement of C3A cell metabolism for usage in bioartificial liver support systems. J Hepatol. 2004;41:599-605. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Harimoto N, Taketomi A, Kitagawa D, Kuroda Y, Itoh S, Gion T, Tanaka S, Shirabe K, Shimada M, Maehara Y. The newly established human hepatocyte cell line: application for the bioartificial liver. J Hepatol. 2005;42:557-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Li H, Gao Y, Xue GZ, Yang JZ. The feasibility of human liver cell lines in China used as cell materials of bioartificial liver. Zhongguo Linchuang Kangfu. 2004;8: 240-241. |
| 15. | Jones JH. Primates and the evolution of long, slow life histories. Curr Biol. 2011;21:R708-R717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 16. | Kalpana K, Ong HS, Soo KC, Tan SY, Prema Raj J. An improved model of galactosamine-induced fulminant hepatic failure in the pig. J Surg Res. 1999;82:121-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 17. | Schmitz V, Dombrowski F, Prieto J, Qian C, Diehl L, Knolle P, Sauerbruch T, Caselmann WH, Spengler U, Leifeld L. Induction of murine liver damage by overexpression of CD40 ligand provides an experimental model to study fulminant hepatic failure. Hepatology. 2006;44:430-439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | van de Kerkhove MP, Hoekstra R, van Gulik TM, Chamuleau RA. Large animal models of fulminant hepatic failure in artificial and bioartificial liver support research. Biomaterials. 2004;25:1613-1625. [PubMed] |
| 19. | Terblanche J, Hickman R. Animal models of fulminant hepatic failure. Dig Dis Sci. 1991;36:770-774. [PubMed] |
| 20. | Jian GD, Zhang Z, Wang Y, Zhou HC, Zhao YC, Pan MX, Gao Y. The security research of new human hepatocytes hybrid bioartificial liver. Zhongguo Zuzhi Gongcheng Yanjiu Yu Linchuang Kangfu. 2011;15:2129-2132. |
| 21. | Ju SY, Cho KA, Cho SJ, Jung YJ, Woo SY, Seoh JY, Han HS, Ryu KH. Effect of hypoxic treatment on bone marrow cells that are able to migrate to the injured liver. Cell Biol Int. 2009;33:31-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | Schroeder IS, Wiese C, Truong TT, Rolletschek A, Wobus AM. Differentiation analysis of pluripotent mouse embryonic stem (ES) cells in vitro. Methods Mol Biol. 2009;530:219-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Zhang H, Liu Z, Li R, Wang D, Liu W, Li J, Yu H, Zhang F, Dou K. Transplantation of embryonic small hepatocytes induces regeneration of injured liver in adult rat. Transplant Proc. 2009;41:3887-3892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Fonsato V, Herrera MB, Buttiglieri S, Gatti S, Camussi G, Tetta C. Use of a rotary bioartificial liver in the differentiation of human liver stem cells. Tissue Eng Part C Methods. 2010;16:123-132. [PubMed] |
| 25. | Navarro-Alvarez N, Soto-Gutierrez A, Kobayashi N. Hepatic stem cells and liver development. Methods Mol Biol. 2010;640:181-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Sielaff TD, Hu MY, Amiot B, Rollins MD, Rao S, McGuire B, Bloomer JR, Hu WS, Cerra FB. Gel-entrapment bioartificial liver therapy in galactosamine hepatitis. J Surg Res. 1995;59:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 27. | Flendrig LM, Calise F, Di Florio E, Mancini A, Ceriello A, Santaniello W, Mezza E, Sicoli F, Belleza G, Bracco A. Significantly improved survival time in pigs with complete liver ischemia treated with a novel bioartificial liver. Int J Artif Organs. 1999;22:701-709. [PubMed] |
| 28. | Cuervas-Mons V, Colás A, Rivera JA, Prados E. In vivo efficacy of a bioartificial liver in improving spontaneous recovery from fulminant hepatic failure: a controlled study in pigs. Transplantation. 2000;69:337-344. [PubMed] |
| 29. | Flendrig LM, Chamuleau RA, Maas MA, Daalhuisen J, Hasset B, Kilty CG, Doyle S, Ladiges NC, Jörning GG, la Soe JW. Evaluation of a novel bioartificial liver in rats with complete liver ischemia: treatment efficacy and species-specific alpha-GST detection to monitor hepatocyte viability. J Hepatol. 1999;30:311-320. [PubMed] |
| 30. | Ortiz M, Jacas C, Córdoba J. Minimal hepatic encephalopathy: diagnosis, clinical significance and recommendations. J Hepatol. 2005;42 Suppl:S45-S53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 192] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 31. | Bajaj JS. Management options for minimal hepatic encephalopathy. Expert Rev Gastroenterol Hepatol. 2008;2:785-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 32. | Ndraha S, Hasan I, Simadibrata M. The effect of L-ornithine L-aspartate and branch chain amino acids on encephalopathy and nutritional status in liver cirrhosis with malnutrition. Acta Med Indones. 2011;43:18-22. [PubMed] |