Published online Nov 28, 2014. doi: 10.3748/wjg.v20.i44.16786
Revised: April 17, 2014
Accepted: May 29, 2014
Published online: November 28, 2014
Processing time: 294 Days and 23 Hours
This study was conducted to explore the feasibility of partial pancreatic head resection and Roux-en-Y pancreatic jejunostomy for the treatment of benign tumors of the pancreatic head (BTPH). From November 2006 to February 2009, four patients (three female and one male) with a mean age of 34.3 years (range: 21-48 years) underwent partial pancreatic head resection and Roux-en-Y pancreatic jejunostomy for the treatment of BTPH (diameters of 3.2-4.5 cm) using small incisions (5.1-7.2 cm). Preoperative symptoms include one case of repeated upper abdominal pain, one case of drowsiness and two cases with no obvious preoperative symptoms. All four surgeries were successfully performed. The mean operative time was 196.8 min (range 165-226 min), and average blood loss was 138.0 mL (range: 82-210 mL). The mean postoperative hospital stay was 7.5 d (range: 7-8 d). In one case, the main pancreatic duct was injured. Pathological examination confirmed that one patient suffered from mucinous cystadenoma, one exhibited insulinoma, and two patients had solid-pseudopapillary neoplasms. There were no deaths or complications observed during the perioperative period. All patients had no signs of recurrence of the BTPH within a follow-up period of 48-76 mo and had good quality of life without diabetes. Partial pancreatic head resection with Roux-en-Y pancreatic jejunostomy is feasible in selected patients with BTPH.
Core tip: This study elucidated an innovative technique for local pancreatic head resection and Roux-en-Y pancreatic jejunostomy in four patients with benign tumors of the pancreatic head and showed that local resection of the pancreatic head in combination with Roux-en-Y pancreatic jejunostomy not only completely resected the pancreatic tumor but also retained optimal pancreatic function and reduced the incidence of pancreatic leakage.
- Citation: Yuan CH, Tao M, Jia YM, Xiong JW, Zhang TL, Xiu DR. Duodenum-preserving resection and Roux-en-Y pancreatic jejunostomy in benign pancreatic head tumors. World J Gastroenterol 2014; 20(44): 16786-16792
- URL: https://www.wjgnet.com/1007-9327/full/v20/i44/16786.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i44.16786
Pancreaticoduodenectomy, duodenum-preserving pancreatic head resection, and pancreatic head tumor removal are the main surgical procedures in most cases of benign tumors of the pancreatic head (BTPH) based on specific circumstances of lesions and the experience level of each surgeon. In recent years, the surgical removal of pancreatic head tumors and duodenum-preserving pancreatic head resection has been gradually replacing the traditional approach of pancreaticoduodenectomy[1,2]. The aim of the present study was to elucidate an innovative technique for partial pancreatic head resection and Roux-en-Y pancreatic jejunostomy used in four patients with BTPH. Furthermore, the recurrence of disease and post-operative quality of life were observed in a follow-up exam.
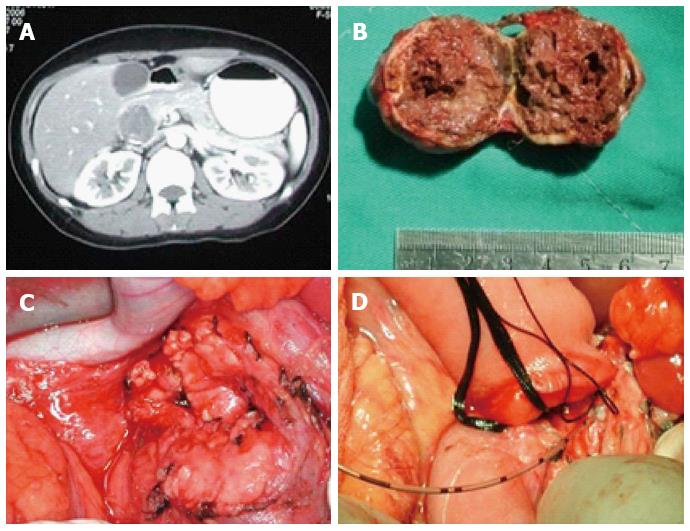
A 31-year-old female was routinely examined after she was admitted to the hospital and diagnosed with a pancreatic tumor by B-ultrasonic examination. The B-ultrasonic wave images revealed a hypoechoic mass at 3.3 cm × 2.8 cm in the pancreatic head. In association with a computed tomography (CT) scan, a low density mass at 3.5 cm × 3.2 cm was detected in the pancreatic head. The tumor displayed an ill-defined borderline and striped calcified lesions (Figure 1A). Preoperative examination showed that the serum tumor markers alpha-fetoprotein (AFP), carcinoembryonic antigen (CEA), and carbohydrate antigen (CA)199 were within normal ranges. However, both intraoperative frozen section analysis and pathological examination confirmed the presence of a solid pseudopapillary tumor of the pancreas (Figure 1B). The patient underwent partial pancreatic head resection and Roux-en-Y pancreaticojejunostomy (Figure 1C and D). The wound surface area of the pancreas was 6.3 cm × 6.5 cm. Because the tumor was very close to the main pancreatic duct, approximately 1.5 cm of the duct was removed. The proximal pancreatic end of the main pancreatic duct was ligated, and a stent was placed in the distal duct. The operation was performed in 210 min, and the amount of intraoperative blood loss was 155 mL. The patient had a smooth, uncomplicated postoperative recovery, started taking oral food on day 3 and was discharged on day 7 after the surgery. There was no recurrence of the tumor observed during the follow-up period of 48-76 mo. Moreover, quality of life for this case was satisfactory, and fasting blood glucose levels were normal.
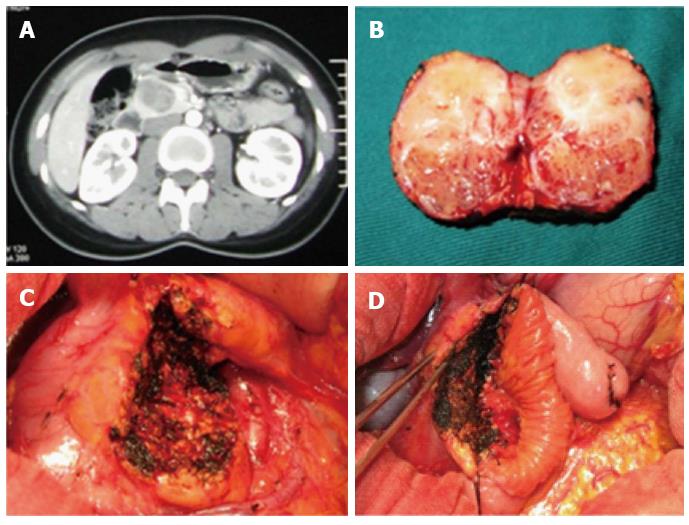
A 21-year-old female was diagnosed with a pancreatic tumor in a B-ultrasonic examination after her hospital stay. Contrast-enhanced abdominal CT scan of the upper abdomen showed oval-shaped lesions in the uncinate process of the pancreas with a diameter of approximately 4.2 cm, and an uneven density was significantly strengthened around the enhanced loci (Figure 2A). Preoperative examination showed that the serum tumor markers AFP, CEA, and CA199 were within the normal range. In the surgical exploration, the size of the uncinate process of the pancreas was 3.6 cm × 3.5 cm (Figure 2B). The patient underwent partial pancreatic head resection and Roux-en-Y pancreatic jejunostomy (Figure 2C and D). The wound surface area of the pancreas was 7.0 cm × 6.5 cm. The operation was performed in 226 min, and the amount of intraoperative blood loss was 210 mL. A solid pseudopapillary tumor of the pancreas was confirmed in postoperative pathology outcomes. The patient had a smooth postoperative recovery with no complications, started taking oral food on day 3, and was discharged on day 8 after the surgery. Recurrence of the tumor was not observed during the follow-up period of 54 mo. Moreover, a good quality of life was associated with fasting blood glucose in the normal range.
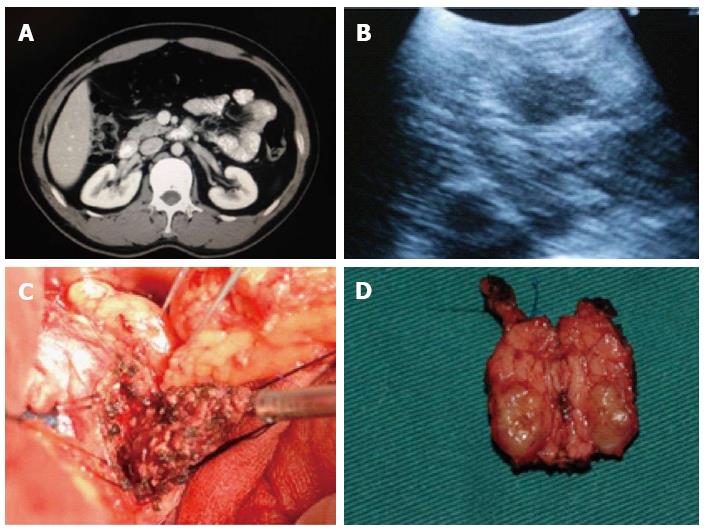
A 37 year-old male had symptoms that included intermittent drowsiness, dizziness, malaise, irritability, sweating, slurred speech, and cognitive impairment without any obvious reason for 15 mo prior to a hospital stay. He was diagnosed with carbon monoxide (CO) poisoning in a local hospital, and his blood glucose level reached 0.8 mmol/L. The patient’s symptoms were well controlled after treatment. However, the symptoms recurred frequently, and he was admitted to our hospital for further treatment. Levels of fasting glucose, serum insulin and C-peptide were shown as 1.9 mmol/L, 83.5 miu/L, and 1140.4 pmol/L, respectively. Enhanced abdominal CT scan of the upper abdomen showed oval-shaped lesions in the uncinate process of the pancreas with a diameter of approximately 3.3 cm, whereas the density around the enhanced loci was significantly strengthened (Figure 3A). During the surgical procedure, ultrasound was used to identify the uncinate process of the pancreas (Figure 3B). The patient underwent partial pancreatic head resection of the tumor (3.2 cm × 3.5 cm) (Figure 3C) and Roux-en-Y pancreaticojejunostomy (Figure 3D). The wound surface area of the pancreas was 5.2 cm × 5.0 cm. Insulinoma was diagnosed intraoperatively by a postsurgical pathology examination. The duration of the surgery was 186 min, and the amount of intraoperative blood loss was 105 mL. The patient had a smooth postoperative recovery with no pancreatic leakage. The serum levels of his fasting glucose, insulin, and C-peptide were 5.5 mmol/L, 12.5 miu/L, and 320.8 pmol/L, respectively, on day 7, and he was discharged on day 8 after surgery. No recurrence of the tumor was observed during the postoperative follow-up period of 50 mo. The patient maintained good quality of life, and fasting blood glucose, insulin, and C-peptide were within the normal range.
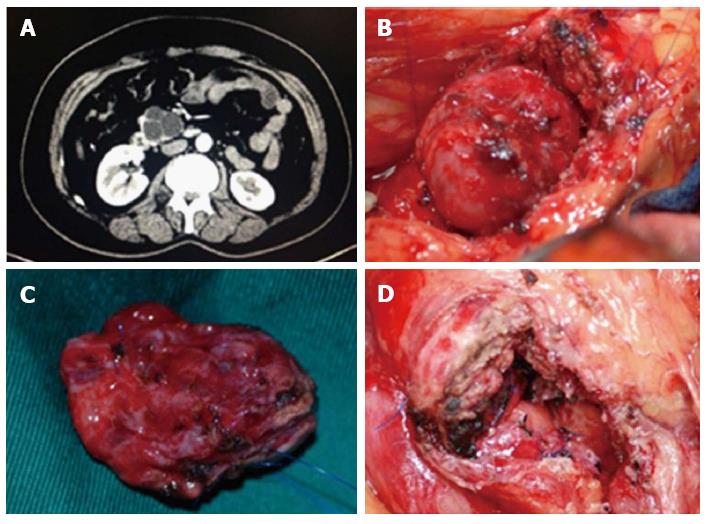
A 48-year-old female had a one month history of intermittent abdominal pain before her visit. Ultrasonic evaluation showed “low or no echo placeholder beneath the right bottom of the pancreas.” Enhanced abdominal CT scan (Figure 4A) and enhanced magnetic resonance imaging (MRI) indicated the uncinate process of the multilocular cystic placeholder (diameter of 4.3 cm). Preoperative examination showed that the serum tumor markers AFP, CEA, and CA199 were within the normal range. In the surgical exploration, the uncinate process of the cystic placeholder was measured at 4.5 cm × 4.8 cm (Figure 4B). The patient underwent partial pancreatic head resection and Roux-en-Y pancreaticojejunostomy (Figure 4C and D). The wound surface area of the pancreas was 7.2 cm × 6.1 cm. Pancreatic mucinous cystic adenoma was confirmed in intraoperative and postsurgical pathological examination. The operation was performed in 165 min, and the amount of intraoperative blood loss was 82 mL. The patient had a smooth postoperative recovery with no complications, and was discharged on day 7 after the surgery. No return of the tumor was observed during the postoperative follow-up period of 48 mo, and her upper abdominal pain symptoms disappeared. The patient maintained good quality of life, and fasting plasma glucose was within the normal range.
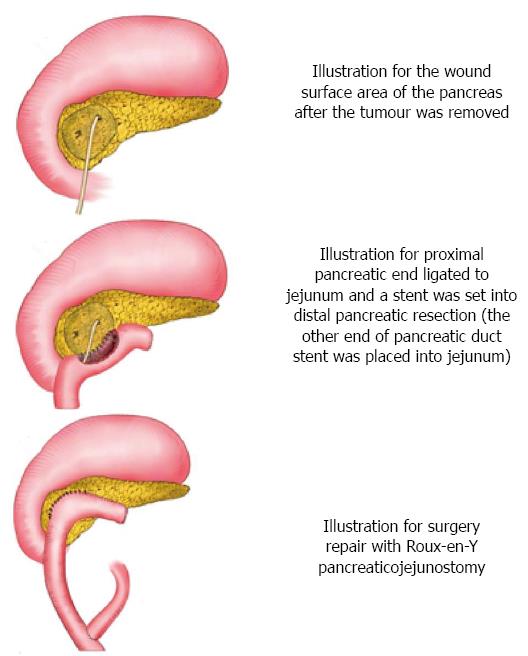
This project focuses on four patients who underwent pancreatic head resection and Roux-en-Y pancreaticojejunostomy (Table 1). This is a retrospective study approved by the Peking University Third Hospital Institutional Review Board. The surgical technical details and outcomes were analyzed. App operations were performed using a supraumbilical transverse incision under general anesthesia. First, a paramedian incision was made on the upper right side of the abdomen. Isolation and exposure: The gastrocolic and duodenum ligaments were slit to expose the pancreas. The duodenum and the pancreatic head region were found. The side peritoneum from the duodenal bulb to the descending part was isolated from the inferior vena cava. The tumor was detected by preoperative enhanced CT/MRI, intraoperative exploration, and ultrasound when necessary. The tumor was carefully isolated from normal pancreatic tissue to avoid injury or ligation of the common bile duct and the pancreatic duct. Specifically, with preserving the right gastroepiploic artery, the anterosuperior pancreaticoduodenal artery was identified and divided. The origin of the posterior superior pancreaticoduodenal artery was identified and the attached pancreatic tissues were separated downward, preserving the vessels. Leaving both the posterior superior pancreaticoduodenal artery and common bile duct intact, the pancreatic tissues surrounding the common bile duct and intervening between the posterior superior pancreaticoduodenal artery and the common bile duct were carefully dissected. The tumor within the head of the pancreas, as well as some pancreatic tissue in the pancreatic head, was completely removed from the tightly attached parapapillary area of the second portion of the duodenum, and the tumor tissue was prepared for frozen section biopsy to confirm the benign tumor. Pancreaticoduodenectomy would have been carried out if a malignant tumor was proven. Further management of surgical-site bleeding: If the tumor was close to the main pancreatic duct and the pancreatic duct was clearly injured, we had to carry out ligation of the proximal pancreatic duct and set up an internal stent at the distal position (subject to Roux-en-Y pancreatic jejunostomy). If there was no injury found in the main pancreatic duct, Roux-en-Y pancreatic jejunostomy was directly performed. Roux-en-Y pancreatic jejunostomy: We cut off the jejunum at the location 15 cm away from Treitz ligament, pulled the distal end to the pancreas through the rear of the colon, sutured the transected end, and constructed side-to-side anastomosis of the pancreas wound with the jejunum (pancreatic duct stent placed into jejunum). Then, end-to-side anastomosis was performed between the transected ends of jejunum and the location of approximately 40 cm from the anastomotic stoma, and a drainage tube was placed under the pancreatic anastomotic stoma. Figure 5 is the schematic sketch of some key steps.
| Sex | Age (yr) | Symptoms | Tests | Preoperative diagnosis | Operation time (min) | Blood loss (mL) | Postoperative hospital stay (d) | Pathology | Size (cm) | |
| Case 1 | F | 31 | Incidental | US CT | SPT | 210 | 155 | 7 | SPT | 3.5 |
| Case 2 | F | 21 | Incidental | US CT | SPT or MCN | 226 | 210 | 8 | SPT | 3.6 |
| Case 3 | M | 37 | Hypoglycemia | US CT | Insulinoma | 186 | 105 | 8 | Insulinoma | 3.5 |
| Case 4 | F | 48 | Abdominal pain | US CT MRI | MCN | 165 | 82 | 7 | MCN | 4.8 |
| Mean (range) | 34.3 (21-48) | 196.8 (165-226) | 138.0 (82-210) | 7.5 (7-8) | 3.85(3.5-4.8) |
BTPH includes pancreatic exocrine tumors (serous cystic adenoma, mucinous cystadenoma, intraductal papillary mucinous tumor, and solid pseudopapillary tumor) and endocrine tumors (insulinoma, pancreatic gastrinoma, and no function neuroendocrine tumors)[3]. With the development of diagnostic imaging techniques, many pancreatic lesions now can be confirmed as benign lesions before surgery[4,5]. It is the continual pursuit of the surgeon to maximize the integration of the pancreas, gastrointestinal tract, and biliary tract organs to facilitate the functional recovery and preservation of the organ while regulating a balance between tumor removal and injury control[6]. In patient selection for specific surgical procedures, the surgeon should conduct a comprehensive assessment based on local changes around the lesion with its systemic response and based on the patients’ understanding of their family history of the disease[7]. Because pathological types of pancreatic lesions are diverse and complex, it may be effective to make a decision on organ preservation when a surgery is applied to a patient. Currently, the main procedures for treating BTPH include pancreaticoduodenectomy, duodenum-preserving pancreatic head resection, and surgical excision of pancreatic cancer[8].
The main surgical approach for pancreatic head tumors has been pancreaticoduodenectomy. Because pancreaticoduodenectomy has a high incidence of complications, organ damage, and loss of exocrine and endocrine pancreatic function, it is controversial as to whether pancreaticoduodenectomy should be conducted for low-grade malignant lesions and benign lesions[9]. The surgical excision of pancreatic cancer is suitable for benign tumors growing outside of the pancreas with a small tumor bed and the ability to avoid injury of the main pancreatic duct during the removal process. Kiely et al[10] and Crippa et al[2] conducted enucleation of benign tumors of the pancreas in 11 and 61 patients, and the incidence of postoperative pancreatic fistula was 27% and 38%, respectively[10]. The postoperative incidence of pancreatic leakage in the surgical excision of pancreatic cancer was 10% higher than in pancreaticoduodenectomy[6]. The following presents the main reasons for a higher incidence of pancreatic leakage remaining in the surgical excision: (1) tumor excision from the deep pancreatic parenchyma, particularly for a tumor of the dorsal pancreas, which can easily injure the main pancreatic duct; (2) an unnoticed injury of the duct after pancreatic ductal ligation; (3) the vice pancreatic duct course varied greatly, which was susceptible to traumatic injury; (4) partial resection of the tumor resulted in a relatively recessed pancreatic section base, which hinders a clear vision of the small pancreatic duct section with possible ligation completed, easily leading to postoperative pancreatic leakage; and (5) the tumor subjected to resection is normally located in the periphery of the pancreatic tissue and does not block the main pancreatic duct. The pancreas is not susceptible to chronic inflammation, but the pancreas and pancreatic duct are soft and can rupture or tear. This was not conducive to achieving healing of the transected ends with wound closure.
How to control the extent of surgical injury and reduce the incidence of pancreatic defects and leakage has been a pressing issue for this surgical treatment[11]. To maintain the advantages of surgical excision of the pancreatic cancer as well as to reduce the incidence of pancreatic leakage, we conducted partial pancreatic head resection and pancreatic Roux-en-Y anastomosis reconstruction for the selected cases. We summarized the indications for the surgery with the following causes: (1) tumor size of 3-5 cm; (2) large surface area wound highly susceptible to pancreatic leakage after partial resection; and (3) clear damage during surgery, the proximal pancreatic duct was ligated, and a stent needed to be placed in the distal pancreatic duct by pancreaticojejunostomy. In some cases, although there was not an explicit statement on pancreatic injury, the tumor was close to the pancreatic duct; therefore, we could not eliminate the possibility that the pancreatic duct was damaged. The modified operation completely removed the lesion in the tissue and reconstructed the digestive tract with Roux-en-Y pancreatic jejunostomy, which may simplify the surgical procedures and reduce postoperative complications. Prior to surgery, a field lesion assessment should be performed using detailed images, especially with an enhanced CT scan, and an appropriate approach of surgical treatment should be chosen after judging the relationship of the lesions between the main pancreatic duct and the surrounding vessels. The diagnosis must be clearly made. The analysis on quick frozen section was performed when it was difficult to determine the nature of the lesions. If a malignant tumor was demonstrated, radical dissection would have been chosen[12]. For instance, special attention should be paid to prevent the ligation of the main pancreatic duct during the local excision of tumors; otherwise, postoperative complications will be increased. In the process of tumor removal, we did not over-free the duodenum and tissues at the rear of the pancreas to prevent pancreatic penetration, which may increase surgical risk and even failure.
In this retrospective study, four cases received local pancreatic resection and surgical wound repair with Roux-en-Y pancreatic jejunostomy. We found that the procedure had the following benefits: preservation of organ function; no injury of the biliary system; intact stomach and duodenum; precise dissection of the peripancreas; and maximization of the retention of a healthy pancreas, thereby avoiding or reducing the risk of postoperative diabetes. The four patients did not suffer from postoperative diabetes or pancreatic leakage. The injury was under control, thus reducing or avoiding pancreatic leakage caused by pancreatic duct injury in the process of local tumor resection. The drainage channel from the pancreas into the digestive tract was rebuilt by precise and exact pancreaticojejunostomy; therefore, the impaired pancreatic exocrine function was restored. It was not necessary to reconstruct the biliary system to avoid bile leakage. All of these factors may improve surgical procedure safety and good preservation and injury control of the pancreas.
The authors suggested that the local resection of the pancreatic head and the surgical wound repair with Roux-en-Y pancreatic jejunostomy may be beneficial in reducing complications; these benefits should be recognized, and the main pancreatic duct should be protected during surgery. The intraoperative detection of tumors using B-ultrasonic scans can be performed when necessary. It was necessary to keep the tumor border 2-3 mm away from the main pancreatic duct to ensure a clean cutting edge without main pancreatic duct injury[13]. Tumor size was not an absolute indication for local excision procedure. Instead, tumor location, the size of its base section, and the relationship between the tumor and the main pancreatic duct are important factors required for the consideration of local excision[14]. During the surgery, the injured blood vessels or small pancreatic duct should be ligated or sutured with appropriate management of the pancreatic trauma. If there is a clear pancreatic duct injury, ligation should be performed at the proximal end and a stent should be placed at the distal end of the pancreatic duct. If B-ultrasonic images examination for preoperative assessment of tumor location show that the tumor is close to the pancreatic duct, surgery should be managed by stent implantation with nasal endoscopy. This technique was helpful not only to search for the pancreatic duct in the surgery but also to reduce pancreatic injury and to examine whether there was any pancreatic duct injury. However, this operation was invasive, with an increased risk of pancreatitis; therefore, risk and benefits must be fully considered before choosing this method.
In summary, our study indicates that a 3-5 cm BTPH with a large pancreatic wound after local resection existed at a high risk of pancreatic leakage. Local resection of the pancreatic head in combination with Roux-en-Y pancreatic jejunostomy not only completely resected the pancreatic tumor but also retained optimal pancreatic function and reduced the incidence of pancreatic leakage.
Four patients (three female and one male) with a mean age of 34.3 years presented with benign tumors of the pancreatic head with diameters of 3.2-4.5 cm.
Benign tumor of the pancreatic head.
CO poisoning.
While the preoperative serum tumor markers alpha-fetoprotein, carcinoembryonic antigen, and carbohydrate antigen 199 were within the normal ranges in case 1, case 2 and case 4, the levels of fasting glucose, serum insulin and C-peptide of case 3 were shown as 1.9 mmol/L, 83.5 miu/L and 1140.4 pmol/L, respectively.
B-ultrasonic examination, computed tomography scan, and enhanced magnetic resonance imaging were used to assist the diagnosis of benign tumors of the pancreatic head in the 4 cases.
Intraoperative frozen section analysis and pathological examination confirmed the presence of solid pseudopapillary tumors of the pancreas in case 2.
The 4 patients were cured by partial pancreatic head resection in combination with Roux-en-Y pancreaticojejunostomy.
How to maintain the advantages of surgical excision of the pancreatic cancer as well as to effectively reduce the incidence of pancreatic leakage is a pressing issue that is unclear for this surgical treatment.
Benign tumors of the pancreatic head include exocrine tumors (serous cystic adenoma, mucinous cystadenoma, intraductal papillary mucinous tumor, and solid pseudopapillary tumor) and endocrine tumors (insulinoma, pancreatic gastrinoma, and no function neuroendocrine tumors).
This case report indicates that the local resection of the pancreatic head in combination with Roux-en-Y pancreatic jejunostomy not only completely resected the pancreatic tumor but also retained optimal pancreatic function and reduced the incidence of pancreatic leakage.
In this manuscript, the authors report 4 cases of duodenum-preserving pancreatic head resection for benign pancreatic lesions. Previous reports on duodenum-preserving pancreatic resections claimed clinical benefits such as less complication rates and shorter hospital stay, low hospital mortality rate of < 1% and superior long-term outcomes.
P- Reviewer: Meshikhes AWN S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Yoon WJ, Brugge WR. Pancreatic cystic neoplasms: diagnosis and management. Gastroenterol Clin North Am. 2012;41:103-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Crippa S, Bassi C, Salvia R, Falconi M, Butturini G, Pederzoli P. Enucleation of pancreatic neoplasms. Br J Surg. 2007;94:1254-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 120] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 3. | Al-Haddad M, Schmidt MC, Sandrasegaran K, Dewitt J. Diagnosis and treatment of cystic pancreatic tumors. Clin Gastroenterol Hepatol. 2011;9:635-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 4. | Spinelli KS, Fromwiller TE, Daniel RA, Kiely JM, Nakeeb A, Komorowski RA, Wilson SD, Pitt HA. Cystic pancreatic neoplasms: observe or operate. Ann Surg. 2004;239:651-67; discussion 651-67;. [PubMed] |
| 5. | Fernández-del Castillo C, Targarona J, Thayer SP, Rattner DW, Brugge WR, Warshaw AL. Incidental pancreatic cysts: clinicopathologic characteristics and comparison with symptomatic patients. Arch Surg. 2003;138:427-43; discussion 427-43;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 426] [Cited by in RCA: 413] [Article Influence: 18.8] [Reference Citation Analysis (0)] |
| 6. | Steinberg WM, Barkin JS, Bradley EL, DiMagno EP, Layer P, Tenner S, Andersen DK, Reber HA. Can neoplastic cystic masses in the head of the pancreas be safely and adequately removed without a whipple resection? Pancreas. 2009;38:721-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 7. | Matsuoka L, Parekh D. The minimally invasive approach to surgical management of pancreatic diseases. Gastroenterol Clin North Am. 2012;41:77-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Farkas G, Leindler L, Daróczi M, Farkas G. Prospective randomised comparison of organ-preserving pancreatic head resection with pylorus-preserving pancreaticoduodenectomy. Langenbecks Arch Surg. 2006;391:338-342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 85] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 9. | Köninger J, Seiler CM, Sauerland S, Wente MN, Reidel MA, Müller MW, Friess H, Büchler MW. Duodenum-preserving pancreatic head resection--a randomized controlled trial comparing the original Beger procedure with the Berne modification (ISRCTN No. 50638764). Surgery. 2008;143:490-498. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 10. | Kiely JM, Nakeeb A, Komorowski RA, Wilson SD, Pitt HA. Cystic pancreatic neoplasms: enucleate or resect? J Gastrointest Surg. 2003;7:890-897. [PubMed] |
| 11. | Callery MP, Pratt WB, Vollmer CM. Prevention and management of pancreatic fistula. J Gastrointest Surg. 2009;13:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 12. | Hackert T, Werner J, Büchler MW. Postoperative pancreatic fistula. Surgeon. 2011;9:211-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 13. | Tucker ON, Crotty PL, Conlon KC. The management of insulinoma. Br J Surg. 2006;93:264-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 14. | Hiramoto JS, Feldstein VA, LaBerge JM, Norton JA. Intraoperative ultrasound and preoperative localization detects all occult insulinomas; discussion 1025-6. Arch Surg. 2001;136:1020-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |