Published online Dec 7, 2014. doi: 10.3748/wjg.v20.i45.16996
Revised: July 9, 2014
Accepted: August 13, 2014
Published online: December 7, 2014
Processing time: 188 Days and 4.6 Hours
Portal hypertension (PH) plays an important role in the natural history of cirrhosis, and is associated with several clinical consequences. The introduction of transjugular intrahepatic portosystemic shunts (TIPS) in the 1980s has been regarded as a major technical advance in the management of the PH-related complications. At present, polytetrafluoroethylene-covered stents are the preferred option over traditional bare metal stents. TIPS is currently indicated as a salvage therapy in patients with bleeding esophageal varices who fail standard treatment. Recently, applying TIPS early (within 72 h after admission) has been shown to be an effective and life-saving treatment in those with high-risk variceal bleeding. In addition, TIPS is recommended as the second-line treatment for secondary prophylaxis. For bleeding gastric varices, applying TIPS was able to achieve hemostasis in more than 90% of patients. More trials are needed to clarify the efficacy of TIPS compared with other treatment modalities, including cyanoacrylate injection and balloon retrograde transvenous obliteration of gastric varices. TIPS should also be considered in bleeding ectopic varices and refractory portal hypertensive gastropathy. In patients with refractory ascites, there is growing evidence that TIPS not only results in better control of ascites, but also improves long-term survival in appropriately selected candidates. In addition, TIPS is a promising treatment for refractory hepatic hydrothorax. However, the role of TIPS in the treatment of hepatorenal and hepatopulmonary syndrome is not well defined. The advantage of TIPS is offset by a risk of developing hepatic encephalopathy, the most relevant post-procedural complication. Emerging data are addressing the determination the optimal time and patient selection for TIPS placement aiming at improving long-term treatment outcome. This review is aimed at summarizing the published data regarding the application of TIPS in the management of complications related to PH.
Core tip: Transjugular intrahepatic portosystemic shunts (TIPS) has been proven to be an effective treatment modality for complications related to portal hypertension (PH). Currently, several efforts are now focusing on improving its efficacy by investigating the significance of the timing of the procedure and the characteristics of patients. This article gives an overview of the TIPS procedure as well as a summary of recent evidences regarding its clinical application in the management of PH-related complications.
- Citation: Siramolpiwat S. Transjugular intrahepatic portosystemic shunts and portal hypertension-related complications. World J Gastroenterol 2014; 20(45): 16996-17010
- URL: https://www.wjgnet.com/1007-9327/full/v20/i45/16996.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i45.16996
Portal hypertension (PH) is one of the common causes of death among patients with cirrhosis, and is defined by an increase of 5 mmHg above the upper normal limit in the pressure gradient between the portal vein and the inferior vena cava (portal pressure gradient: PPG)[1-3]. PH becomes clinically significant when PPG exceeds a threshold of 10 mmHg[3-5]. The development of PH results in the formation of portal-systemic collaterals that divert part of the portal blood flow to the systemic circulation, which directly gives rise to several clinical consequences, including variceal bleeding, ascites, and hepatorenal syndrome[5,6]. Therefore, pharmacological therapy or intervention that will result in a decline in PPG is the preferred treatment approach for these patients.
Transjugular intrahepatic portosystemic shunt (TIPS) is a percutaneously created low-resistance channel between the portal vein and the hepatic vein. The goal of TIPS is to reduce portal pressure by shunting blood from the portal to the systemic circulation, bypassing the liver. Physiologically, TIPS generates hemodynamic changes similar to those observed in surgical portosystemic shunts. Currently, TIPS has become overwhelmingly preferred over the traditional surgical shunts due to its less invasive technique, reduction in complications, and faster recovery time. This review focuses on the clinical application of TIPS placement in the management of PH-related complications.
The primary function of TIPS is to create a low-resistance shunt between the intrahepatic portion of the portal vein and the hepatic vein from a transjugular approach. Most authors cite Rosch as the principal inventor of TIPS, who originally described transjugular approach portal venography[7]. Later, in 1982, Colapinto et al[8] was the first to report the clinical use of TIPS with balloon angioplasty in expanding the intrahepatic tract. Nonetheless, this technique still resulted in poor patency of the created tract. To ameliorate this, an expandable metal stent was placed across the shunt to maintain its patency[9].
Taking into account its invasive technique and complications, the indication of TIPS should be firmly addressed before performing the procedure. In general, TIPS is recommended only in patients with PH-related complications who fail conservative treatment. The initial pre-procedural evaluation consists of a thorough review of clinical history and physical examination to identify procedural risks. Laboratory studies should be assessed prior to the procedure. These should encompass a complete blood count, coagulation, and metabolic panels, including serum electrolytes, creatinine, and liver function test. With a cirrhotic liver, vascular anatomy can be dramatically altered. Therefore, cross-sectional imaging studies with computer tomography, magnetic resonance imaging, or ultrasound with color Doppler should also be obtained to facilitate the anatomical orientation and to document hepatic and portal vein patency. Abdominal paracentesis and/or thoracentesis should be performed in patients with tense ascites or hepatic hydrothorax. This allows the liver to drop down into a more natural position and facilitates the portal vein puncture[10]. Those with a previous history of hepatic encephalopathy should have the encephalopathy treated, and their mental status optimized before performing the procedure. In subjects with suspected or known cardiac disease, an echocardiography should be done to rule out systolic or diastolic cardiac failure. So far, evidence from a single clinical trial has failed to show any benefit in prophylactic antibiotics to reduce peri-procedural infections[11]. However, in clinical practice, as these patients are poorly tolerated to infection, it is generally recommended that prophylactic antibiotics be administered during the procedure[12].
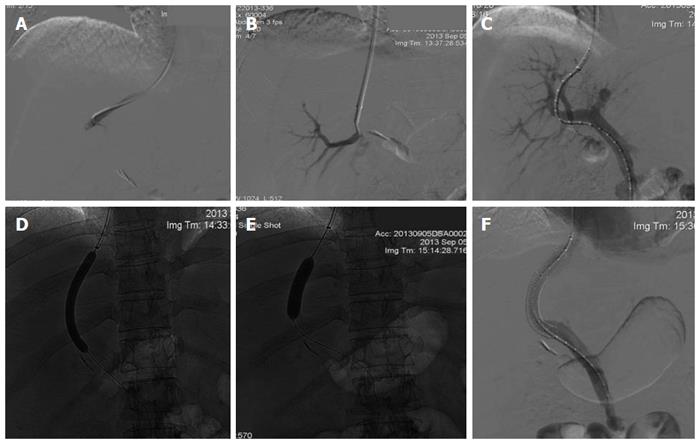
TIPS is performed mostly under conscious sedation, but in the case of prolonged duration or an unstable patient, general anesthesia is preferred. The success rate of achieving portal decompression appears to be more than 90% in most series, and several reviews have recently described detailed step-by-step methods of TIPS placement[13-15]. In brief, a catheter is introduced via the jugular vein, passing the right atrium into the hepatic vein. A needle inserted through the catheter is then used to puncture the liver parenchyma, and enters the portal vein branch. Once the portal vein has been cannulated, portal pressure measurement and venography are measured. Next, the intrahepatic parenchymal tract is dilated by an angioplasty balloon. Subsequently, either a bare metal or polytetrafluoroethylene (PTFE)-covered stent is deployed across the created tract. Normally, a 10- or 12-mm stent diameter is chosen for adult patients. Finally, the post-TIPS portosystemic pressure gradient (PSG) is calculated. If needed, further balloon dilatation can be performed to obtain the desired PSG (Figure 1).
Traditionally, many radiologists calculate PSG by subtracting the right atrial pressure (RAP) from the portal pressure. However, inferior vena cava pressure (IVCP) should theoretically be used as the internal zero reference instead of RAP, due to the gradient between the portal vein and the inferior vena cava better reflecting the pressure difference between the portal and systemic venous systems. In a recent study, La Mura et al[16] performed a trial comparing PSG by using RAP and IVCP as the internal zero in 99 patients with TIPS placement. Compared with IVCP, using RAP as the internal zero resulted in an undesirable increase in mean PSG of 2.5 mmHg (95%CI: 2.0-2.9; P < 0.001), which led to an unnecessary dilatation of TIPS in 20% of patients.
PTFE-covered stents are specifically designed stent grafts for TIPS, consisting of a 4-8-cm long proximal covered part for the intrahepatic portion and a 2-cm long uncovered caudal part that lies in the portal vein. The PTFE is a trilaminar structure with slight porosity and is impermeable to liquid bile, preventing the occlusion of the lumen by ingrowth of tissue from the surrounding liver. A recent meta-analysis of six trials (one prospective and five retrospective) comparing TIPS placement with PTFE-covered and bare metal stents for treating PH-related complications showed that the covered stent was superior in terms of achieving shunt patency (HR = 0.28; 95%CI 0.20-0.35), lower risk of hepatic encephalopathy (HR = 0.65; 95%CI: 0.45-0.86), and better survival (data derived from four observational studies; HR = 0.76; 95%CI: 0.58-0.94)[17]. Indeed, to date, there are two randomized controlled trials evaluating this issue (the first one was also included in the previously mentioned meta-analysis)[18-20]. In both trials, the PTFE-covered stent was superior to the bare metal stent in maintaining long-term shunt patency with 2-year shunt dysfunction rates of 24%-44% and 64%, respectively. Notably, one study demonstrated that the incidence of clinical relapse (variceal bleeding or ascites) and post-TIPS hepatic encephalopathy was lower in those assigned to the covered stent[19]. Theoretically, better shunt patency should result in a higher rate of hepatic encephalopathy. The significantly lower rate of hepatic encephalopathy in the PTFE-covered stent group could possibly be explained by the fact that those with uncovered stent experienced higher numbers of clinical relapses, hospitalizations, and re-interventions, which eventually aggravated hepatic encephalopathy. However, no survival advantage of the covered stent over the bare metal stent was shown in both trials. Interestingly, Perarnau et al[20] have recently reported that, despite the higher cost of PTFE-covered stents, both groups were comparable with respect to cost-effectiveness. Currently, a PTFE-covered stent is advocated as the preferable option by the American Association for the Study of Liver Diseases (AASLD) guidelines[21].
Many complications can occur during or after the TIPS implantation procedure. Complications related to the puncture site include intraperitoneal hemorrhage, portal vein perforation, and hepatic artery or bile duct injury, which may lead to fistula formation. The incidence of these fatal complications is reported to be 0.5%-4.3%[22]. Ultrasonographic guidance of the portal vein puncture and a clear knowledge of vascular anatomy of the liver are recommended in order to correctly portal vein access, thus avoiding non-target organ puncture.
The most relevant clinical complication is hepatic encephalopathy, which is related to an increased shunting of blood away from the liver to the systemic circulation. The incidence of new onset or worsening hepatic encephalopathy is reported to be 20%-31%[22-24]. Several parameters have been documented as a predictor of post-TIPS encephalopathy: hypoalbuminemia, older age, previous encephalopathy, and encephalopathy at the time of TIPS creation[23,25,26]. The management of post-TIPS encephalopathy is similar to that of portosystemic encephalopathy in other settings[22,24]. Most patients respond to standard treatment and a correction of identified precipitating factors. In refractory cases, a reduction of the shunt’s diameter should be considered[22,23,27].
The contraindications of TIPS according to AASLD guidelines are shown in Table 1[3]. TIPS should not be performed in patients who have severe heart failure or pulmonary hypertension due to an increased risk of life-threatening pulmonary congestion. In cases of relative contraindications, the risk of the procedure should be weighed against the benefit. Moreover, the overall prognosis of the patient must be considered prior to the procedure.
| Absolute | Relative |
| Congestive heart failure | Hepatoma especially if central |
| Multiple hepatic cysts | Obstruction of all hepatic veins |
| Uncontrolled systemic infection or sepsis | Portal vein thrombosis |
| Unrelieved biliary obstruction | Severe coagulopathy (INR > 5) |
| Severe pulmonary hypertension | Thrombocytopenia of < 20000/cm3 |
| Moderate pulmonary hypertension |
Several clinical scoring systems have been proposed to predict the outcome in patients undergoing TIPS. The Child-Pugh score was originally developed to estimate the risk of postoperative mortality in patients with esophageal varices[28]. It has been validated as a useful predictor of short-term and long-term survival after TIPS placement. However, the major drawback of the Child-Pugh score is its “ceiling effect”, which fails to differentiate among patients with severe liver dysfunction. In addition, it incorporates two subjective parameters: ascites and hepatic encephalopathy, which could be influenced by interobserver variability. In 2000, Malinchoc et al[29] first described the Mayo Clinic risk score for cirrhotic patients considered for TIPS. In this study, patients who had a score > 1.8 had a median survival of 2.8 mo compared with 1.3 years in those who had a score < 1.8. Subsequently, a slight modification of the Mayo Clinic risk score was introduced and became known as the Model For End-Stage Liver Disease (MELD) score[30]. The accuracy of the MELD score in predicting short-term mortality in patients with cirrhosis has been shown in several studies[31]. Currently, the MELD score has gained wide acceptance as a prognostic model in patients with end-stage liver disease. Regarding post-TIPS outcome, the MELD score was found to be superior to the Child-Pugh score in predicting the long-term survival[32-34]. Indeed, it has been shown that patients with a MELD score > 18 had significantly worse outcome after TIPS placement compared with those with MELD score ≤ 18[33]. A recent study reported a 5-year survival of more than 80% in patients undergoing TIPS for variceal bleeding with a pre-TIPS MELD score < 10[35].
Esophageal varices are present in approximately 50% of patients with cirrhosis, and most directly result from PH. As portal pressure increases, venous connections between the portal and systemic circulation arise[6]. It has been well documented that esophageal varices develop in cirrhotic patients who have a hepatic venous pressure gradient (HVPG) of at least 10-12 mmHg. Esophageal varices occur at a rate of 7% per year, and the 1-year rate of first variceal hemorrhage is approximately 12% (5% for small varices and 15% for large varices)[1,2]. In those who bleed, there is a 15%-20% chance of death within 6 wk after a bleeding episode. Moreover, the 1-year rebleeding rate is approximately 60% with a mortality rate of up to 33%. Accordingly, bleeding esophageal varices is the most lethal complication among cirrhotic patients[4].
In the current guidelines, it is suggested that primary prophylaxis to prevent first variceal bleeding should be administered in patients with medium to large varices, and in patients with small varices that are associated with a high risk of hemorrhage (Child C cirrhosis or presence of red wale marks). The treatment of choice is either non-selective beta-blockers (NSBBs) or endoscopic variceal ligation (EVL)[1,3]. Due to the fact that these patients are at relatively low risk of bleeding, strategies for primary prophylaxis should be minimally invasive. In addition, reports using surgical portocaval shunts, which generate the same physiologic changes as TIPS, for primary prophylaxis resulted in more frequent hepatic encephalopathy and higher mortality[1,36]. Therefore, TIPS is generally not recommended for this purpose[3].
The current standard of care for patients with acute variceal bleeding is a combination of early vasoactive drug administration and endoscopic therapy (preferably EVL), together with a short course of prophylactic antibiotics[1,3]. The initial hemostasis rate is approximately 80%-90% in most series. In those whom standard treatment fails, an effective rescue treatment is urgently needed. A review of 15 studies evaluating TIPS as a salvage therapy for refractory variceal bleeding reported a hemostasis rate of more than 90% with a rebleeding rate of 6%-27%[37]. Current guidelines recommend that TIPS is indicated in those who fail standard treatment or who develop a severe early rebleeding episode[3,38].
It is worth mentioning that, despite successful bleeding control after TIPS placement, a significant number of patients are still at a high risk of dying[39-41]. A study published in 2001 showed that rescue TIPS was able to control bleeding in 90% of patients with refractory variceal hemorrhage, but was accompanied by 30- and 60-d mortality rates of 29% and 35%, respectively[40]. The major cause of death was deterioration of liver function associated with multi-organ failure and sepsis. The study by Bañares et al[42], which included 56 patients with refractory variceal bleeding treated with TIPS, demonstrated that the actuarial probability of survival at 30 d was significantly lower in Child-Pugh class C than class A or B (48% vs 90%; P < 0.001). In this study, the presence of ascites, hepatic encephalopathy, and serum albumin level before TIPS placement were the independent factors associated with the risk of post-procedural mortality. Indeed, other clinical parameters have been documented as a prognostic factor of poor survival and treatment failure such as a MELD score > 18 or HVPG > 20[43]. This is of particular importance as it indicates that the overall prognosis of patients with acute severe variceal bleeding is mainly related to their general condition, especially the degree of liver dysfunction. This finding exhibited the existence of a group of patients that are too ill, and will die even if TIPS is successfully performed. Hence, these patients should be differentially considered as a high-risk population that deserves a more prompt and effective treatment approach. Accordingly, early utilization of TIPS as a potential life-saving treatment in this selected high-risk population has been suggested.
A randomized controlled trial by Monescillo et al[44] assigned 116 patients with high-risk bleeding esophageal varices (HVPG > 20 mmHg measured within 24 h after admission) to receive early TIPS or standard medical treatment. In this study, the early TIPS group had a significantly better outcome in terms of treatment failure, and in-hospital and 1-year survival. Indeed, the 1-year survival rate increased from 35% in the standard treatment to 62% in the early TIPS group. Nevertheless, routine HVPG measurement in the setting of acute variceal bleeding is clinically impractical.
A multicenter trial published in 2010 by García-Pagán et al[45] included 63 patients with high-risk variceal bleeding: Child C (CTP ≤ 13) or Child B cirrhosis with active bleeding at endoscopy. All patients were randomly assigned to receive early TIPS with PTFE-covered stents within 72 h after admission (n = 32) or vasoactive drug plus EVL (n = 31). In the standard treatment group, 14/31 patients reached primary endpoint (failure to control acute bleeding or rebleeding), and when the endpoint was reached, their MELD score increased from 18.8 ± 6.4 to 22.6 ± 11. Of which, TIPS was precluded in five patients due to advanced liver failure, and subsequently, all of them died. In the remaining nine patients, TIPS was performed as a rescue in seven, and four of them died within 36 d despite hemostasis being achieved. In contrast, only one patient in the early TIPS group reached the primary endpoint. Importantly, the early TIPS group had lower 1-year rebleeding or treatment failure rate, and better 1-year survival compared to the standard treatment group. The numbers needed to treat to prevent one treatment failure and one death were 2.1 (1.4-4) and 4 (2.1-50), respectively. Later, the benefit of early TIPS in patients with high-risk variceal bleeding was confirmed in a retrospective post-randomized controlled trial surveillance reported by the same authors[46]. The results of all three trials are shown in Table 2.
| Findings | Ref. | |||||
| Monescillo et al[44], 2004 | García-Pagán et al[45], 2010 | Garcia-Pagán et al[46], 2012 | ||||
| Early TIPS | Standard care | Early TIPS | Standard care | Early TIPS | Standard care | |
| Patients | 26 | 26 | 32 | 31 | 45 | 30 |
| Treatment failure1 | 12% | 50% | 3% | 45% | 7% | 50% |
| Early rebleeding (< 5 d) | 4% | 12% | 3% | 13% | 2% | 13% |
| 1-yr mortality rate | 31% | 65% | 14% | 39% | 14%3 | 30% |
| Development of hepatic encephalopathy2 | 31% | 35% | 25% | 39% | 51% | 50% |
Interestingly, applying early TIPS did not result in an increased rate of hepatic encephalopathy in all three trials. The possible explanation is that most reported episodes of hepatic encephalopathy were precipitated by an episode of bleeding. A recent study has evaluated the economic implication of applying TIPS early, and found that it was a cost-effective intervention in patients with high-risk variceal bleeding[47]. Currently, the Baveno V consensus suggests that early TIPS (within 72 h) should be considered in patients with a high-risk of treatment failure after pharmacologic and endoscopic therapy[38]. However, the applicability of this strategy to patients with less severe liver dysfunction, or in a center with less expertise in TIPS implantation, is still a matter of debate[48].
Currently, it is generally suggested that secondary prophylaxis to prevent rebleeding from esophageal varices should be initiated in all patients who survive the first episode of variceal bleeding. A combination of NSBBs and EVL is the preferred treatment option. Hence, failure of secondary prophylaxis occurs in 10%-15% of patients[1,6]. Many clinical trials have evaluated the efficacy of TIPS for the prevention of variceal rebleeding. A meta-analysis of 12 randomized controlled trials showed that TIPS was more effective than endoscopic intervention with/without pharmacologic therapy in secondary prophylaxis of variceal bleeding (OR = 0.32; 95%CI: 0.24-0.43; P < 0.00001)[49]. However, all-cause mortality was comparable between the two groups (OR = 1.17; 95%CI: 0.85-1.61; P = 0.33). As expected, the rate of new onset or worsening hepatic encephalopathy was higher in the TIPS group (OR = 2.21; 95%CI: 1.61-3.03; P < 0.00001). It should be taken into account that most trials were conducted before the introduction of the PTFE-covered stent, and the endoscopic therapy used was sclerotherapy. As mentioned earlier, it has been well documented that the PTFE-covered stent offers an advantage over the bare metal stent in terms of the post-implantation rate of shunt patency, rebleeding, and possibly long-term survival[18-20]. Accordingly, even though clinical trials comparing TIPS using a PTFE-covered stent and the current standard treatment are lacking, the disadvantage of TIPS in secondary prophylaxis would be overcome by using PTFE-covered stents.
A surgical portosystemic shunt procedure is recommended as an alternative treatment for secondary prophylaxis in patients with well-compensated cirrhosis[1,6]. To date, two trials have compared TIPS and surgical shunts in the prevention of rebleeding from esophageal varices[50,51]. The first study reported a higher rebleeding rate in the TIPS group compared with the group that received an 8-mm portocaval H-graft shunt without any significant difference in mortality. Another study demonstrated that TIPS and a distal splenorenal shunt were comparable in terms of rebleeding rate and overall mortality. However, due to the prostheses used in both trials being bare metal stents, as expected, patients assigned to TIPS had a significantly higher rate of shunt thrombosis requiring re-intervention. Again, this finding suggests that using a covered stent would result in a lower rate of shunt dysfunction, and a better long-term outcome. In conclusion, despite the previously mentioned drawbacks, TIPS is generally recommended as the second-line treatment in secondary prophylaxis of variceal bleeding for those who fail medical and endoscopic intervention[21,38].
Several lines of evidence suggest that hemodynamic response (a decrease in HVPG from baseline) after pharmacological therapy is the strongest predictor of variceal bleeding and rebleeding in cirrhotic patients with PH[52]. Indeed, after a rebleeding episode, the mortality rate approaches 30%[1,2]. Previous study reported that patients who were classified as hemodynamic responders maintained a low rebleeding rate while on drug therapy, whereas the rebleeding rate was substantially higher in hemodynamic non-responders (approximately 40% at 2 years)[53]. Another prospective trial showed that, in hemodynamic non-responders, the rebleeding rate reached 87.5% after a median duration of 28 mo, despite adding EVL as an adjunctive treatment, compared to 34% in those who were hemodynamic responders to NSBBs with/without nitrate[54]. Additionally, a reduction in HVPG was strongly associated with a decreased risk of developing spontaneous bacterial peritonitis and death[55,56]. In 2006, González et al[57] conducted a prospective study in 50 patients with cirrhosis presenting with acute variceal bleeding. After treatment of an acute bleeding episode, all patients underwent HVPG measurement, and received secondary prophylaxis with NSBBs and nitrates. After a mean time of 15.6 d, a second HVPG was measured and 9 (18%) patients were classified as hemodynamic non-responders (a reduction of HVPG < 10% from baseline). Of these, preemptive TIPS was performed in all nine patients, and none of them re-bled during a mean follow-up of 22 mo. Unfortunately, this study did not include a control group. More data are needed to clarify whether TIPS might play role as a preemptive treatment before the development of the first rebleeding episode in patients who are at high risk of rebleeding, particularly in HVPG non-responders.
The prevalence of gastric varices (GV) in patients with PH is 15%-20%, which is significantly lower than that of esophageal varices. However, the bleeding from GV is more severe, requiring more blood transfusions, and has a higher mortality rate[58,59]. Sarin et al[60] have categorized GV based on its location and association with esophageal varices. The incidence of bleeding is highest in gastroesophageal varices type 2 (GOV2) and isolated gastric varices type 1 (IGV1), commonly known as cardiofundal varices. Unfortunately, most published trials evaluating treatment of GV have included different types of GV, of which less than half of the recruited patients had cardiofundal varices.
Current recommendation suggests that endoscopic obliteration with cyanoacrylate (CA) glue injection is the treatment of choice in patients with bleeding GV[38]. Most uncontrolled series reported an initial hemostasis rate of over 90%, and a rebleeding rate of 20%-30%[59,61]. To date, a small number of case series have shown that TIPS was also effective in more than 90% of patients with refractory GV bleeding, with a 6-mo rebleeding rate of 25%[39,62]. A retrospective study by Mahadeva et al[63] included 43 patients with bleeding GV (GOV1/GOV2: 28/15) treated with CA (n = 23) or TIPS (n = 20). In this study, the initial rebleeding rate was significantly lower in the TIPS group (15% vs 30%; P = 0.005), without any difference in overall mortality. Currently, TIPS has been advocated by standard guidelines as a rescue treatment for bleeding GV if endoscopic therapy is not possible or after a single failure of endoscopic therapy[3,38]. Regarding secondary prophylaxis, currently there is only one prospective randomized controlled trial from Taiwan showing that TIPS was more effective than CA injection in the prevention of rebleeding from GV, with a rebleeding rate of 11% and 38%, respectively (P = 0.014)[64]. Unfortunately, no survival difference was shown in this study. More randomized control trials are needed to confirm this finding. It has been documented that GV may bleed despite a PSG < 12 mmHg, which may be a result of the presence of gastro-renal and spleno-renal shunts[65]. Interestingly, one uncontrolled study has shown that the advantage of TIPS on survival in patients with bleeding GV was limited to those with a pre-TIPS PSG > 12 mmHg[66]. Therefore, some experts recommend that additional embolization of collaterals feeding GV should be performed at the time of TIPS placement. However, two small retrospective studies failed to show any benefit of adding embolization with TIPS over TIPS alone in patients with bleeding GV[67,68].
In recent years, balloon retrograde transvenous obliteration of gastric varices (BRTO) has become a promising modality to treat GV, particularly in Asian countries[69]. From a hemodynamic standpoint, BRTO is different from TIPS as the portosystemic collaterals (mostly gastro-renal shunts) are occluded by a balloon, followed by an injection of sclerosant agent. Thus, several reports have shown that BRTO causes an increase in portal pressure, and aggravation of esophageal varices because of the obliteration of spontaneous portosystemic shunt caused by the procedure[70,71]. So far, there are only few trials comparing BRTO and TIPS in the treatment of acute GV bleeding. The first study, which randomly assigned 15 patients with active GV bleeding and presence of gastro-renal shunt by imaging to receive BRTO or TIPS, failed to show any significant difference in the initial hemostasis and rebleeding rates between the two groups[72]. Recently, Sabri et al[73] conducted a retrospective study in 50 patients with bleeding GV treated with TIPS or BRTO. The authors found that the rebleeding rate was lower in BRTO group (0% vs 11% at 1 year) without any statistical significance. There was no difference in the rate of hepatic encephalopathy between those treated with TIPS and BRTO in either trial. As mentioned earlier, the advantage of BRTO is offset by an aggravation in PH, which results in an increase in variceal size and ascites. Interestingly, a recent study has shown that an additional TIPS placement results in a protective effect against the development of ascites, hepatic hydrothorax, and rebleeding in bleeding GV patients treated with BRTO[74]. Whether CA, TIPS, and/or BRTO is the best therapeutic option of bleeding GV merits further study. Currently, it is recommended that decision-making should be individualized and based on the patient’s characteristics and vascular anatomy, along with the local expertise[61,75].
Ectopic varices are dilated portosystemic collaterals of the gastrointestinal mucosa outside the gastroesophageal region. These variceal veins can occur along the entire gastrointestinal tract[76]. The clinical spectrum of bleeding ectopic varices varies from asymptomatic, occult bleeding to massive bleeding with or without hypovolemic shock[77]. Various therapeutic modalities have been documented in case reports and case series as an effective treatment of ectopic varices. However, as randomized controlled trials comparing different treatment approaches are lacking, there is no general guideline for the management of ectopic varices.
Several reports have shown that TIPS was effective for the treatment of bleeding ectopic varices, with a rebleeding rate that varied from 17% to 37%[78-80]. Most rebleeding episodes were related to shunt dysfunction and responded to shunt intervention. The largest series was reported in 2008, which included 27 patients with ectopic varices (43% rectal varices, 29% stomal varices, and 14% duodenal varices) treated with TIPS (five patients also received selective variceal embolization as an adjunctive treatment)[81]. Of which, TIPS was performed in an emergency setting to control acute bleeding in nine patients, and hemostasis was achieved in six (67%). During follow-up, rebleeding from ectopic varices occurred in five (21%) patients. Interestingly, three patients re-bled despite patent shunts and low PSG, and bleeding was successfully controlled in two patients by additional thrombin injections.
It has been documented that additional selective embolization of varices at the time of TIPS placement reduced the rebleeding rate in patients with ectopic varices[78,81]. However, due to the retrospective nature of the studies and given that there were no controlled groups, it is impossible to draw any firm conclusion. Whether additional embolization of varices should be routinely performed in all patients with ectopic varices undergoing TIPS warrants further study.
Portal hypertensive gastropathy (PHG) occurs in up to 70% of patients with PH[82]. Histological characteristics of PHG are vascular ectasia of the mucosal and submucosal capillaries without inflammation[83,84]. Clinically, PHG can cause chronic gastrointestinal bleeding requiring repeated transfusion[82]. Vasoconstrictors or NSBBs have been documented to be an effective treatment for PHG[85,86]. However, data of portal decompression for PHG are lacking. So far, two case series have shown that TIPS placement gives rise to an endoscopic resolution in more than 85% of cirrhotic patients with PHG[87,88]. In addition, there was a significantly lower transfusion requirement after TIPS placement in patients with severe PHG (0.6 ± 0.8 vs 2.9 ± 2.0, P = 0.04).
Gastric antral vascular ectasia (GAVE), also known as watermelon stomach, is a gastric mucosal lesion that should be differentiated from severe PHG[82]. Besides vascular ectasia, as found in PHG, the histological features of GAVE consist of spindle cell proliferation and fibrohyalinosis[89,90]. Typically, GAVE is characterized by the presence of red marks varying in size, which are predominantly located in the gastric antrum. Even though there is no correlation between GAVE and PH, about 30% of affected patients have concomitant liver cirrhosis[91,92]. Based on available information, treatments aiming at lowering portal pressure (including TIPS) do not appear to be beneficial in reducing either acute or chronic bleeding from GAVE[87,91,93].
Ascites is a common problem in patients with decompensated cirrhosis. Splanchnic vasodilatation and the activation of the sympathetic nervous system and the renin-angiotensin-aldosterone system are the proposed mechanism. Typically, patients with ascites are treated with diet modification and diuretics; however, ascites are refractory to medical therapy in 5%-10% of cases[94,95]. Refractory ascites is associated with poor quality of life, high risk of spontaneous bacterial peritonitis, and hepatorenal syndrome. The available therapeutic options for patients with refractory ascites are serial large-volume paracentesis (LVP), TIPS, surgical portosystemic shunts, and finally, in eligible candidates, liver transplantation[96].
Unlike secondary prevention of variceal bleeding, the target HVPG that needs to be achieved when the indication is refractory ascites is unclear[27]. This uncertainty is not surprising, because ascites formation in cirrhosis is not only the direct consequence of PH, but also the changes in the renal and neurohormonal systems.
Currently, six randomized controlled trials have compared TIPS and LVP in the management of refractory ascites (Table 3)[97-102]. As shown, TIPS was superior to LVP in terms of controlling ascites, but the impact of TIPS on survival was uncertain. Three out of six trials demonstrated a survival benefit of TIPS over LVP, whereas TIPS had no effect on survival in the other two trials. In the remaining trial, survival was better in patients allocated to LVP. Four meta-analyses of the five earliest controlled trials have been published, which again yielded conflicting results regarding long-term outcomes[103-106]. This discrepancy could be explained by distinct selection criteria of the included patients and the difference in the technical success rate of the procedures[27]. Indeed, all trials that favored TIPS have included patients with more preserved liver and kidney function. The most recently published study, which excluded patients with CTP ≥ 11, Cr ≥ 1.9 mg/dL and serum bilirubin > 3 mg/dL, showed that TIPS significantly improved long-term survival compared with LVP plus albumin infusion[99].
| Ref. | Number of patients | Complete resolution of ascites within 6 mo1 | Treatment failure2 | Survival at 2 yr | Newly developed or severe encephalopathy | |||||||||
| TIPS | LVP | TIPS | LVP | P-value | TIPS | LVP | P-value | TIPS | LVP | P-value | TIPS | LVP | P-value | |
| Lebrec et al[98] 1996 | 13 | 12 | 38% | 0% | < 0.05 | NR | 29% | 60% | 0.03 | 45% | 0% | < 0.05 | ||
| Rössle et al[100], 2000 | 29 | 31 | 52% | 16% | 0.001 | 10% | 48% | 0.001 | 58% | 32% | 0.11 | 23% | 13% | NS |
| Ginès et al[97], 2002 | 35 | 35 | NR | 49% | 83% | 0.003 | 26% | 30% | 0.51 | 60% | 34% | 0.03 | ||
| Sanyal et al[102], 2003 | 52 | 57 | NR | 42% | 84% | < 0.001 | 35% | 33% | 0.84 | 38% | 21% | 0.058 | ||
| Salerno et al[101], 2004 | 33 | 33 | 60%3 | 3% | < 0.001 | 21% | 57% | 0.001 | 59% | 29% | 0.021 | 61% | 39% | NS |
| Narahara et al[99], 2011 | 30 | 30 | 30% | 0% | < 0.005 | 13% | 80% | < 0.001 | 64% | 35% | < 0.005 | 67% | 17% | < 0.001 |
In 2007, Salerno et al[107] sought to eliminate the heterogeneity among studies by performing a meta-analysis of individual patient data from four controlled trials. The result showed that the actuarial transplant-free survival was better in patients allocated to TIPS compared to LVP (1- and 2-year LT-free survival of 63.1% and 49% vs 52.5% and 35.2%, respectively; P = 0.035). Recurrence of ascites was reported in 42% of patients allocated to TIPS, and 89% of patients allocated to LVP (P < 0.0001). By multivariate analysis, allocation to TIPS (HR = 0.61; 95%CI: 0.41-0.91; P = 0.015), older age (HR = 1.02; 95%CI: 1.00-1.05; P = 0.041), serum bilirubin (HR = 1.22; 95%CI: 1.03-1.46; P = 0.022), and low serum sodium (HR = 0.95; 95%CI: 0.92-0.99; P = 0.03) were independently associated with mortality. Of note, the positive effects of TIPS on survival were shown across all subgroups of patients classified according to different MELD score. As expected, the average number of cases of hepatic encephalopathy was significantly higher in patients allocated to TIPS (1.13 ± 1.93 vs 0.63 ± 1.18; P = 0.006); however, the cumulative probability of developing first episode of hepatic encephalopathy was similar between two groups.
Supporting the idea that better selection of patients can improve post-TIPS outcome, a combination of serum bilirubin < 3 mg/dL and platelet count > 75 × 109/L has been found as a simple predictive tool of good outcome in patients with refractory ascites treated with TIPS[108]. The actuarial 1-year survival rate in patients with both platelet count > 75 × 109/L and bilirubin level < 3 mg/dL was 73.1% compared to 31.2% in patients with platelet count < 75 × 109/L or bilirubin level > 3 mg/L. This model has been internally and externally validated by the same authors in other cohorts, and its reproducibility has been confirmed. Recently, baseline serum creatinine has been reported as the only independent predictor of no response and survival in patients with refractory ascites undergoing TIPS[109]. Current AASLD guidelines suggest that TIPS should be considered as a treatment option for refractory ascites in appropriately selected patients[21]. Additional studies are needed to clarify in which clinical setting TIPS should be considered as the first-line treatment in refractory ascites.
Hepatic hydrothorax is characterized by transudative recurrent pleural effusion in patients with advanced liver disease and PH. A commonly proposed mechanism is the direct passage of peritoneal fluid through the diaphragmatic defects[110,111]. The first-line treatment consists of salt restriction, diuretics, and repeated thoracentesis. However, a number of patients with hepatic hydrothorax are refractory to initial treatments[96]. These patients warrant consideration of additional therapies, including TIPS, surgical repair of diaphragmatic defects, and pleurodesis.
The efficacy of TIPS in refractory hepatic hydrothorax has been documented in several non-randomized retrospective studies and case reports (Table 4)[112-118]. As shown, the overall clinical response rate varied from 58% to 80%. The largest study was reported by Dhanasekaran et al[118], which included 73 patients with refractory hepatic hydrothorax treated with TIPS. In this study, within 1 mo after TIPS placement, 59%, 20.5%, and 20.5% of patients had complete clinical response, partial response, and no response, respectively. The 1-, 3-, and 5-year survival rates were 48%, 26%, and 15%, respectively. In addition, it has been shown that pre-TIPS MELD score (HR = 1.9; 95%CI: 1.0-3.7; P = 0.039) and clinical response after TIPS (HR = 2.5; 95%CI: 1.4-4.5; P = 0.003) were independent factors associated with overall survival[118]. Indeed, median survival in those who had pre-TIPS MELD < 15 was significantly higher than those with pre-TIPS MELD ≥ 15 (875 vs 180 d; P = 0.035).
| Ref. | No. of patients | Child-Pugh or MELD score | Efficacy (complete/partial response) | 30-d mortality | 1-yr survival | Predictor of mortality |
| Strauss et al[112], 1994 | 5 | C: 5 | 80%/20% | NR | NR | NR |
| Gordon et al[113], 1997 | 24 | B/C: 5/19 | 58%/21% | 21% | NR | Child C |
| Non-response | ||||||
| Jeffries et al[114], 1998 | 12 | A/B/C: 1/5/6 | 42%/17% | 25% | NR | Age > 65 |
| Siegerstetter et al[115], 2001 | 40 | B/C: 24/16 | 71%/11% | NR | 64% | Age > 60 |
| Spencer et al[116], 2002 | 21 | B/C: 7/14 | 63%/11% | 29% | NR | Multiple comorbidities |
| Wilputte et al[117], 2007 | 28 | B/C: 12/16 | 57%/11% | 14% | 41% | Child-Pugh score |
| Dhanasekaran et al[118], 2010 | 73 | MELD < 15: 32.8% | 59%/20.5%1 | 19% | 48% | Pre-TIPS MELD score Non-response |
| MELD > 15: 67.2% | 60%/15%2 |
Currently, there are no randomized trials comparing TIPS with other second-line treatments. Nonetheless, based on current evidence, TIPS placement results in a good control of hepatic hydrothorax, and it also results in a mobilization of ascites, which is the source of accumulated fluid. TIPS is thus the preferred treatment for refractory hepatic hydrothorax[96,119]. However, similar to refractory ascites, the benefit of TIPS in patients with refractory hepatic hydrothorax may be limited to those who are younger and have preserved baseline liver function.
Hepatorenal syndrome (HRS) is a severe complication in patients with decompensated cirrhosis. HRS develops as a result of severe splanchnic vasodilatation and circulatory dysfunction, which lead to intense renal vasoconstriction[96]. Type 1 HRS is defined as the development of rapidly progressive renal failure in the setting of a precipitating event. In contrast, type 2 HRS is characterized by slowly progressing or steady renal failure and refractory ascites[120].
To date, several studies have evaluated the role of TIPS placement in patients with HRS[120]. TIPS has been shown to improve kidney function (urinary sodium excretion and serum creatinine), and hemodynamic parameters in patients with HRS[121,122]. Plasma renin activity, aldosterone and noradrenaline concentration decrease significantly within 4-6 mo after the procedure. The largest study was reported in 2000, which included 41 patients with HRS[123]. Thirty-one out of 41 patients were submitted to TIPS (HRS type 1/2 = 14/17), whereas TIPS was precluded in the remaining 10 due to severe liver dysfunction. Apart from the positive effect on renal function, the TIPS group had better 3-mo survival compared to the non-TIPS group (63% and 10%, respectively). Indeed, considering only the subgroup of patients with type 1 HRS, which carries a grave prognosis, TIPS placement still resulted in better survival. However, the benefit of TIPS in this study was hampered by the selection bias toward the intervention arm. Additionally, in 2004, Wong et al[124] reported that TIPS could be used as a bridging therapy before liver transplantation in patients with HRS type 1 who initially responded to vasoconstrictor treatment.
It should be kept in mind that, typically, patients with HRS, particularly type 1, suffer from severe liver failure, which has been regarded as a contraindication to TIPS placement. Accordingly, the clinical applicability of TIPS in these patients is considerably low. In summary, the well-established benefit of TIPS is limited to hemodynamic derangement with an inconclusive advantage on survival. Together with a limited applicability of the procedure, TIPS is therefore recommended only in a selected group of patients with HRS and/or in candidates for liver transplantation.
Hepatopulmonary syndrome (HPS) is characterized by an intrapulmonary vasodilatation causing abnormal gas exchange in the setting of PH or severe liver dysfunction. HPS is relatively common in cirrhotic patients awaiting liver transplantation[125,126]. Currently, liver transplantation is the only effective treatment for HPS, resulting in complete resolution of gas-exchange abnormalities in more than 80% of patients[127,128].
Physiologically, TIPS aggravates existing hyperdynamic circulation in cirrhotic patients with PH, which could trigger or increase pulmonary vasodilatation, and adversely effect pulmonary gas exchange. Several case reports have evaluated the role of TIPS in HPS, which yielded conflicting results. Some case reports have shown that applying TIPS could improve pulmonary gas exchange or achieve clinical resolution, while others demonstrated a negative outcome[129-133]. This discrepancy might be caused by the distinctive clinical features of included patients, and the fact that TIPS was performed for indications other than HPS in the majority of them. The largest case series, which included three patients with advanced HPS treated with TIPS, failed to show any improvement in pulmonary gas exchange[134]. Finally, one patient in this study underwent liver transplantation and had a resolution of intrapulmonary vasodilatation. Hence, currently, there is not sufficient evidence to support the use of TIPS for HPS.
In recent years, TIPS has gained wide acceptance as a treatment for severe or refractory complications of portal hypertension. The advantage of TIPS is that it produces a greater and more rapid decrease in portal pressure than other treatment modalities. Currently, TIPS is of paramount importance in the treatment armamentarium for bleeding esophageal and gastric varices, particularly in those who fail standard treatment. In addition, TIPS has emerged as a recommended treatment modality in patients with refractory ascites and hepatic hydrothorax. Nevertheless, the application of TIPS is offset by an increased risk of hepatic encephalopathy. Future trials should focus on optimizing the appropriate timing and patient selection to achieve positive long-term outcomes after the procedure.
P- Reviewer: Qin JM, Takaki A S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Garcia-Tsao G, Bosch J. Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med. 2010;362:823-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 639] [Cited by in RCA: 640] [Article Influence: 42.7] [Reference Citation Analysis (0)] |
| 2. | Bosch J, Berzigotti A, Garcia-Pagan JC, Abraldes JG. The management of portal hypertension: rational basis, available treatments and future options. J Hepatol. 2008;48 Suppl 1:S68-S92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 198] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Garcia-Tsao G, Sanyal AJ, Grace ND, Carey W. Prevention and management of gastroesophageal varices and variceal hemorrhage in cirrhosis. Hepatology. 2007;46:922-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1229] [Cited by in RCA: 1210] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 4. | Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology. 2008;134:1715-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 240] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 5. | de Franchis R, Primignani M. Natural history of portal hypertension in patients with cirrhosis. Clin Liver Dis. 2001;5:645-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 231] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | O’Brien J, Triantos C, Burroughs AK. Management of varices in patients with cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:402-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 33] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Rösch J, Hanafee WN, Snow H. Transjugular portal venography and radiologic portacaval shunt: an experimental study. Radiology. 1969;92:1112-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 218] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Colapinto RF, Stronell RD, Gildiner M, Ritchie AC, Langer B, Taylor BR, Blendis LM. Formation of intrahepatic portosystemic shunts using a balloon dilatation catheter: preliminary clinical experience. AJR Am J Roentgenol. 1983;140:709-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 83] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Palmaz JC, Sibbitt RR, Reuter SR, Garcia F, Tio FO. Expandable intrahepatic portacaval shunt stents: early experience in the dog. AJR Am J Roentgenol. 1985;145:821-825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 70] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 10. | Bhogal HK, Sanyal AJ. Using transjugular intrahepatic portosystemic shunts for complications of cirrhosis. Clin Gastroenterol Hepatol. 2011;9:936-946; quiz e123. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Deibert P, Schwarz S, Olschewski M, Siegerstetter V, Blum HE, Rössle M. Risk factors and prevention of early infection after implantation or revision of transjugular intrahepatic portosystemic shunts: results of a randomized study. Dig Dis Sci. 1998;43:1708-1713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Darcy M. Evaluation and management of transjugular intrahepatic portosystemic shunts. AJR Am J Roentgenol. 2012;199:730-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 13. | Boyer TD. Transjugular intrahepatic portosystemic shunt: current status. Gastroenterology. 2003;124:1700-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 97] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Clark TW. Stepwise placement of a transjugular intrahepatic portosystemic shunt endograft. Tech Vasc Interv Radiol. 2008;11:208-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Fidelman N, Kwan SW, LaBerge JM, Gordon RL, Ring EJ, Kerlan RK. The transjugular intrahepatic portosystemic shunt: an update. AJR Am J Roentgenol. 2012;199:746-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 16. | La Mura V, Abraldes JG, Berzigotti A, Erice E, Flores-Arroyo A, García-Pagán JC, Bosch J. Right atrial pressure is not adequate to calculate portal pressure gradient in cirrhosis: a clinical-hemodynamic correlation study. Hepatology. 2010;51:2108-2116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Yang Z, Han G, Wu Q, Ye X, Jin Z, Yin Z, Qi X, Bai M, Wu K, Fan D. Patency and clinical outcomes of transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stents versus bare stents: a meta-analysis. J Gastroenterol Hepatol. 2010;25:1718-1725. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 111] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 18. | Bureau C, Garcia-Pagan JC, Otal P, Pomier-Layrargues G, Chabbert V, Cortez C, Perreault P, Péron JM, Abraldes JG, Bouchard L, Bilbao JI, Bosch J, Rousseau H, Vinel JP. Improved clinical outcome using polytetrafluoroethylene-coated stents for TIPS: results of a randomized study. Gastroenterology. 2004;126:469-475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 338] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 19. | Bureau C, Pagan JC, Layrargues GP, Metivier S, Bellot P, Perreault P, Otal P, Abraldes JG, Peron JM, Rousseau H, Bosch J, Vinel JP. Patency of stents covered with polytetrafluoroethylene in patients treated by transjugular intrahepatic portosystemic shunts: long-term results of a randomized multicentre study. Liver Int. 2007;27:742-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 220] [Cited by in RCA: 225] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 20. | Perarnau JM, Le Gouge A, Nicolas C, d’Alteroche L, Borentain P, Saliba F, Minello A, Anty R, Chagneau-Derrode C, Bernard PH. Covered vs. uncovered stents for transjugular intrahepatic portosystemic shunt: a randomized controlled trial. J Hepatol. 2014;60:962-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 152] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 21. | Boyer TD, Haskal ZJ. The Role of Transjugular Intrahepatic Portosystemic Shunt (TIPS) in the Management of Portal Hypertension: update 2009. Hepatology. 2010;51:306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 405] [Article Influence: 27.0] [Reference Citation Analysis (1)] |
| 22. | Ripamonti R, Ferral H, Alonzo M, Patel NH. Transjugular intrahepatic portosystemic shunt-related complications and practical solutions. Semin Intervent Radiol. 2006;23:165-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (1)] |
| 23. | Riggio O, Angeloni S, Salvatori FM, De Santis A, Cerini F, Farcomeni A, Attili AF, Merli M. Incidence, natural history, and risk factors of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt with polytetrafluoroethylene-covered stent grafts. Am J Gastroenterol. 2008;103:2738-2746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 205] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 24. | Riggio O, Nardelli S, Moscucci F, Pasquale C, Ridola L, Merli M. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. Clin Liver Dis. 2012;16:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 118] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 25. | Masson S, Mardini HA, Rose JD, Record CO. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunt insertion: a decade of experience. QJM. 2008;101:493-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 26. | Somberg KA, Riegler JL, LaBerge JM, Doherty-Simor MM, Bachetti P, Roberts JP, Lake JR. Hepatic encephalopathy after transjugular intrahepatic portosystemic shunts: incidence and risk factors. Am J Gastroenterol. 1995;90:549-555. [PubMed] |
| 27. | Rössle M. TIPS: 25 years later. J Hepatol. 2013;59:1081-1093. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 287] [Article Influence: 23.9] [Reference Citation Analysis (0)] |
| 28. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5490] [Cited by in RCA: 5735] [Article Influence: 110.3] [Reference Citation Analysis (2)] |
| 29. | Malinchoc M, Kamath PS, Gordon FD, Peine CJ, Rank J, ter Borg PC. A model to predict poor survival in patients undergoing transjugular intrahepatic portosystemic shunts. Hepatology. 2000;31:864-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1967] [Cited by in RCA: 2067] [Article Influence: 82.7] [Reference Citation Analysis (0)] |
| 30. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3462] [Cited by in RCA: 3676] [Article Influence: 153.2] [Reference Citation Analysis (0)] |
| 31. | Durand F, Valla D. Assessment of prognosis of cirrhosis. Semin Liver Dis. 2008;28:110-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 218] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 32. | Angermayr B, Cejna M, Karnel F, Gschwantler M, Koenig F, Pidlich J, Mendel H, Pichler L, Wichlas M, Kreil A. Child-Pugh versus MELD score in predicting survival in patients undergoing transjugular intrahepatic portosystemic shunt. Gut. 2003;52:879-885. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 227] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 33. | Ferral H, Gamboa P, Postoak DW, Albernaz VS, Young CR, Speeg KV, McMahan CA. Survival after elective transjugular intrahepatic portosystemic shunt creation: prediction with model for end-stage liver disease score. Radiology. 2004;231:231-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 113] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 34. | Schepke M, Roth F, Fimmers R, Brensing KA, Sudhop T, Schild HH, Sauerbruch T. Comparison of MELD, Child-Pugh, and Emory model for the prediction of survival in patients undergoing transjugular intrahepatic portosystemic shunting. Am J Gastroenterol. 2003;98:1167-1174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 130] [Cited by in RCA: 131] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 35. | Heinzow HS, Lenz P, Köhler M, Reinecke F, Ullerich H, Domschke W, Domagk D, Meister T. Clinical outcome and predictors of survival after TIPS insertion in patients with liver cirrhosis. World J Gastroenterol. 2012;18:5211-5218. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 36. | Conn HO, Lindenmuth WW, May CJ, Ramsby GR. Prophylactic portacaval anastomosis. Medicine (Baltimore). 1972;51:27-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 133] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 37. | Vangeli M, Patch D, Burroughs AK. Salvage tips for uncontrolled variceal bleeding. J Hepatol. 2002;37:703-704. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 66] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 38. | de Franchis R. Revising consensus in portal hypertension: report of the Baveno V consensus workshop on methodology of diagnosis and therapy in portal hypertension. J Hepatol. 2010;53:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1066] [Cited by in RCA: 1030] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 39. | Chau TN, Patch D, Chan YW, Nagral A, Dick R, Burroughs AK. “Salvage” transjugular intrahepatic portosystemic shunts: gastric fundal compared with esophageal variceal bleeding. Gastroenterology. 1998;114:981-987. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 189] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 40. | Azoulay D, Castaing D, Majno P, Saliba F, Ichaï P, Smail A, Delvart V, Danaoui M, Samuel D, Bismuth H. Salvage transjugular intrahepatic portosystemic shunt for uncontrolled variceal bleeding in patients with decompensated cirrhosis. J Hepatol. 2001;35:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 134] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 41. | D’Amico G, Luca A. TIPS is a cost effective alternative to surgical shunt as a rescue therapy for prevention of recurrent bleeding from esophageal varices. J Hepatol. 2008;48:387-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Bañares R, Casado M, Rodríguez-Láiz JM, Camúñez F, Matilla A, Echenagusía A, Simó G, Piqueras B, Clemente G, Cos E. Urgent transjugular intrahepatic portosystemic shunt for control of acute variceal bleeding. Am J Gastroenterol. 1998;93:75-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 46] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 43. | Moitinho E, Escorsell A, Bandi JC, Salmerón JM, García-Pagán JC, Rodés J, Bosch J. Prognostic value of early measurements of portal pressure in acute variceal bleeding. Gastroenterology. 1999;117:626-631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 342] [Cited by in RCA: 305] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 44. | Monescillo A, Martínez-Lagares F, Ruiz-del-Arbol L, Sierra A, Guevara C, Jiménez E, Marrero JM, Buceta E, Sánchez J, Castellot A. Influence of portal hypertension and its early decompression by TIPS placement on the outcome of variceal bleeding. Hepatology. 2004;40:793-801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 353] [Cited by in RCA: 328] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 45. | García-Pagán JC, Caca K, Bureau C, Laleman W, Appenrodt B, Luca A, Abraldes JG, Nevens F, Vinel JP, Mössner J, Bosch J. Early use of TIPS in patients with cirrhosis and variceal bleeding. N Engl J Med. 2010;362:2370-2379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 826] [Cited by in RCA: 842] [Article Influence: 56.1] [Reference Citation Analysis (0)] |
| 46. | Garcia-Pagán JC, Di Pascoli M, Caca K, Laleman W, Bureau C, Appenrodt B, Luca A, Zipprich A, Abraldes JG, Nevens F, Vinel JP, Sauerbruch T, Bosch J. Use of early-TIPS for high-risk variceal bleeding: results of a post-RCT surveillance study. J Hepatol. 2013;58:45-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 198] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 47. | Harman DJ, McCorry RB, Jacob RP, Lim TR, O’Neill R, Ryder SD, James MW, Aithal GP, Guha IN. Economic modelling of early transjugular intrahepatic portosystemic shunt insertion for acute variceal haemorrhage. Eur J Gastroenterol Hepatol. 2013;25:201-207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 48. | Thabut D, Rudler M, Lebrec D. Early TIPS with covered stents in high-risk patients with cirrhosis presenting with variceal bleeding: are we ready to dive into the deep end of the pool? J Hepatol. 2011;55:1148-1149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 49. | Khan S, Tudur Smith C, Williamson P, Sutton R. Portosystemic shunts versus endoscopic therapy for variceal rebleeding in patients with cirrhosis. Cochrane Database Syst Rev. 2006;CD000553. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 50. | Rosemurgy AS, Serafini FM, Zweibel BR, Black TJ, Kudryk BT, Nord HJ, Goode SE. Transjugular intrahepatic portosystemic shunt vs. small-diameter prosthetic H-graft portacaval shunt: extended follow-up of an expanded randomized prospective trial. J Gastrointest Surg. 2000;4:589-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 60] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 51. | Henderson JM, Boyer TD, Kutner MH, Galloway JR, Rikkers LF, Jeffers LJ, Abu-Elmagd K, Connor J. Distal splenorenal shunt versus transjugular intrahepatic portal systematic shunt for variceal bleeding: a randomized trial. Gastroenterology. 2006;130:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 151] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 52. | D’Amico G, Garcia-Pagan JC, Luca A, Bosch J. Hepatic vein pressure gradient reduction and prevention of variceal bleeding in cirrhosis: a systematic review. Gastroenterology. 2006;131:1611-1624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 350] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 53. | Bureau C, Péron JM, Alric L, Morales J, Sanchez J, Barange K, Payen JL, Vinel JP. “A La Carte” treatment of portal hypertension: Adapting medical therapy to hemodynamic response for the prevention of bleeding. Hepatology. 2002;36:1361-1366. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 54. | Augustin S, González A, Badia L, Millán L, Gelabert A, Romero A, Segarra A, Martell M, Esteban R, Guardia J. Long-term follow-up of hemodynamic responders to pharmacological therapy after variceal bleeding. Hepatology. 2012;56:706-714. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Abraldes JG, Tarantino I, Turnes J, Garcia-Pagan JC, Rodés J, Bosch J. Hemodynamic response to pharmacological treatment of portal hypertension and long-term prognosis of cirrhosis. Hepatology. 2003;37:902-908. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 367] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 56. | Turnes J, Garcia-Pagan JC, Abraldes JG, Hernandez-Guerra M, Dell’Era A, Bosch J. Pharmacological reduction of portal pressure and long-term risk of first variceal bleeding in patients with cirrhosis. Am J Gastroenterol. 2006;101:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 153] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 57. | González A, Augustin S, Pérez M, Dot J, Saperas E, Tomasello A, Segarra A, Armengol JR, Malagelada JR, Esteban R. Hemodynamic response-guided therapy for prevention of variceal rebleeding: an uncontrolled pilot study. Hepatology. 2006;44:806-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 58. | Kim T, Shijo H, Kokawa H, Tokumitsu H, Kubara K, Ota K, Akiyoshi N, Iida T, Yokoyama M, Okumura M. Risk factors for hemorrhage from gastric fundal varices. Hepatology. 1997;25:307-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 63] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 59. | Tripathi D, Ferguson JW, Therapondos G, Plevris JN, Hayes PC. Review article: recent advances in the management of bleeding gastric varices. Aliment Pharmacol Ther. 2006;24:1-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Sarin SK, Lahoti D, Saxena SP, Murthy NS, Makwana UK. Prevalence, classification and natural history of gastric varices: a long-term follow-up study in 568 portal hypertension patients. Hepatology. 1992;16:1343-1349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 847] [Article Influence: 25.7] [Reference Citation Analysis (42)] |
| 61. | Garcia-Pagán JC, Barrufet M, Cardenas A, Escorsell A. Management of gastric varices. Clin Gastroenterol Hepatol. 2014;12:919-928.e1; quiz e51-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 62. | Barange K, Péron JM, Imani K, Otal P, Payen JL, Rousseau H, Pascal JP, Joffre F, Vinel JP. Transjugular intrahepatic portosystemic shunt in the treatment of refractory bleeding from ruptured gastric varices. Hepatology. 1999;30:1139-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 119] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 63. | Mahadeva S, Bellamy MC, Kessel D, Davies MH, Millson CE. Cost-effectiveness of N-butyl-2-cyanoacrylate (histoacryl) glue injections versus transjugular intrahepatic portosystemic shunt in the management of acute gastric variceal bleeding. Am J Gastroenterol. 2003;98:2688-2693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (2)] |
| 64. | Lo GH, Liang HL, Chen WC, Chen MH, Lai KH, Hsu PI, Lin CK, Chan HH, Pan HB. A prospective, randomized controlled trial of transjugular intrahepatic portosystemic shunt versus cyanoacrylate injection in the prevention of gastric variceal rebleeding. Endoscopy. 2007;39:679-685. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 222] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 65. | Jalan R, Redhead DN, Forrest EH, Hayes PC. Relationship between directly measured portal pressure gradient and variceal hemorrhage. Am J Gastroenterol. 1995;90:1994-1996. [PubMed] |
| 66. | Tripathi D, Therapondos G, Jackson E, Redhead DN, Hayes PC. The role of the transjugular intrahepatic portosystemic stent shunt (TIPSS) in the management of bleeding gastric varices: clinical and haemodynamic correlations. Gut. 2002;51:270-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 163] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 67. | Gaba RC, Bui JT, Cotler SJ, Kallwitz ER, Mengin OT, Martinez BK, Berkes JL, Carrillo TC, Knuttinen MG, Owens CA. Rebleeding rates following TIPS for variceal hemorrhage in the Viatorr era: TIPS alone versus TIPS with variceal embolization. Hepatol Int. 2010;4:749-756. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 68. | Xiao T, Chen L, Chen W, Xu B, Long Q, Li R, Li L, Peng Z, Fang D, Wang R. Comparison of transjugular intrahepatic portosystemic shunt (TIPS) alone versus TIPS combined with embolotherapy in advanced cirrhosis: a retrospective study. J Clin Gastroenterol. 2011;45:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 37] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 69. | Saad WE. Balloon-occluded retrograde transvenous obliteration of gastric varices: concept, basic techniques, and outcomes. Semin Intervent Radiol. 2012;29:118-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 70. | Ninoi T, Nishida N, Kaminou T, Sakai Y, Kitayama T, Hamuro M, Yamada R, Nakamura K, Arakawa T, Inoue Y. Balloon-occluded retrograde transvenous obliteration of gastric varices with gastrorenal shunt: long-term follow-up in 78 patients. AJR Am J Roentgenol. 2005;184:1340-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 191] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 71. | Chikamori F, Kuniyoshi N, Kawashima T, Takase Y. Gastric varices with gastrorenal shunt: combined therapy using transjugular retrograde obliteration and partial splenic embolization. AJR Am J Roentgenol. 2008;191:555-559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 47] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 72. | Choi YH, Yoon CJ, Park JH, Chung JW, Kwon JW, Choi GM. Balloon-occluded retrograde transvenous obliteration for gastric variceal bleeding: its feasibility compared with transjugular intrahepatic portosystemic shunt. Korean J Radiol. 2003;4:109-116. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 104] [Cited by in RCA: 114] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 73. | Sabri SS, Abi-Jaoudeh N, Swee W, Saad WE, Turba UC, Caldwell SH, Angle JF, Matsumoto AH. Short-term rebleeding rates for isolated gastric varices managed by transjugular intrahepatic portosystemic shunt versus balloon-occluded retrograde transvenous obliteration. J Vasc Interv Radiol. 2014;25:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 74. | Saad WE, Wagner CC, Lippert A, Al-Osaimi A, Davies MG, Matsumoto AH, Angle JF, Caldwell S. Protective value of TIPS against the development of hydrothorax/ascites and upper gastrointestinal bleeding after balloon-occluded retrograde transvenous obliteration (BRTO). Am J Gastroenterol. 2013;108:1612-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 75. | Caldwell S. Gastric varices: is there a role for endoscopic cyanoacrylates, or are we entering the BRTO era? Am J Gastroenterol. 2012;107:1784-1790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 76. | Almadi MA, Almessabi A, Wong P, Ghali PM, Barkun A. Ectopic varices. Gastrointest Endosc. 2011;74:380-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 77. | Sato T, Akaike J, Toyota J, Karino Y, Ohmura T. Clinicopathological features and treatment of ectopic varices with portal hypertension. Int J Hepatol. 2011;2011:960720. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (35)] |
| 78. | Vangeli M, Patch D, Terreni N, Tibballs J, Watkinson A, Davies N, Burroughs AK. Bleeding ectopic varices--treatment with transjugular intrahepatic porto-systemic shunt (TIPS) and embolisation. J Hepatol. 2004;41:560-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 150] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 79. | Haskal ZJ, Scott M, Rubin RA, Cope C. Intestinal varices: treatment with the transjugular intrahepatic portosystemic shunt. Radiology. 1994;191:183-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 80. | Saad WE, Lippert A, Saad NE, Caldwell S. Ectopic varices: anatomical classification, hemodynamic classification, and hemodynamic-based management. Tech Vasc Interv Radiol. 2013;16:158-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 103] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 81. | Kochar N, Tripathi D, McAvoy NC, Ireland H, Redhead DN, Hayes PC. Bleeding ectopic varices in cirrhosis: the role of transjugular intrahepatic portosystemic stent shunts. Aliment Pharmacol Ther. 2008;28:294-303. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 82. | Ripoll C, Garcia-Tsao G. The management of portal hypertensive gastropathy and gastric antral vascular ectasia. Dig Liver Dis. 2011;43:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 83. | McCormack TT, Sims J, Eyre-Brook I, Kennedy H, Goepel J, Johnson AG, Triger DR. Gastric lesions in portal hypertension: inflammatory gastritis or congestive gastropathy? Gut. 1985;26:1226-1232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 380] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 84. | Cubillas R, Rockey DC. Portal hypertensive gastropathy: a review. Liver Int. 2010;30:1094-1102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 85. | Pérez-Ayuso RM, Piqué JM, Bosch J, Panés J, González A, Pérez R, Rigau J, Quintero E, Valderrama R, Viver J. Propranolol in prevention of recurrent bleeding from severe portal hypertensive gastropathy in cirrhosis. Lancet. 1991;337:1431-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 166] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 86. | Bruha R, Marecek Z, Spicak J, Hulek P, Lata J, Petrtyl J, Urbanek P, Taimr P, Volfova M, Dite P. Double-blind randomized, comparative multicenter study of the effect of terlipressin in the treatment of acute esophageal variceal and/or hypertensive gastropathy bleeding. Hepatogastroenterology. 2002;49:1161-1166. [PubMed] |
| 87. | Kamath PS, Lacerda M, Ahlquist DA, McKusick MA, Andrews JC, Nagorney DA. Gastric mucosal responses to intrahepatic portosystemic shunting in patients with cirrhosis. Gastroenterology. 2000;118:905-911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 118] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 88. | Mezawa S, Homma H, Ohta H, Masuko E, Doi T, Miyanishi K, Takada K, Kukitsu T, Sato T, Niitsu Y. Effect of transjugular intrahepatic portosystemic shunt formation on portal hypertensive gastropathy and gastric circulation. Am J Gastroenterol. 2001;96:1155-1159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 55] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 89. | Fuccio L, Mussetto A, Laterza L, Eusebi LH, Bazzoli F. Diagnosis and management of gastric antral vascular ectasia. World J Gastrointest Endosc. 2013;5:6-13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 69] [Cited by in RCA: 75] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 90. | Payen JL, Calès P, Voigt JJ, Barbe S, Pilette C, Dubuisson L, Desmorat H, Vinel JP, Kervran A, Chayvialle JA. Severe portal hypertensive gastropathy and antral vascular ectasia are distinct entities in patients with cirrhosis. Gastroenterology. 1995;108:138-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 129] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 91. | Spahr L, Villeneuve JP, Dufresne MP, Tassé D, Bui B, Willems B, Fenyves D, Pomier-Layrargues G. Gastric antral vascular ectasia in cirrhotic patients: absence of relation with portal hypertension. Gut. 1999;44:739-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 116] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 92. | Gostout CJ, Viggiano TR, Ahlquist DA, Wang KK, Larson MV, Balm R. The clinical and endoscopic spectrum of the watermelon stomach. J Clin Gastroenterol. 1992;15:256-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 169] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 93. | Nardone G, Rocco A, Balzano T, Budillon G. The efficacy of octreotide therapy in chronic bleeding due to vascular abnormalities of the gastrointestinal tract. Aliment Pharmacol Ther. 1999;13:1429-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 114] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 94. | Moore CM, Van Thiel DH. Cirrhotic ascites review: Pathophysiology, diagnosis and management. World J Hepatol. 2013;5:251-263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 78] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 95. | Wong F. Management of ascites in cirrhosis. J Gastroenterol Hepatol. 2012;27:11-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 96. | Runyon BA. Introduction to the revised American Association for the Study of Liver Diseases Practice Guideline management of adult patients with ascites due to cirrhosis 2012. Hepatology. 2013;57:1651-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 514] [Cited by in RCA: 519] [Article Influence: 43.3] [Reference Citation Analysis (1)] |
| 97. | Ginès P, Uriz J, Calahorra B, Garcia-Tsao G, Kamath PS, Del Arbol LR, Planas R, Bosch J, Arroyo V, Rodés J. Transjugular intrahepatic portosystemic shunting versus paracentesis plus albumin for refractory ascites in cirrhosis. Gastroenterology. 2002;123:1839-1847. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 401] [Cited by in RCA: 364] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 98. | Lebrec D, Giuily N, Hadengue A, Vilgrain V, Moreau R, Poynard T, Gadano A, Lassen C, Benhamou JP, Erlinger S. Transjugular intrahepatic portosystemic shunts: comparison with paracentesis in patients with cirrhosis and refractory ascites: a randomized trial. French Group of Clinicians and a Group of Biologists. J Hepatol. 1996;25:135-144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 314] [Cited by in RCA: 288] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 99. | Narahara Y, Kanazawa H, Fukuda T, Matsushita Y, Harimoto H, Kidokoro H, Katakura T, Atsukawa M, Taki Y, Kimura Y. Transjugular intrahepatic portosystemic shunt versus paracentesis plus albumin in patients with refractory ascites who have good hepatic and renal function: a prospective randomized trial. J Gastroenterol. 2011;46:78-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 100. | Rössle M, Ochs A, Gülberg V, Siegerstetter V, Holl J, Deibert P, Olschewski M, Reiser M, Gerbes AL. A comparison of paracentesis and transjugular intrahepatic portosystemic shunting in patients with ascites. N Engl J Med. 2000;342:1701-1707. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 380] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 101. | Salerno F, Merli M, Riggio O, Cazzaniga M, Valeriano V, Pozzi M, Nicolini A, Salvatori F. Randomized controlled study of TIPS versus paracentesis plus albumin in cirrhosis with severe ascites. Hepatology. 2004;40:629-635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 249] [Cited by in RCA: 236] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 102. | Sanyal AJ, Genning C, Reddy KR, Wong F, Kowdley KV, Benner K, McCashland T. The North American Study for the Treatment of Refractory Ascites. Gastroenterology. 2003;124:634-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 282] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 103. | D’Amico G, Luca A, Morabito A, Miraglia R, D’Amico M. Uncovered transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis. Gastroenterology. 2005;129:1282-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 135] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 104. | Deltenre P, Mathurin P, Dharancy S, Moreau R, Bulois P, Henrion J, Pruvot FR, Ernst O, Paris JC, Lebrec D. Transjugular intrahepatic portosystemic shunt in refractory ascites: a meta-analysis. Liver Int. 2005;25:349-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 97] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 105. | Albillos A, Bañares R, González M, Catalina MV, Molinero LM. A meta-analysis of transjugular intrahepatic portosystemic shunt versus paracentesis for refractory ascites. J Hepatol. 2005;43:990-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 110] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 106. | Saab S, Nieto JM, Lewis SK, Runyon BA. TIPS versus paracentesis for cirrhotic patients with refractory ascites. Cochrane Database Syst Rev. 2006;CD004889. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 107. | Salerno F, Cammà C, Enea M, Rössle M, Wong F. Transjugular intrahepatic portosystemic shunt for refractory ascites: a meta-analysis of individual patient data. Gastroenterology. 2007;133:825-834. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 396] [Cited by in RCA: 366] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 108. | Bureau C, Métivier S, D’Amico M, Péron JM, Otal P, Pagan JC, Chabbert V, Chagneau-Derrode C, Procopet B, Rousseau H, Bosch J, Vinel JP. Serum bilirubin and platelet count: a simple predictive model for survival in patients with refractory ascites treated by TIPS. J Hepatol. 2011;54:901-907. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 109. | Taki Y, Kanazawa H, Narahara Y, Itokawa N, Kondo C, Fukuda T, Harimto H, Matsushita Y, Kidokoro H, Katakura T. Predictive factors for improvement of ascites after transjugular intrahepatic portosystemic shunt in patients with refractory ascites. Hepatol Res. 2014;44:871-877. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 110. | Krok KL, Cárdenas A. Hepatic hydrothorax. Semin Respir Crit Care Med. 2012;33:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 111. | Singh A, Bajwa A, Shujaat A. Evidence-based review of the management of hepatic hydrothorax. Respiration. 2013;86:155-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 112. | Strauss RM, Martin LG, Kaufman SL, Boyer TD. Transjugular intrahepatic portal systemic shunt for the management of symptomatic cirrhotic hydrothorax. Am J Gastroenterol. 1994;89:1520-1522. [PubMed] |
| 113. | Gordon FD, Anastopoulos HT, Crenshaw W, Gilchrist B, McEniff N, Falchuk KR, LoCicero J, Lewis WD, Jenkins RL, Trey C. The successful treatment of symptomatic, refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt. Hepatology. 1997;25:1366-1369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 144] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 114. | Jeffries MA, Kazanjian S, Wilson M, Punch J, Fontana RJ. Transjugular intrahepatic portosystemic shunts and liver transplantation in patients with refractory hepatic hydrothorax. Liver Transpl Surg. 1998;4:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 115. | Siegerstetter V, Deibert P, Ochs A, Olschewski M, Blum HE, Rössle M. Treatment of refractory hepatic hydrothorax with transjugular intrahepatic portosystemic shunt: long-term results in 40 patients. Eur J Gastroenterol Hepatol. 2001;13:529-534. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 132] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 116. | Spencer EB, Cohen DT, Darcy MD. Safety and efficacy of transjugular intrahepatic portosystemic shunt creation for the treatment of hepatic hydrothorax. J Vasc Interv Radiol. 2002;13:385-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 80] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 117. | Wilputte JY, Goffette P, Zech F, Godoy-Gepert A, Geubel A. The outcome after transjugular intrahepatic portosystemic shunt (TIPS) for hepatic hydrothorax is closely related to liver dysfunction: a long-term study in 28 patients. Acta Gastroenterol Belg. 2007;70:6-10. [PubMed] |
| 118. | Dhanasekaran R, West JK, Gonzales PC, Subramanian R, Parekh S, Spivey JR, Martin LG, Kim HS. Transjugular intrahepatic portosystemic shunt for symptomatic refractory hepatic hydrothorax in patients with cirrhosis. Am J Gastroenterol. 2010;105:635-641. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 119. | European Association for the Study of the Liver. EASL clinical practice guidelines on the management of ascites, spontaneous bacterial peritonitis, and hepatorenal syndrome in cirrhosis. J Hepatol. 2010;53:397-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1131] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 120. | Wong F. Recent advances in our understanding of hepatorenal syndrome. Nat Rev Gastroenterol Hepatol. 2012;9:382-391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 121. | Guevara M, Ginès P, Bandi JC, Gilabert R, Sort P, Jiménez W, Garcia-Pagan JC, Bosch J, Arroyo V, Rodés J. Transjugular intrahepatic portosystemic shunt in hepatorenal syndrome: effects on renal function and vasoactive systems. Hepatology. 1998;28:416-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 251] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 122. | Testino G, Ferro C, Sumberaz A, Messa P, Morelli N, Guadagni B, Ardizzone G, Valente U. Type-2 hepatorenal syndrome and refractory ascites: role of transjugular intrahepatic portosystemic stent-shunt in eighteen patients with advanced cirrhosis awaiting orthotopic liver transplantation. Hepatogastroenterology. 2003;50:1753-1755. [PubMed] |
| 123. | Brensing KA, Textor J, Perz J, Schiedermaier P, Raab P, Strunk H, Klehr HU, Kramer HJ, Spengler U, Schild H. Long term outcome after transjugular intrahepatic portosystemic stent-shunt in non-transplant cirrhotics with hepatorenal syndrome: a phase II study. Gut. 2000;47:288-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 334] [Cited by in RCA: 286] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 124. | Wong F, Pantea L, Sniderman K. Midodrine, octreotide, albumin, and TIPS in selected patients with cirrhosis and type 1 hepatorenal syndrome. Hepatology. 2004;40:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 273] [Cited by in RCA: 227] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 125. | Zhang J, Fallon MB. Hepatopulmonary syndrome: update on pathogenesis and clinical features. Nat Rev Gastroenterol Hepatol. 2012;9:539-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 126. | Fallon MB, Krowka MJ, Brown RS, Trotter JF, Zacks S, Roberts KE, Shah VH, Kaplowitz N, Forman L, Wille K. Impact of hepatopulmonary syndrome on quality of life and survival in liver transplant candidates. Gastroenterology. 2008;135:1168-1175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 242] [Cited by in RCA: 202] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 127. | Machicao VI, Balakrishnan M, Fallon MB. Pulmonary complications in chronic liver disease. Hepatology. 2014;59:1627-1637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 126] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 128. | Gupta S, Castel H, Rao RV, Picard M, Lilly L, Faughnan ME, Pomier-Layrargues G. Improved survival after liver transplantation in patients with hepatopulmonary syndrome. Am J Transplant. 2010;10:354-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 121] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 129. | Allgaier HP, Haag K, Ochs A, Hauenstein KH, Jeserich M, Krause T, Heilmann C, Gerok W, Rössle M. Hepato-pulmonary syndrome: successful treatment by transjugular intrahepatic portosystemic stent-shunt (TIPS). J Hepatol. 1995;23:102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 130. | Corley DA, Scharschmidt B, Bass N, Somberg K, Gold W. Lack of efficacy of TIPS for hepatopulmonary syndrome. Gastroenterology. 1997;113:728-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 55] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 131. | Lasch HM, Fried MW, Zacks SL, Odell P, Johnson MW, Gerber DA, Sandhu FS, Fair JH, Shrestha R. Use of transjugular intrahepatic portosystemic shunt as a bridge to liver transplantation in a patient with severe hepatopulmonary syndrome. Liver Transpl. 2001;7:147-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 40] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 132. | Riegler JL, Lang KA, Johnson SP, Westerman JH. Transjugular intrahepatic portosystemic shunt improves oxygenation in hepatopulmonary syndrome. Gastroenterology. 1995;109:978-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 75] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 133. | Selim KM, Akriviadis EA, Zuckerman E, Chen D, Reynolds TB. Transjugular intrahepatic portosystemic shunt: a successful treatment for hepatopulmonary syndrome. Am J Gastroenterol. 1998;93:455-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 134. | Martinez-Palli G, Drake BB, Garcia-Pagan JC, Barbera JA, Arguedas MR, Rodriguez-Roisin R, Bosch J, Fallon MB. Effect of transjugular intrahepatic portosystemic shunt on pulmonary gas exchange in patients with portal hypertension and hepatopulmonary syndrome. World J Gastroenterol. 2005;11:6858-6862. [PubMed] |