Published online Dec 14, 2014. doi: 10.3748/wjg.v20.i46.17345
Revised: April 1, 2014
Accepted: July 22, 2014
Published online: December 14, 2014
Processing time: 292 Days and 17.3 Hours
Neovascularization was reported to arise early in the adenoma-carcinoma sequence in colorectal cancer (CRC), and the importance of angiogenesis in cancer progression has been established. Computed tomography (CT) perfusion (CTP) based on high temporal resolution CT images enables evaluation of hemodynamics of tissue in vivo by modeling tracer kinetics. CTP has been reported to characterize tumor angiogenesis, and to be a sensitive marker for predicting recurrence or survival in CRC. In this review, we will discuss the biomarker value of CTP in the management of CRC patients.
Core tip: The importance of angiogenesis, which is an essential process of colorectal cancer (CRC), has been established. Therefore, the in vivo measure of angiogenesis such as computed tomography (CT) perfusion (CTP) technique can be a robust imaging biomarker in CRC. Especially, CTP certainly fulfils the criteria necessary for prospective validation as a clinical trial end point, because CT is a stable platform, broadly available, and non-invasive. Therefore, we believe that CTP will plays an important role in the management of CRC, providing patients more personalized and effective treatment.
- Citation: Hayano K, Fujishiro T, Sahani DV, Satoh A, Aoyagi T, Ohira G, Tochigi T, Matsubara H, Shuto K. Computed tomography perfusion imaging as a potential imaging biomarker of colorectal cancer. World J Gastroenterol 2014; 20(46): 17345-17351
- URL: https://www.wjgnet.com/1007-9327/full/v20/i46/17345.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i46.17345
Colorectal cancer (CRC) is the second leading cause of cancer death in the United States[1]. About 70% of patients are operated with curative intent; however, up to 30% of these patients will relapse subsequently, usually within 2-3 years[2,3].
In CRC, neovascularization was reported to arise early in the adenoma-carcinoma sequence[4]. Angiogenesis is an essential process of CRC, and plays an important role in the process of growth and metastasis[5,6]. Previous studies reported that angiogenesis could be a useful prognostic marker in almost all carcinomas, including CRC[5-7]. However, currently, the methods for assessing angiogenesis in clinical practice have relied mainly on immunohistological analysis of postoperative specimens such as microvessel density (MVD) counting. The disadvantage of this analysis is that they assess anatomical data only, and can be used only for postoperative specimens. Therefore, the methods, which enable quantitative measure of angiogenesis in in vivo, are highly expected to be a robust biomarker for the management of CRC patients in clinical practice.
Computed tomography perfusion (CTP), which can quantify tumor vascularity by measuring the temporal changes in tissue attenuation following intravenous contrast administration, is readily incorporated into the existing CT protocol, and enables evaluation of hemodynamics of tissue in vivo by modeling tracer kinetics[8,9]. Generally, a small volume of contrast material (40-70 mL depending on concentration) is injected at high flow injection rate (> 4 mL/s), and followed by 20-40 mL of saline flush at a similar flow rate to obtain a narrow bolus for optimal CTP analysis. Both tissue and vascular enhancement by contrast material can be measured and traced over time (45-120 s depending on kinetic model and parameter), and mathematic models such as compartmental or deconvolution analysis for contrast material exchange have been applied to quantify the tissue vascular physiology[10]. Perfusion parameters are dependent on the scan protocol and the kinetic model for perfusion analysis[9,11], but the common CTP parameters were blood flow (BF), blood volume (BV), mean transit time (MTT), and permeability surface area product (PS)[12-16]. CTP has emerged as an important imaging biomarker to evaluate tissue vascular physiology and tumor biology, which has been reported to associate with tumor characterization, survival, and therapy response in CRC[13-15,17,18]. Furthermore, with the increasing use of the neoadjuvant chemoraditaion therapy (CRT) for rectal cancer and the targeted therapy including antiangiogenic therapy in recent various oncology trials, there is a renewed interest in the use of CTP as a surrogate endpoint for monitoring early therapeutic response or predicting treatment outcome[18-22]. Although still considered a research tool in the realm of oncology, CTP offers several potential clinical applications; and therefore, its integration into routine clinical practice is a distinct possibility because most CT scanners now come equipped with sophisticated hardware platforms coupled with powerful and user-friendly software packages for tissue perfusion analysis.
