Published online Dec 7, 2014. doi: 10.3748/wjg.v20.i45.17179
Revised: July 20, 2014
Accepted: September 29, 2014
Published online: December 7, 2014
Processing time: 200 Days and 4.1 Hours
AIM: To evaluate our experience of the clinical management of spontaneous isolated superior mesenteric artery dissection (ISMAD).
METHODS: From January 2008 to July 2013, 18 patients with ISMAD were retrospectively analyzed, including 7 patients who received conservative therapy, 9 patients who received reconstruction with bare stents, and 2 patients who underwent surgical treatment. The decision to intervene was based on anatomic suitability, patient comorbidities and symptoms.
RESULTS: Intestinal ischemia-related symptoms completely resolved in 7 patients who received conservative therapy. Stent placement was successful in 9 patients. Of the 9 patients who received endovascular stenting, abdominal pain was alleviated after the procedure and gradually disappeared within 3 d. Follow-up computed tomography and computed tomography angiography were available in all patients during the first month and the first year after the procedure, which revealed patent stent and patent involved superior mesenteric artery branches with complete obliteration of the dissection lesion. In the 2 patients who underwent surgical treatment, good clinical efficacy was also observed.
CONCLUSION: ISMAD may be managed successfully in a variety of ways based on the clinical symptoms. ISMAD should be treated by conservative management as the first-line option, however, in those with bowel necrosis or imminent arterial rupture during conservative therapy, endovascular or surgical therapy is indicated.
Core tip: Therapeutic options for isolated superior mesenteric artery dissection include conservative management, endovascular treatments or open surgery. In this small series, conservative therapy was indicated for asymptomatic patients or those with short-term symptoms, while endovascular or surgical therapy was recommended for those with clinical or imaging evidence of bowel necrosis or imminent arterial rupture. Percutaneous endovascular reconstruction with bare stent implantation is a feasible treatment choice with a high success rate and good clinical outcome.
- Citation: Lv PH, Zhang XC, Wang LF, Chen ZL, Shi HB. Management of isolated superior mesenteric artery dissection. World J Gastroenterol 2014; 20(45): 17179-17184
- URL: https://www.wjgnet.com/1007-9327/full/v20/i45/17179.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i45.17179
Since the first description by Bauersfeld[1] in 1947, occasional cases of spontaneous isolated superior mesenteric artery dissection (ISMAD) have been reported. However, early identification in the acute stage is increasingly possible due to the development of advanced imaging technology, in particular, abdominal computed tomography (CT) scanning[2,3]. Therapeutic options include conservative management[4-7], endovascular treatments or open surgery such as bypass or direct surgical reconstruction of the lesion, although no consensus has been reached with regard to the best treatment modality[8-13]. From January 2008 to July 2013, 18 patients with ISMAD received treatment in our center. We adopted a treatment algorithm based on the current literature and report our results in these patients.
We retrospectively reviewed the medical history of 18 patients who were diagnosed with ISMAD from January 2008 to July 2013 in our institution. There were 14 males and 4 females with a mean age of 49.8 years (range, 36-66 years). All patients presented with sudden upper abdominal, low back pain, nausea, vomiting, bloody stools, and diarrhea. Of the 18 patients in this series, 6 had hypertension, 7 had dyslipidemia, 2 had diabetes and 9 had a history of smoking. Presenting symptoms and risk factors in these 18 patients are shown in Table 1.
| Patients age (yr/sex) | Symptoms | Risk factors | Percentage of true lumen (%) | Treatment outcome | Follow-up (mo) |
| 46/M | Abdominal pain | Smoking | 25 | Pain resolution | 32 |
| 56/M | Abdominal pain | Hypertension, diabetes | 40 | Pain resolution | 28 |
| 66/M | Abdominal pain radiating to the back | Hypertension | 60 | Pain resolution | 33 |
| 40/M | Abdominal pain, nausea, vomiting | Smoking, hypertension | 0 | Pain resolution | 11 |
| 48/M | Abdominal pain | Smoking | 45 | Pain resolution | 32 |
| 52/M | Abdominal pain, Diarrhea | Dyslipidemia | 36 | Pain resolution | 32 |
| 61/F | Abdominal pain | Smoking | 48 | Pain resolution | 14 |
| 43/M | Abdominal pain, bloody stools | Smoking, hypertension | 30 | Pain resolution | 26 |
| 49/M | Abdominal pain | Smoking | 0 | Pain resolution | 16 |
| 48/M | Abdominal pain | Smoking | 10 | Pain resolution | 16 |
| 36/F | Abdominal pain, bloody stools | Dyslipidemia | 62 | Pain resolution | 22 |
| 39/M | Abdominal pain | Hypertension | 0 | Pain resolution | 18 |
| 44/M | Abdominal pain | Hypertension, diabetes | 20 | Pain resolution | 22 |
| 45/M | Abdominal pain | Dyslipidemia | 8 | Pain resolution | 28 |
| 58/F | Abdominal pain | Smoking, dyslipidemia | 20 | Pain resolution | 17 |
| 52/M | Abdominal pain, nausea, vomiting | Dyslipidemia | 52 | Pain resolution | 12 |
| 55/M | Abdominal pain | Dyslipidemia | 12 | Pain resolution | 18 |
| 58/F | Abdominal pain | Smoking, dyslipidemia | 20 | Pain resolution | 14 |
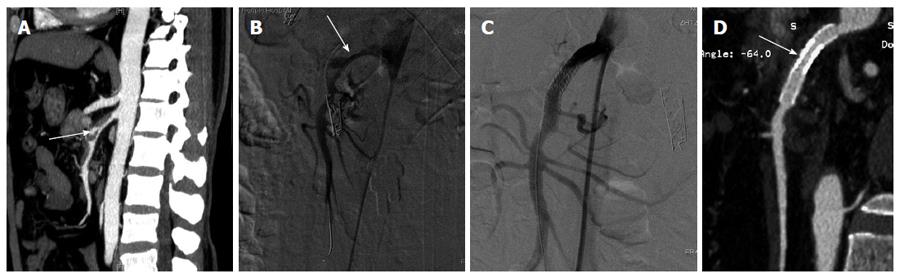
ISMAD was detected via upper abdominal contrast-enhanced CT examination in all patients. The distance from the superior mesenteric artery (SMA) ostium to the origin of the dissection was measured using 3-dimensional (3D) reconstructions of CT scans. The percentage of true lumen compression by the false lumen was also calculated from CT scans. The diagnosis of ISMAD was made when one of the following signs was seen in the SMA on the initial CT: (1) intimal flap and contrast enhancement within the false lumen (Figure 1A); or (2) a crescent-shaped area along the wall of the SMA with higher attenuation than blood, showing no contrast enhancement after injection of contrast material.
Conservative therapy was performed in 7 patients focusing on antiplatelet therapy, blood pressure control, bowel rest, intravenous fluids, and nutritional support with or without antithrombotic therapy. Abdominal pain was gradually alleviated within 7 d in these 7 patients. Percutaneous endovascular reconstruction with bare stents was carried out in 9 patients with persistent abdominal pain despite conservative treatment or signs of bowel ischemia (bloody stools). Two patients underwent surgical treatment. One of these two patients, who failed conservative and endovascular therapy, underwent dissecting aneurysm resection plus vascular prosthesis grafting. The other patient underwent emergent surgery with thrombectomy plus partial intimectomy due to persistent acute abdominal pain and bloody stools. All patients received antithrombotic therapy (anticoagulant, n = 6, antiplatelet agents n = 8, both n = 4).
Under local anesthesia, a 7F short vascular sheath was inserted into the right femoral artery. Selective superior mesenteric arterial angiography was performed with a 4-5F RH catheter (TERUMO, Japan) and the patient was given a bolus of intravenous heparin (50 IU/kg) followed by an infusion to maintain full heparinization (500-1000 IU/h). A 6F or 7F guiding catheter (Envoy; Cordis) was used as an access channel into the proximal SMA. After successfully cannulating a branch of the SMA with a guide-wire (Cordis, United States), a balloon-expandable stent or a self-expanding stent was advanced over the guide-wire and was deployed covering the entire dissected segment. The stents used included various types of self-expanding stents (PRECISE, Cordis; WALLSTENT, Boston Scientific; RX Acculink, Abbott) and balloon-expandable renal stents (Medtronic, Inc). Patients who underwent endovascular bare stent placement were instructed to take aspirin (150 mg/d) and clopidogrel (75 mg/d) orally for 6 mo postoperatively.
Follow-up data were obtained at outpatient examinations twice or three times per year. Imaging follow-up using CT was routinely performed once during the first month, and once within the following year.
As described by Yun et al[14], ISMAD was classified into three types based on the presence of dissection re-entry and SMA main trunk patency at initial diagnosis. Four patients were type I, five were type IIa, six were type IIb, and three were type III. The entry sites were all located within a short distance of the ostium. The distance from the SMA ostium to the origin of the dissection ranged from 0.6 cm to 4.8 cm (mean, 2.1 cm). The medium percentage of true lumen compression by the false lumen was 27.1% (range: 0%-62%) (Table 1). Two patients had aneurysmal dilatation of the SMA at the time of presentation. The diameter of these SMA aneurysms was 1.0 and 1.3 cm, respectively.
Stent placement was successful in 9 patients. A single stent was used in six patients, the overlapping stent technique was used in two patients (Figure 1), and the triple overlapping technique in one patient (Table 2). The diameter of the dissection proximal normal artery, the length of the dissection and the SMA branches involved are shown in Table 2.
| Patients | Proximal arterial diameter (mm) | Length of dissection (mm) | Number of branches involved | Stent size | Stent type |
| 1 | 5 | 23 | 1 | 6 × 18 | Balloon-expandable (Palmaz Blue) |
| 7 × 30 | self-expanding (Precise) | ||||
| 4 | 5.6 | 30 | 2 | 6 × 40 | Self-expanding (Precise) |
| 8 | 6 | 15 | 0 | 7 × 30 | Self-expanding (Precise) |
| 10 | 7 | 35 | 2 | 7 × 18 | Balloon-expandable (Palmaz Blue) |
| 8 × 40 | self-expanding (Precise) | ||||
| 8 × 40 | Self-expanding (Precise) | ||||
| 13 | 5.5 | 10 | 0 | 6 × 18 | Balloon-expandable (Palmaz Blue) |
| 14 | 5.4 | 18 | 1 | 6 × 30 | self-expanding (Precise) |
| 15 | 6.4 | 28 | 1 | 7 × 15 | Balloon-expandable (Palmaz Blue) |
| 8 × 30 | self-expanding (Precise) | ||||
| 17 | 7 | 19 | 1 | 7 × 30 | Self-expanding (Precise) |
| 18 | 5.8 | 22 | 1 | 6 × 30 | Self-expanding (Precise) |
Intestinal ischemia-related symptoms completely resolved in the patients who received conservative therapy. Of the 9 patients receiving endovascular stenting, abdominal pain was alleviated after the procedure and gradually disappeared within 3 d. Follow-up CT and CTA were available in all patients during the first month and the first year after the procedure, which revealed patent stent and patent involved SMA branches with complete obliteration of the dissection lesion. The patient with ISMAD who underwent resection plus vascular prosthesis grafting and another patient who underwent thrombectomy plus intimectomy recovered well, without intestinal ischemia-related symptoms.
Although an increasing number of ISMADs have been reported in recent years due to the development of advanced imaging technology[2,3], the natural history of ISMAD is not well understood due to the complexity and rarity of the condition. In the present study, six patients (6/18) presented with hypertension, however, there was no atherosclerotic change in the orifice and the proximal segment of SMA. It seems that different mechanisms are involved in ISMAD as compared with aortic dissection. With regard to the causes of ISMAD, hemodynamic abnormalities in the SMA at the transitional point may be a major cause of ISMAD[15-17].
Isolated SMA dissections are most commonly diagnosed by CT scanning, but may also be diagnosed by ultrasound imaging, MRI, or angiogram[10]. The clinical presentation in our ISMAD patients was acute abdominal pain. This pain is related to stenosis of the true lumen causing mesenteric ischemia[18], or to rupture of the dissection causing a mesenteric hematoma[3]. Yun et al[14] classified ISMADs into three types based on their imaging findings. In our eighteen ISMAD patients, four were type I, five were type IIa, six were type IIb, and two were type III. Endovascular procedures were performed in type I (1), type IIb (4), type IIa (3) and type III (1). Surgery was required in two patients (type III). This indicated that ISMAD with SMA occlusion can be monitored carefully and the patient can undergo endovascular or surgical treatment if necessary. Therefore, the treatment of ISMAD should be partially based on imaging findings[19].
The treatment methods for ISMAD are not universally agreed[19-22]. In the present study, symptomatic patients were treated by conservative management as first-line treatment, including hypotensive and antiplatelet measures, bowel rest, intravenous fluids, and nutritional support, which showed favorable efficacy in 7 patients in whom abdominal pain was gradually alleviated within 5 d. However, if conditions such as aneurysmal false lumen dilation, rupture of artery, persistent abdominal pain, or ISMAD-induced severe intestinal ischemia during conservative therapy are observed, surgical or interventional therapy is indicated. Surgery was required when there was non-viable bowel or where endovascular techniques were considered inappropriate or failed. The surgical procedure for ISMAD included thrombectomy and intima resection.
Recently, interest has centered on endovascular treatment with the use of bare stents for ISMAD[10,16,19,22-25]. There are two aims of endovascular treatment:one is to prevent the extension of dissection and the other is to increase blood flow into the small intestine by obliterating the false lumen[9,16]. The choice of stent diameter was based on the proximal normal artery diameter. The types of bare stents were determined according to the operator’s preference and availability of the stent. In our study, we chose the self-expandable carotid bare metal stent, due to its radial strength, multisegmental design, and good compliance. This stent is generally appropriate for the SMA, which is characterized by weakened vascular wall and curved original site[19]. A balloon-expandable stent (Palmaz Blue) is also useful if the dissection is short.
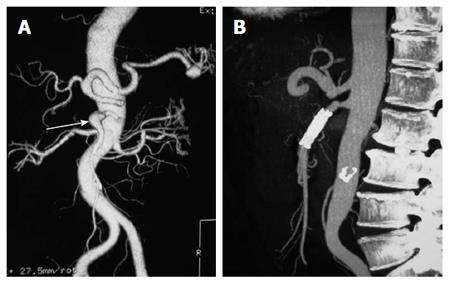
A bare stent is sometimes sufficient for compressing the septum of an ISMAD, opening the stenotic segment and increasing inflow to remedy the ischemia, and with gradual endothelialization of the stent, the thrombosis or the occlusion inside the false lumen can be observed. However, in some cases, such as ruptured, expanding, or large pseudo-aneurysms, and in cases of remodeling failure with single stent treatment, multiple stents are required. In our multiple stent series, 3 ISMADs were treated by a balloon-expandable stent plus a self-expandable stent, however, 1 ISMAD with a pseudoaneurysm was treated using one self-expandable stent (Figure 2). Our experience showed excellent immediate and long-term (11-32 mo) results[14]. The patency rates of the involved stented SMA and SMA branches were determined in all patients during the first month and the first year after the procedure.