Needless to say, imaging plays an important role in the management of patients with CRC. And CT has become the main diagnostic tool in tumor evaluation, including diagnosis, staging, or monitoring of anticancer therapies because of the relatively low cost and wide availability. In this review, we discuss how CTP can be applied to the management of CRC as an imaging biomarker, reviewing novel clinical approaches of CTP in assessing response to the treatment or predicting survival in CRC patients.
In patients with a locally advanced rectal cancer, neoadjuvant CRT has been recommended, and such neoadjuvant therapy is useful for decreasing the tumor stage to facilitate curative resection and to decrease the rate of recurrence[22,23]. Thus, it is highly desirable and beneficial to develop the noninvasive diagnostic tool to monitor or predict the response to CRT.
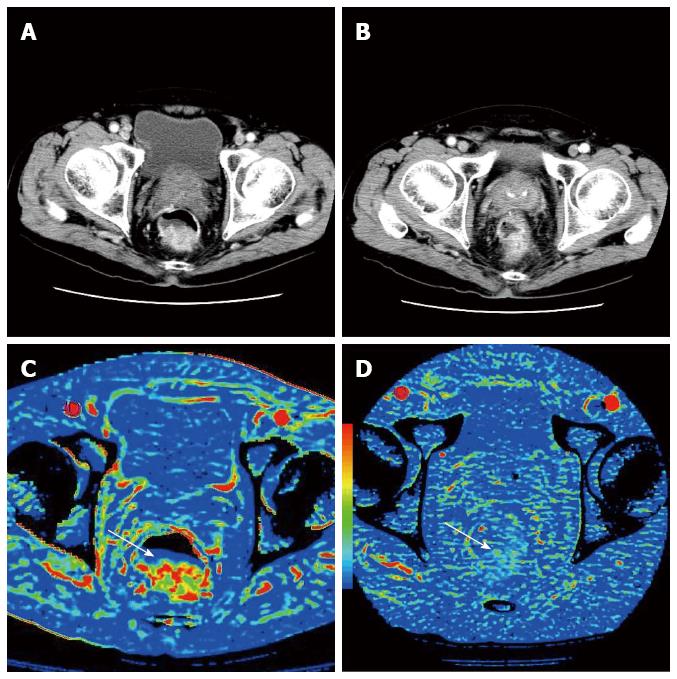
There are several CTP studies reporting perfusion changes of rectal cancer during CRT (Table 1)[13,24,25]. These previous reports demonstrated that BF and BV decreased, whereas MTT increased after CRT (Figure 1). Similar perfusion changes were also reported in CTP studies of head and neck cancer and esophageal cancer treated with CRT[26,27]. These changes may reflect the fibrosis induced by CRT. The tissue fibrosis leads directly to compression of tumor capillaries and increased flow resistance, which leads to decrease in BF and BV, and increase in MTT[14,28]. Another assumption is that these perfusion changes may reflect reduction in MVD. Johansson et al[29] showed that irradiation caused decrease in MVD in a rat glioma model.
| Ref. | Year | Number of patients | Parameter changes | Response Prediction |
| Sahani et al[13] | 2005 | 15 | BF/decrease; MTT/increase | High baseline BF and low MTT associated with poor response |
| (12 responders vs 3 non-responders) | ||||
| Bellomi et al[25] | 2007 | 20 | BF, BV, PS/decrease | High baseline BF and BV associated with good response |
| (17 responders vs 7 non-responders) | ||||
| Curvo-Semedo et al[24] | 2012 | 25 | BF, BV, PS/decrease; MTT/increase | High baseline BF and low MTT associated with poor response |
| (5 responders vs 15 non-responders) |
Previous papers also reported that baseline perfusion values could predict the response to the CRT[24,25]. However, their results are controversial. Bellomi et al[25] showed baseline BF and BV in non-responders to be significantly lower and MTT significantly higher than in responders, while Curvo-Semedo et al[24] showed baseline BF in non-responder to be significantly higher and MTT significantly lower than in responders. The reasons for these discrepancies might be because they used different reference standards to assess response, different patient selection criteria and different kinetic models. The small sample size of these studies also might affect their results. It is highly beneficial to predict the response to CRT so that non-responders could avoid the side effects associated with intensive therapeutic regimens, and therefore, predictive value of CTP in the response to CRT should be evaluated in a prospective study with a larger sample size.
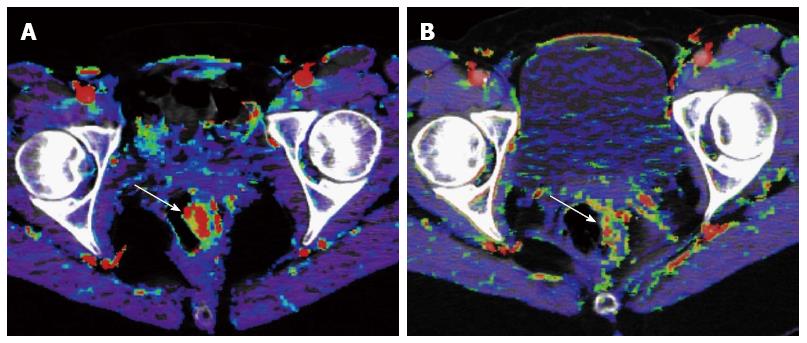
Tumor angiogenesis provides an attractive target for anticancer therapy. Previous clinical trials have established that the addition of antiangiogenic agents to chemotherapy significantly improves survival compared with chemotherapy alone in first-line and second-line treatment of metastatic CRC[30,31]. These studies have shown followings: (1) in the treatment of metastatic CRC, the addition of the VEGF-directed antibody, bevacizumab, to chemotherapy significantly improve outcome in comparison with chemotherapy alone; and (2) bevacizumab has minimum activity as a single agent. Based on these findings, further understanding of the mechanism of the interaction between antiangiogenic agents and chemotherapy is highly beneficial for the personalized therapy in CRC. In addition, the morphologic assessment has a difficulty in distinguishing viable tumor from necrotic or fibrotic tissue because molecular targeted agents suppress tumor growth by downregulating angiogenesis without causing much morphologic change. Thus, there is an increasing interest in the in vivo quantification of angiogenesis based on images such as CTP. A few CTP papers concerning antiangiogenic therapy have been published in CRC (Table 2)[18,32,33]. Willett et al[18,32] reported that tumor BF and BV decreased within two weeks of the initiation of bevacizumab alone, which is a monoclonal antibody that targets VEGF, and they also reported reduction in MVD after the administration of bevacizumab (Figure 2). Anzidei et al[33] reported a CTP study of liver metastases in CRC treated with chemotherapy and bevacizumab. Their study showed reduction in BF and PS after the therapy, but this tendency was not statistically significant. It is assumed that these perfusion changes may reflect the change of MVD, because several previous reports demonstrated positive correlations of BF, BV and PS with MVD[15,34,35]. However, exploitation of the ability of this technique in predicting response to antiangiogenic therapy is still in early stages of development.
| Ref. | Year | Number of patients | Time of CTP after the treatment | Parameter changes |
| Willet et al[18] | 2004 | 6 (primary) | After 12 d of administration of bevacizumab | BF, BV/decrease |
| Willet et al[32] | 2009 | 32 (primary) | After 12 d of administration of bevacizumab | BF, PS/decrease |
| Anzidei et al[33] | 2011 | 18 (liver metastases) | After 181 d of the treatment (including bevacizumab) | BF, BV/slightly decrease (not significant) |
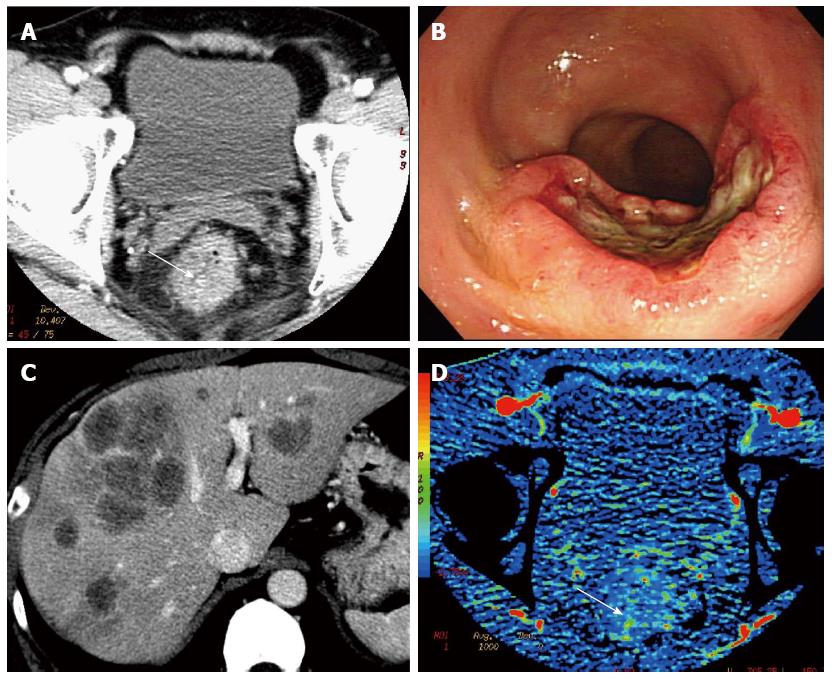
Angiogenesis plays an important role in cancer progression, and could be a useful prognostic marker in almost all carcinomas, including CRC[5-7]. Thus, CTP that can assesses angiogenesis of the tumor in vivo, is highly expected to be an imaging biomarker that can reflect clinical outcome of CRC. CTP has been reported to be a sensitive marker for predicting recurrence or outcome in CRC[14,16]. Goh et al[16] reported that tumor BF measured by perfusion CT was significantly lower in CRC patients who ultimately developed metastatic disease. Our previous CTP study demonstrated that rectal cancer with low BF, which tended to be accompanied by synchronous metastatic lymphnodes or distant metastases (Figure 3), associated with poor overall survival[14]. These authors hypothesized that reduced BF are related to increasing interstitial fluid pressure and hypoxia in tumor tissue[14,16]. Accumulation of excess fluid in the interstitium of tumor and excessive tumor growth in a confined space also leads to increased solid tissue pressure and elevated interstitial fluid pressure, which in turn cause capillary compression, and may reduce blood flow[36,37]. Regarding to relationship between CTP and tissue oxygenation, Haider et al[38] reported that the tumor blood flow on CTP correlated positively with tumor oxygenation in cervical cancer. Hypoxic environment plays an important role in cancer progression with promoting oncogenic mutations, cell survival, and more aggressive behavior in tumors[39]. Therefore, low BF tumor may correlate with poor survival. But we have to say that CTP measurements to clinical outcomes remain limited. These reports are based on small and single center studies. Therefore, we need to confirm these results with larger multicenter trials.
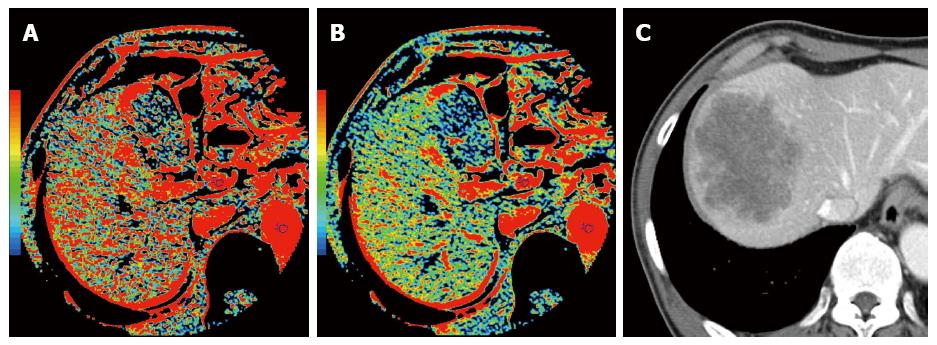
Several studies have suggested the relationship between tumor progression and hemodynamic changes in the liver. In 1983, Leveson et al[40,41] reported that gastrointestinal cancer patients with simultaneous liver metastasis exhibited a higher hepatic arterial blood flow measured with scintigraphy. In CRC with simultaneous liver metastases, Leen et al[42] demonstrated a significant increase in the hepatic arterial blood flow and a significant decrease in the portal blood flow compared with those observed in healthy volunteers using doppler ultrasonography study. The CTP technique was also applied to this investigation of the relationship between tumor progression and hemodynamic changes in the liver. In liver CTP studies of patients with known metastatic disease, increased arterial perfusion has been shown by Miles et al[43] and Blomley et al[44]. Cuenod et al[45] reported that hemodynamic changes, including decrease in the portal blood flow and increase in the mean transit time, could be detected using CTP in the rats with occult liver metastases. In CRC with simultaneous liver metastases, Leggett et al[46] reported that the hepatic arterial blood flow significantly increased and the portal blood flow decreased by using CTP. In CTP study of esophageal cancer, Fujishiro et al[47] reported that the preoperative hepatic arterial blood flow might be a useful predictive marker for the future metastases. Therefore, CTP derived hemodynamic change in the hepatic blood flow has a potential to become a novel imaging biomarker to predict recurrence or metastases in CRC patients (Figure 4). A tumor-related circulating vasoactive mediator may contribute to this global perfusion change[48], and further supportive work is necessary[49].
Data relating CTP to clinical outcomes in CRC still remain limited. Relatively small, single center studies have suggested that CTP parameters may reflect clinical outcome in CRC[14,16]. Thus, there is a need to confirm these results with prospective and larger multicenter trials. As CT technology has reached maturity, further consideration has to be given to the direction of CTP research. As an imaging biomarker, CTP certainly fulfils the criteria necessary for prospective validation as a clinical trial end point, because CT is a stable platform, widely available, and non-invasive. Therefore, we believe that CTP technique will play an important role in the management of patients with CRC as a key imaging technique in clinical practice, providing patients more personalized treatment.
P- Reviewer: Caboclo LF, Cai SJ, Kato J S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9215] [Cited by in RCA: 9855] [Article Influence: 821.3] [Reference Citation Analysis (4)] |
| 2. | Sargent DJ, Wieand HS, Haller DG, Gray R, Benedetti JK, Buyse M, Labianca R, Seitz JF, O’Callaghan CJ, Francini G. Disease-free survival versus overall survival as a primary end point for adjuvant colon cancer studies: individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol. 2005;23:8664-8670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 491] [Cited by in RCA: 522] [Article Influence: 26.1] [Reference Citation Analysis (0)] |
| 3. | Sargent DJ, Patiyil S, Yothers G, Haller DG, Gray R, Benedetti J, Buyse M, Labianca R, Seitz JF, O’Callaghan CJ. End points for colon cancer adjuvant trials: observations and recommendations based on individual patient data from 20,898 patients enrolled onto 18 randomized trials from the ACCENT Group. J Clin Oncol. 2007;25:4569-4574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 193] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 4. | Rak J, Mitsuhashi Y, Bayko L, Filmus J, Shirasawa S, Sasazuki T, Kerbel RS. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55:4575-4580. [PubMed] |
| 5. | Des Guetz G, Uzzan B, Nicolas P, Cucherat M, Morere JF, Benamouzig R, Breau JL, Perret GY. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823-1832. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 255] [Cited by in RCA: 284] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 6. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6437] [Cited by in RCA: 6480] [Article Influence: 259.2] [Reference Citation Analysis (0)] |
| 7. | Jain RK, Carmeliet P. SnapShot: Tumor angiogenesis. Cell. 2012;149:1408-1408.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 8. | Axel L. Cerebral blood flow determination by rapid-sequence computed tomography: theoretical analysis. Radiology. 1980;137:679-686. [PubMed] |
| 9. | Miles KA, Hayball M, Dixon AK. Colour perfusion imaging: a new application of computed tomography. Lancet. 1991;337:643-645. [PubMed] |
| 10. | García-Figueiras R, Goh VJ, Padhani AR, Baleato-González S, Garrido M, León L, Gómez-Caamaño A. CT perfusion in oncologic imaging: a useful tool? AJR Am J Roentgenol. 2013;200:8-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 11. | Kambadakone AR, Sahani DV. Body perfusion CT: technique, clinical applications, and advances. Radiol Clin North Am. 2009;47:161-178. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 163] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 12. | Kambadakone AR, Sharma A, Catalano OA, Hahn PF, Sahani DV. Protocol modifications for CT perfusion (CTp) examinations of abdomen-pelvic tumors: impact on radiation dose and data processing time. Eur Radiol. 2011;21:1293-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 13. | Sahani DV, Kalva SP, Hamberg LM, Hahn PF, Willett CG, Saini S, Mueller PR, Lee TY. Assessing tumor perfusion and treatment response in rectal cancer with multisection CT: initial observations. Radiology. 2005;234:785-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 219] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Hayano K, Shuto K, Koda K, Yanagawa N, Okazumi S, Matsubara H. Quantitative measurement of blood flow using perfusion CT for assessing clinicopathologic features and prognosis in patients with rectal cancer. Dis Colon Rectum. 2009;52:1624-1629. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Goh V, Halligan S, Daley F, Wellsted DM, Guenther T, Bartram CI. Colorectal tumor vascularity: quantitative assessment with multidetector CT--do tumor perfusion measurements reflect angiogenesis? Radiology. 2008;249:510-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 16. | Goh V, Halligan S, Wellsted DM, Bartram CI. Can perfusion CT assessment of primary colorectal adenocarcinoma blood flow at staging predict for subsequent metastatic disease? A pilot study. Eur Radiol. 2009;19:79-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 68] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Goh V, Halligan S, Hugill JA, Gartner L, Bartram CI. Quantitative colorectal cancer perfusion measurement using dynamic contrast-enhanced multidetector-row computed tomography: effect of acquisition time and implications for protocols. J Comput Assist Tomogr. 2005;29:59-63. [PubMed] |
| 18. | Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1468] [Cited by in RCA: 1441] [Article Influence: 68.6] [Reference Citation Analysis (0)] |
| 19. | Koukourakis MI, Mavanis I, Kouklakis G, Pitiakoudis M, Minopoulos G, Manolas C, Simopoulos C. Early antivascular effects of bevacizumab anti-VEGF monoclonal antibody on colorectal carcinomas assessed with functional CT imaging. Am J Clin Oncol. 2007;30:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 20. | Tai JH, Tessier J, Ryan AJ, Hoffman L, Chen X, Lee TY. Assessment of acute antivascular effects of vandetanib with high-resolution dynamic contrast-enhanced computed tomographic imaging in a human colon tumor xenograft model in the nude rat. Neoplasia. 2010;12:697-707. [PubMed] |
| 21. | Ren Y, Fleischmann D, Foygel K, Molvin L, Lutz AM, Koong AC, Jeffrey RB, Tian L, Willmann JK. Antiangiogenic and radiation therapy: early effects on in vivo computed tomography perfusion parameters in human colon cancer xenografts in mice. Invest Radiol. 2012;47:25-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1109] [Article Influence: 69.3] [Reference Citation Analysis (2)] |
| 23. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4454] [Article Influence: 212.1] [Reference Citation Analysis (1)] |
| 24. | Curvo-Semedo L, Portilha MA, Ruivo C, Borrego M, Leite JS, Caseiro-Alves F. Usefulness of perfusion CT to assess response to neoadjuvant combined chemoradiotherapy in patients with locally advanced rectal cancer. Acad Radiol. 2012;19:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | Bellomi M, Petralia G, Sonzogni A, Zampino MG, Rocca A. CT perfusion for the monitoring of neoadjuvant chemotherapy and radiation therapy in rectal carcinoma: initial experience. Radiology. 2007;244:486-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 150] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 26. | Hayano K, Shuto K, Satoh A, Aoyagi T, Narushima K, Gunji H, Kono T, Yanagawa N, Okazumi S, Matsubara H. Tumor blood flow change measured by CT perfusion during chemoradiation therapy (CRT) for monitoring response and predicting survival in patients with esophageal cancer. Esophagus. 2014;11:72-79. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 27. | Surlan-Popovic K, Bisdas S, Rumboldt Z, Koh TS, Strojan P. Changes in perfusion CT of advanced squamous cell carcinoma of the head and neck treated during the course of concomitant chemoradiotherapy. AJNR Am J Neuroradiol. 2010;31:570-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 28. | Satoh A, Shuto K, Okazumi S, Ohira G, Natsume T, Hayano K, Narushima K, Saito H, Ohta T, Nabeya Y. Role of perfusion CT in assessing tumor blood flow and malignancy level of gastric cancer. Dig Surg. 2010;27:253-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | Johansson M, Bergenheim AT, Widmark A, Henriksson R. Effects of radiotherapy and estramustine on the microvasculature in malignant glioma. Br J Cancer. 1999;80:142-148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 30. | Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7832] [Cited by in RCA: 7723] [Article Influence: 367.8] [Reference Citation Analysis (1)] |
| 31. | Hurwitz HI, Fehrenbacher L, Hainsworth JD, Heim W, Berlin J, Holmgren E, Hambleton J, Novotny WF, Kabbinavar F. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol. 2005;23:3502-3508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 491] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 32. | Willett CG, Duda DG, di Tomaso E, Boucher Y, Ancukiewicz M, Sahani DV, Lahdenranta J, Chung DC, Fischman AJ, Lauwers GY. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J Clin Oncol. 2009;27:3020-3026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 387] [Article Influence: 24.2] [Reference Citation Analysis (0)] |
| 33. | Anzidei M, Napoli A, Zaccagna F, Cartocci G, Saba L, Menichini G, Cavallo Marincola B, Marotta E, Di Mare L, Catalano C. Liver metastases from colorectal cancer treated with conventional and antiangiogenetic chemotherapy: evaluation with liver computed tomography perfusion and magnetic resonance diffusion-weighted imaging. J Comput Assist Tomogr. 2011;35:690-696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 34. | d’Assignies G, Couvelard A, Bahrami S, Vullierme MP, Hammel P, Hentic O, Sauvanet A, Bedossa P, Ruszniewski P, Vilgrain V. Pancreatic endocrine tumors: tumor blood flow assessed with perfusion CT reflects angiogenesis and correlates with prognostic factors. Radiology. 2009;250:407-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 193] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 35. | Chen Y, Zhang J, Dai J, Feng X, Lu H, Zhou C. Angiogenesis of renal cell carcinoma: perfusion CT findings. Abdom Imaging. 2010;35:622-628. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 63] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Helmlinger G, Netti PA, Lichtenbeld HC, Melder RJ, Jain RK. Solid stress inhibits the growth of multicellular tumor spheroids. Nat Biotechnol. 1997;15:778-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 601] [Cited by in RCA: 509] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 37. | Griffon-Etienne G, Boucher Y, Brekken C, Suit HD, Jain RK. Taxane-induced apoptosis decompresses blood vessels and lowers interstitial fluid pressure in solid tumors: clinical implications. Cancer Res. 1999;59:3776-3782. [PubMed] |
| 38. | Haider MA, Milosevic M, Fyles A, Sitartchouk I, Yeung I, Henderson E, Lockwood G, Lee TY, Roberts TP. Assessment of the tumor microenvironment in cervix cancer using dynamic contrast enhanced CT, interstitial fluid pressure and oxygen measurements. Int J Radiat Oncol Biol Phys. 2005;62:1100-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 39. | Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2218] [Cited by in RCA: 2371] [Article Influence: 169.4] [Reference Citation Analysis (0)] |
| 40. | Leveson SH, Wiggins PA, Nasiru TA, Giles GR, Robinson PJ, Parkin A. Improving the detection of hepatic metastases by the use of dynamic flow scintigraphy. Br J Cancer. 1983;47:719-721. [PubMed] |
| 41. | Leveson SH, Wiggins PA, Giles GR, Parkin A, Robinson PJ. Deranged liver blood flow patterns in the detection of liver metastases. Br J Surg. 1985;72:128-130. [PubMed] |
| 42. | Leen E, Goldberg JA, Robertson J, Sutherland GR, McArdle CS. The use of duplex sonography in the detection of colorectal hepatic metastases. Br J Cancer. 1991;63:323-325. [PubMed] |
| 43. | Miles KA, Hayball MP, Dixon AK. Functional images of hepatic perfusion obtained with dynamic CT. Radiology. 1993;188:405-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 249] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 44. | Blomley MJ, Coulden R, Dawson P, Kormano M, Donlan P, Bufkin C, Lipton MJ. Liver perfusion studied with ultrafast CT. J Comput Assist Tomogr. 1995;19:424-433. [PubMed] |
| 45. | Cuenod C, Leconte I, Siauve N, Resten A, Dromain C, Poulet B, Frouin F, Clément O, Frija G. Early changes in liver perfusion caused by occult metastases in rats: detection with quantitative CT. Radiology. 2001;218:556-561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 123] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 46. | Leggett DA, Kelley BB, Bunce IH, Miles KA. Colorectal cancer: diagnostic potential of CT measurements of hepatic perfusion and implications for contrast enhancement protocols. Radiology. 1997;205:716-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 92] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 47. | Fujishiro T, Shuto K, Hayano K, Satoh A, Kono T, Ohira G, Tohma T, Gunji H, Narushima K, Tochigi T. Preoperative hepatic CT perfusion as an early predictor for the recurrence of esophageal squamous cell carcinoma: initial clinical results. Oncol Rep. 2014;31:1083-1088. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 48. | Carter R, Anderson JH, Cooke TG, Baxter JN, Angerson WJ. Splanchnic blood flow changes in the presence of hepatic tumour: evidence of a humoral mediator. Br J Cancer. 1994;69:1025-1026. [PubMed] |
| 49. | Pandharipande PV, Krinsky GA, Rusinek H, Lee VS. Perfusion imaging of the liver: current challenges and future goals. Radiology. 2005;234:661-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 228] [Article Influence: 11.4] [Reference Citation Analysis (0)] |