In conclusion, symptomatic ISMADs should be treated by conservative management, however, in patients with persistent abdominal, ISMAD-induced severe intestinal ischemia, and chronic intestinal ischemia-related symptoms during conservative therapy, rupture of artery, and obvious aneurysmal false lumen dilation at high risk of rupture, endovascular or surgical therapy is indicated. Although a larger sample and expanded follow-up are needed, our series shows that percutaneous endovascular bare stent placement can be easily manipulated with a high success rate and can be achieved with excellent prognosis and no severe adverse effects.
We acknowledge the assistance of Su-Ping Geng in collection of patient data, and the assistance of Wen-Nuo Huang.
The natural history of isolated superior mesenteric artery dissection (ISMAD) is not well understood due to the rarity and complexity of the condition. Therapeutic options include conservative management, endovascular treatments or open surgery, although no consensus has been reached with regard to the best treatment modality.
ISMAD may be managed successfully in a variety of ways based on the clinical symptoms.
Percutaneous endovascular reconstruction with bare stent implantation is a feasible treatment choice with a high success rate and good clinical outcome.
In this small series, conservative therapy was mainly indicated for asymptomatic patients or those with short-term symptoms, while endovascular or surgical therapy was recommended for those with persistent intestinal ischemia-related symptoms or rupture of the artery.
The choice of stent diameter was based on the proximal normal artery diameter. The types of bare stents used were determined according to the operator’s preference, availability of the stent, and the degree of difference in the vessel diameter.
This is a good descriptive study in which the authors evaluated their experience of the clinical management of ISMAD. Eighteen patients with ISMAD were retrospectively analyzed. The manuscript is clear and well written. The authors found that ISMAD may be managed successfully in a variety of ways based on the clinical symptoms.
P- Reviewer: Chaturvedi P, Yoon DH S- Editor: Qi Y L- Editor: Webster JR E- Editor: Zhang DN
| 1. | Bauersfeld SR. Dissecting aneurysm of the aorta; a presentation of 15 cases and a review of the recent literature. Ann Intern Med. 1947;26:873-889. [PubMed] |
| 2. | Sakamoto I, Ogawa Y, Sueyoshi E, Fukui K, Murakami T, Uetani M. Imaging appearances and management of isolated spontaneous dissection of the superior mesenteric artery. Eur J Radiol. 2007;64:103-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 177] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 3. | Suzuki S, Furui S, Kohtake H, Sakamoto T, Yamasaki M, Furukawa A, Murata K, Takei R. Isolated dissection of the superior mesenteric artery: CT findings in six cases. Abdom Imaging. 2004;29:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Zerbib P, Perot C, Lambert M, Seblini M, Pruvot FR, Chambon JP. Management of isolated spontaneous dissection of superior mesenteric artery. Langenbecks Arch Surg. 2010;395:437-443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Wu B, Zhang J, Yin MD, Wang L, Song JQ, Li X, Yang D, Duan ZQ, Xin SJ. Isolated superior mesenteric artery dissection: case for conservative treatment and endovascular repair. Chin Med J (Engl). 2009;122:238-240. [PubMed] |
| 6. | Sharma M, Babu CS, Garg S, Rai P. Portal venous system and its tributaries: a radial endosonographic assessment. Endosc Ultrasound. 2012;1:96-107. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 7. | Kim HK, Jung HK, Cho J, Lee JM, Huh S. Clinical and radiologic course of symptomatic spontaneous isolated dissection of the superior mesenteric artery treated with conservative management. J Vasc Surg. 2014;59:465-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 70] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Kwak JW, Paik CN, Lee KM, Chung WC, Jung SH, Kim JE, Baik JH, Yang JM. [Isolated spontaneous dissection of superior mesenteric artery: treated by percutaneous endovascular stent placement]. Korean J Gastroenterol. 2010;55:58-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Wu XM, Wang TD, Chen MF. Percutaneous endovascular treatment for isolated spontaneous superior mesenteric artery dissection: report of two cases and literature review. Catheter Cardiovasc Interv. 2009;73:145-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 10. | Gobble RM, Brill ER, Rockman CB, Hecht EM, Lamparello PJ, Jacobowitz GR, Maldonado TS. Endovascular treatment of spontaneous dissections of the superior mesenteric artery. J Vasc Surg. 2009;50:1326-1332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 89] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 11. | Rossini LG, Ribeiro PA, Rodrigues FC, Filippi SS, Zago Rde R, Schneider NC, Okawa L, Klug WA. Transrectal ultrasound - Techniques and outcomes in the management of intestinal endometriosis. Endosc Ultrasound. 2012;1:23-35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Takayama T, Miyata T, Shirakawa M, Nagawa H. Isolated spontaneous dissection of the splanchnic arteries. J Vasc Surg. 2008;48:329-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 91] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 13. | Froment P, Alerci M, Vandoni RE, Bogen M, Gertsch P, Galeazzi G. Stenting of a spontaneous dissection of the superior mesenteric artery: a new therapeutic approach? Cardiovasc Intervent Radiol. 2004;27:529-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 14. | Yun WS, Kim YW, Park KB, Cho SK, Do YS, Lee KB, Kim DI, Kim DK. Clinical and angiographic follow-up of spontaneous isolated superior mesenteric artery dissection. Eur J Vasc Endovasc Surg. 2009;37:572-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 15. | Park YJ, Park CW, Park KB, Roh YN, Kim DI, Kim YW. Inference from clinical and fluid dynamic studies about underlying cause of spontaneous isolated superior mesenteric artery dissection. J Vasc Surg. 2011;53:80-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 16. | Chu SY, Hsu MY, Chen CM, Yeow KM, Hung CF, Su IH, Shie RF, Pan KT. Endovascular repair of spontaneous isolated dissection of the superior mesenteric artery. Clin Radiol. 2012;67:32-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 17. | Sparks SR, Vasquez JC, Bergan JJ, Owens EL. Failure of nonoperative management of isolated superior mesenteric artery dissection. Ann Vasc Surg. 2000;14:105-109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 93] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 18. | Ghuysen A, Meunier P, Van Damme H, Creemers E, D’orio V. [Isolated spontaneous dissection of the superior mesenteric artery: a case report]. Ann Cardiol Angeiol (Paris). 2008;57:238-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 19. | Zhang X, Sun Y, Chen Z, Li X. Therapeutic regimen options for isolated superior mesenteric artery dissection. Vasc Endovascular Surg. 2012;46:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Cho BS, Lee MS, Lee MK, Choi YJ, Kim CN, Kang YJ, Park JS, Ahn HY. Treatment guidelines for isolated dissection of the superior mesenteric artery based on follow-up CT findings. Eur J Vasc Endovasc Surg. 2011;41:780-785. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 74] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 21. | Guo J, Liu Z, Sun S, Wang S, Ge N, Liu X, Wang G, Liu W. Endosonography-assisted diagnosis and therapy of gastrointestinal submucosal tumors. Endosc Ultrasound. 2013;2:125-133. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 22. | Hwang CK, Wang JY, Chaikof EL. Spontaneous dissection of the superior mesenteric artery. Ann Vasc Surg. 2010;24:254.e1-254.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 23. | Jia Z, Zhao J, Jiang G. Regarding “Management strategy for spontaneous isolated dissection of the superior mesenteric artery based on morphologic classification”. J Vasc Surg. 2014;59:876-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 24. | Jia Z, Zhao J, Jiang G. Comment on endovascular stent placement for treatment of spontaneous isolated dissection of the superior mesenteric artery. Ann Vasc Surg. 2014;28:1081-1082. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 25. | Li N, Lu QS, Zhou J, Bao JM, Zhao ZQ, Jing ZP. Endovascular stent placement for treatment of spontaneous isolated dissection of the superior mesenteric artery. Ann Vasc Surg. 2014;28:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |