Published online Nov 28, 2014. doi: 10.3748/wjg.v20.i44.16559
Revised: May 10, 2014
Accepted: July 22, 2014
Published online: November 28, 2014
Processing time: 275 Days and 23.5 Hours
Autoimmune pancreatitis (AIP) is a distinct form of chronic pancreatitis that is increasingly being reported. The presentation and clinical image findings of AIP sometimes resemble those of several pancreatic malignancies, but the therapeutic strategy differs appreciably. Therefore, accurate diagnosis is necessary for cases of AIP. To date, AIP is classified into two distinct subtypes from the viewpoints of etiology, serum markers, histology, other organ involvements, and frequency of relapse: type 1 is related to IgG4 (lymphoplasmacytic sclerosing pancreatitis) and type 2 is related to a granulocytic epithelial lesion (idiopathic duct-centric chronic pancreatitis). Both types of AIP are characterized by focal or diffuse pancreatic enlargement accompanied with a narrowing of the main pancreatic duct, and both show dramatic responses to corticosteroid. Unlike type 2, type 1 is characteristically associated with increasing levels of serum IgG4 and positive serum autoantibodies, abundant infiltration of IgG4-positive plasmacytes, frequent extrapancreatic lesions, and relapse. These findings have led several countries to propose diagnostic criteria for AIP, which consist of essentially similar diagnostic items; however, several differences exist for each country, mainly due to differences in the definition of AIP and the modalities used to diagnose this disease. An attempt to unite the diagnostic criteria worldwide was made with the publication in 2011 of the international consensus diagnostic criteria for AIP, established at the 2010 Congress of the International Association of Pancreatology (IAP).
Core tip: Autoimmune pancreatitis (AIP) was first reported in Japan in 1995. Since then, a large series of studies has been documented and the concept of AIP is now recognized worldwide. Two distinct subtypes of AIP occur with different incidences in Asian and western countries. Type 1 is often associated with IgG4-related systemic diseases and shares histological features of lymphoplasmacytic sclerosing pancreatitis. Type 2 is usually not associated with IgG4 abnormality and histologically shows idiopathic duct-centric pancreatitis with granulocytic epithelial lesions. Independent diagnostic criteria had previously been used in individual countries, but international consensus diagnostic criteria were published in 2011.
- Citation: Matsubayashi H, Kakushima N, Takizawa K, Tanaka M, Imai K, Hotta K, Ono H. Diagnosis of autoimmune pancreatitis. World J Gastroenterol 2014; 20(44): 16559-16569
- URL: https://www.wjgnet.com/1007-9327/full/v20/i44/16559.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i44.16559
Autoimmune pancreatitis (AIP) was first documented in 1995 by Yoshida et al[1], who reported a case of chronic pancreatitis that fulfilled the definition of an autoimmune disease[2] with respect to hyperglobulinemia, positive serum autoantibody, and steroid response. In 2001, Hamano et al[3] reported increased serum levels of IgG4 in Japanese patients with AIP. This disease is a form of chronic pancreatitis characterized by frequent presentation with obstructive jaundice, simultaneous and/or metachronal occurrences of extrapancreatic lesions, histology of lymphoplasmacytic infiltrates with fibrosis, and a dramatic response to corticosteroids[4-9]. Symptoms, blood test data, and clinical images of the AIP often resemble those of pancreatic cancer (PC)[10-12], malignant lymphoma[1,13], and other types of pancreatitis. Therefore, differential diagnosis must be conducted carefully.
The first diagnostic criteria for AIP were established in Japan in 2002[14], revised in 2006[15], and revised again in 2011(Table 1)[16]. During this period, the concepts of AIP were well recognized worldwide and nationwide diagnostic criteria were proposed in South Korea[17,18], the United States, Germany[19], and Italy[20]. The conditions and methodologies used in each criterion varied; hence, the cases diagnosed as AIP sometimes differed by country. AIP was later revealed to consist of two distinct subtypes: type 1 AIP, which is characterized by histology resembling that of “lymphoplasmacytic sclerosing pancreatitis (LPSP),” and type 2 AIP or “idiopathic duct-centric pancreatitis (IDCP)[21]” with “granulocytic epithelial lesion (GEL)[8,22]”. Type 1 AIP is now considered the pancreatic manifestation of systemic organ disorders termed “IgG4-related diseases (IgG4-RD)[23]”, while type 2 is usually not associated with IgG4 activity or extrapancreatic lesions other than ulcerative colitis (UC). The proportions of type 1 and type 2 AIP vary substantially in western and eastern countries. Consensus meetings have been held and international criteria were established in Asia in 2008[24], and on a worldwide scale (international consensus diagnostic criteria: ICDC) in 2011 (Tables 2-4 and Figures 1-3)[25]. The ICDC are presently evaluated as the most sensitive and specific criteria for diagnosing AIP[26].
| A: Diagnostic items |
| I: Enlargement of the pancreas: |
| (a) Diffuse enlargement |
| (b) Segmental/focal enlargement |
| II: ERP (endoscopic retrograde pancreatography) shows irregular narrowing of the main pancreatic duct |
| III: Serological findings |
| Elevated level of serum IgG4 (≥ 135 mg/dL) |
| IV: Pathological findings: Among (1)-(4) listed below |
| (a) Three or more are observed |
| (b) Two are observed |
| (1) Prominent infiltration of lymphocytes and plasmacytes and fibrosis |
| (2) More than 10 IgG4-positive plasmacytes per high-power microscope field |
| (3) Storiform fibrosis |
| (4) Obliterative phlebitis |
| V: Extra-pancreatic lesions: sclerosing cholangitis, sclerosing dacryoadenitis/sialoadenitis/retroperitoneal fibrosis |
| (a) Clinical lesions |
| Extrapancreatic sclerosing cholangitis, sclerosing dacryoadenitis/ sialoadenitis (Mikulicz disease) or/retroperitoneal fibrosis |
| (b) Pathological lesions |
| Pathological examination shows characteristic features of sclerosing cholangitis, sclerosing dacryoadenitis sialoadenitis or/retroperitoneal fibrosis |
| <Option> Effectiveness of steroid therapy |
| A specialized facility may include in its diagnosis the effectiveness of steroid therapy, once pancreatic or bile duct cancers have been ruled out. When it is difficult to differentiate from malignant conditions, it is desirable to perform cytological examination using an endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). Facile therapeutic diagnosis by steroids should be avoided unless the possibility of malignant tumor has been ruled out by pathological diagnosis. |
| B: Diagnosis |
| I: Definite diagnosis |
| (1) Diffuse type |
| Ia + III/IVb/V (a/b) |
| (2) Segmental/focal type |
| Ib + II + two or more of < III/IVb/V (a/b) > |
| or |
| Ib + II + < III/IVb/V (a/b) > + Option |
| (3) Definite diagnosis by histopathological study |
| IVa |
| II: Probable diagnosis |
| Segmental/focal type: Ib + II + < III/IVb/V(a/b) > |
| III: Possible diagnosis1 |
| Diffuse type: Ia + II + Option |
| Segmental/focal type: Ib + II + Option |
| Diagnosis | Primary basis for diagnosis | Imaging evidence | Collateral evidence |
| Definitive type 1 AIP | Histology | Typical/indeterminate | Histologically confirmed LPSP (level 1 H) |
| Imaging | Typical | Any non-D level 1/level 2 | |
| Indeterminate | Two or more from level 1 (+ level 2 D1) | ||
| Response to steroid | Indeterminate | Level 1 S/OOI + Rt or level 1 D + | |
| Level 2 S/OOI/H + Rt | |||
| Probable type 1 AIP | Indeterminate | Level 2 S/OOI/H + Rt | |
| Criterion | Level 1 | Level 2 | |
| P: Parenchymal imaging | Typical: | Indeterminate (including atypical3): | |
| Diffuse enlargement with delayed enhancement (sometimes associated with rim-like enhancement) | Segmental/focal enlargement with delayed enhancement | ||
| D: Ductal imaging (ERP) | Long (> 1/3 length of the main pancreatic duct) or multiple strictures without marked upstream dilatation | Segmental/focal narrowing without marked upstream dilatation (duct size, < 5 mm) | |
| S: Serology | IgG4, > 2 × upper limit of normal value | IgG4, 1-2 × upper limit of normal value | |
| OOI: Other organ involvement | a or b | a or b | |
| a: Histology of extrapancreatic organs | a: Histology of extrapancreatic organs including endoscopic biopsies of bile duct4: | ||
| Any three of the following: | Both of the following: | ||
| (1) Marked lymphoplasmacytic infiltration with fibrosis and without granulocytic infiltration | (1) Marked lymphoplasmacytic infiltration without granulocytic infiltration | ||
| (2) Storiform fibrosis | (2) Abundant (> 10 cells/HPF) IgG4-positive cells | ||
| (3) Obliterative phlebitis | |||
| (4) Abundant (> 10 cells/HPF) IgG4-positive cells | |||
| b: Typical radiological evidence | b: Physical or radiological evidence | ||
| At least one of the following: | At least one of the following | ||
| (1) Segmental/multiple proximal (hilar/intrahepatic) or proximal and distal bile duct stricture | (1) Symmetrically enlarged salivary/lachrymal glands | ||
| (2) Retroperitoneal fibrosis | (2) Radiological evidence of renal involvement described in association with AIP | ||
| H: Histology of the pancreas | LPSP (core biopsy/resection) | LPSP (core biopsy) | |
| At least 3 of the following: | Any 2 of the following: | ||
| (1) Periductal lymphoplasmacytic infiltrate without granulocytic infiltration | (1) Periductal lymphoplasmacytic infiltrate without granulocytic infiltration | ||
| (2) Obliterative phlebitis | (2) Obliterative phlebitis | ||
| (3) Storiform fibrosis | (3) Storiform fibrosis | ||
| (4) Abundant (> 10 cells/HPF) IgG4-positive cells | (4) Abundant (> 10 cells/HPF) IgG4-positive cells | ||
| Response to steroid (Rt)2 | Diagnostic steroid trial | ||
| Rapid ( ≤ 2 wk) radiologically demonstrable resolution or marked improvement in pancreatic/extrapancreatic manifestations | |||
| Diagnosis | Imaging evidence | Collateral evidence |
| Definitive type 2 AIP | Typical/indeterminate | Histologically confirmed IDCP (level 1 H) or clinical inflammatory bowel disease + level 2 H + Rt |
| Probable type 2 AIP | Typical/indeterminate | Level 2 H/clinical inflammatory bowel disease + Rt |
| Criterion | Level 1 | Level 2 |
| P: Parenchymal imaging | Typical: | Indeterminate (including atypical2): |
| Diffuse enlargement with delayed enhancement (sometimes associated with rim-like enhancement) | Segmental/focal enlargement with delayed enhancement | |
| D: Ductal imaging (ERP) | Long (> 1/3 length of the main pancreatic duct) or multiple strictures without marked upstream dilatation | Segmental/focal narrowing without marked upstream dilatation (duct size, < 5 mm) |
| OOI: Other organ involvement | Clinically diagnosed inflammatory bowel disease | |
| H: Histology of the pancreas (core biopsy/resection) | IDCP | |
| Both of the following: | Both of the following: | |
| (1) Granulocytic infiltration of duct wall (GEL) with or without granulocytic acinar inflammation | (1) Granulocytic and lymphoplasmacytic acinar infiltrate | |
| (2) Absent or scant (0-10 cells/HPF) IgG4-positive cells | (2) Absent or scant (0-10 cells/HPF) IgG4-positive cells | |
| Response to steroid (Rt)1 | Diagnostic steroid trial | |
| Rapid ( ≤ 2 wk) radiologically demonstrable resolution or marked improvement in manifestations | ||
A worldwide survey of AIP[27] indicated that most cases of AIP in Asia fit the histological profile of LPSP, or type 1 AIP, while European and American cases are a mixture of LPSP and idiopathic duct-centric pancreatitis (IDCP)[21,27,28]. The necessity of adequate pancreatic specimens for histology makes accurate diagnosis of IDCP difficult before resection, and this is probably the reason for the limited number of reported cases of type 2 AIP. The two types of AIP also differ in characteristics depending on the geographical distribution, age and gender of the patients, serological findings, association with extra pancreatic lesions, and relapse ratios (Table 5).
| Type 1 AIP | Type 2 AIP | |
| Geographical distributuion | Asia > United States, Europe | Europe > United States > Asia |
| Age at presentation | 60-70 s | 40-50 s |
| Gender | Male >> Female | Male = Female |
| Symptoms | Jaundice, Abdominal pain | Jaundice, Abdominal pain |
| Serology | IgG4, IgG, Autoantibodies | Usually negative |
| Pancreatic images | Enlarged (focal, diffuse) | Enlarged (focal, diffuse) |
| Pancreatic histology | LPSP | IDCP with GEL |
| Extrapancreatic lesions | Sclerosing cholangitis, sialoadenitis, retroperitoneal fibrosis, interstitional nephritis, etc. | Inflammatory bowel disease |
| Steroid response | Excellent | Excellent |
| Relapse | High rate | Rare |
Type 1 AIP is histologically characterized as LPSP and is often associated with: (1) abundant lymphoplasmacytic infiltration with IgG4-positive cells [> 10 cells/high power field (HPF)]; (2) storiform fibrosis; and (3) obliterative phlebitis (Tables 1, 2 and 5). Type 1 AIP frequently occurs in elderly men and is geographically distributed in greater numbers in Asia[29,30] than in western countries[19,20,22,31]. Type 1 AIP is the pancreatic manifestation of IgG4-related disease (IgG4-RD)[23,32]; consequently, a variety of systemic lesions with IgG4-positive cells infiltrates develop simultaneously or metachronously, in association with elevated level of serum IgG or IgG4 (> 135 mg/dL) and positive serum autoantibodies. These systemic lesions include sclerosing cholangitis (60%), sialadenitis (14%), retroperitoneal fibrosis (10%), interstitial pneumonitis (8%), and tubulointerstitial nephritis (8%)[4], and many other organs are recognized as possible targets of IgG4-RD or type 1 AIP5 (Table 6). Response to corticosteroid therapy is usually excellent (97%-98%)[33,34]; however, a high rate of relapse is also observed (56% in 1 year within steroid initiation and 92% within 3 years) (Table 5).
| Close association | Possible association |
| Lachrymal gland inflammation | Hypophysitis |
| Sialoadenitis | Autoimmune neurosensory hearing loss |
| Hilar lymphadenopathy | Uveitis |
| Interstitial pneumonitis | Chronic thyroiditis |
| Sclerosing cholangitis | Pseudotumor (breast, lung, liver) |
| Retroperitoneal fibrosis | Gastric ulcer |
| Tubulointestinal nephritis | Swelling of Papilla of Vater |
| IgG4 hepatopathy | |
| Periaortitis | |
| Prostatitis | |
| Schonlein-Henoch purpura | |
| Autoimmune thrombocytopenia |
Type 2 AIP is regarded as a specific pancreatic disease, characterized histologically by duct-centric pancreatitis with a GEL[21,22,27,35]. Type 2 AIP patients are more frequently diagnosed in western countries, with a younger age of onset and without gender deviation, compared to type 1[36]. Type 2 AIP occasionally coexists with inflammatory bowel disease (16%-30%)[36,37]. Response to steroids is excellent, as in type 1, but type 2 AIP rarely relapse (Table 5)[37].
Patients with type 2 AIP have no serological markers of autoimmunity. Therefore, the classification of type 2 AIP as a clinical entity of AIP is still debated. Nevertheless, the deposition of C3c and IgG in the basement membrane of the pancreatic ducts and acini suggests an immune complex-mediated destruction of ducts and acini in type 2 as well as type 1 AIP[38].
Diagnostic criteria, either nationwide[9,16-20] or international[24,25], consist mostly of common diagnostic items such as image findings of the pancreatic parenchyma, pancreatography, and extrapancreatic lesions; serological findings; histology of the pancreatic lesion; and response to steroid therapy (Tables 1-3). The diagnostic items are very similar, but the method or approach for analyzing each finding varies depending on the country. For instance, in Japan16, endoscopic retrograde pancreatography (ERP) is performed even by general clinicians but is usually precluded in western counties to avoid causing or worsening pancreatitis. In contrast, the Mayo Clinic in the United States[9] routinely performs pancreatic core biopsy for diagnosing AIP. These differences in the methodology seem to reflect the diagnostic criteria or diagnostic algorithm used by individual country[9,16-20].
Focal or diffuse pancreatic enlargement is a common finding in both types of AIP. A dynamic study showed that enhancement of the pancreatic parenchyma is repressed during the arterial to parenchymal phase and is recovered at the portal phase to delayed phase[39]. This enhancement pattern is distinct from that of PC and is applied to contrast-enhanced EUS for the differentiation of AIP and cancer by analyzing time-intensity curves[40,41]. Typically, a linear or band-like structure, depicted as low density by computed tomography (CT) and a hypo-intensity signal by T2-weight magnetic resonance image (MRI), appears at the margin of the enlarged pancreatic parenchyma and is referred to as a “capsule-like rim”, reflecting the fibrous tissue[39,42]. Abdominal ultrasonography (US) and EUS show similar findings to those of early chronic pancreatitis, including hyperechoic foci (91%-100%), hyperechoic strands (30%-81%), lobularity (15%-53%), and a hyperechoic wall of the main pancreatic duct (30%) in cases with AIP, and these findings decrease after steroid therapy[33,43]. Ultrasound of typical diffuse-type AIP shows a diffusely enlarged low-echoic pancreas without ductal dilation, or so-called “sausage-like appearance.” Elastographic studies have revealed inconsistent results regarding the hardness of pancreatic lesions associated with AIP[44,45].
An irregular narrowing of the main pancreatic duct (MPD), but not a complete stenosis or obstruction, is seen in cases of AIP. Nishino et al[46] analyzed the differences in ERP findings between AIP and PC, and found a higher prevalence of narrowing of the MPD for ≥ 3 cm of its length and a higher prevalence for the presence of side branches in the narrowed portion of the MPD in the AIP group than in the PC group (P < 0.001 and P < 0.001, respectively). In addition, an obvious dilation of the MPD (≥ 4 mm) upstream of the lesion was recognized in 87% of the PC cases, but this was seen in only 11% of the AIP cases (P < 0.001). The narrowed portion of the MPD is not visualized by magnetic resonance cholangiopancreatography (MRCP)[47]; however, use of ERP is only mandatory in the Japanese criteria (Table 1). Either MRCP or ERP is acceptable in the Korean criteria17,18 and modality is not specified in the Mayo criteria (HISORt)[9]. The ERCP finding seems to be extremely important in atypical cases[10,33]; for instance, a case that does not show marked shrinkage following steroid therapy[33,48] or a case of PC mimicking[11] or accompanying[12]AIP.
The most sensitive and specific serum marker for type 1 AIP is IgG4 (≥ 135 mg/dL, sensitivity: 86%, specificity to AIP against PC: 96%). However, IgG4 is not actually specific for AIP[5], and elevated serum IgG4 or infiltrations of numerous IgG4-bearing plasma cells have also been reported in cases with PC (10%, 13/135)[49]. Various antibodies appear in the sera of AIP patients, such as anti-lactoferrin antibody, anti-carbonic anhydrase II antibody, antinuclear antibody (ANA), and rheumatoid factor (RF) at respective frequencies of 75%, 55%, 60%, and 20%-30%[50]. The sensitivity of a set of non-specific serum markers (IgG + ANA + RF) (91%) is similar to that of IgG4, but the specificity (61%) is significantly lower than for IgG4[5]. The SS-A (Ro) and SS-B (La) antibodies, which are markers of Sjögren’s syndrome, are rarely seen in AIP patients, giving additional grounds for the idea that sclerosing sialadenitis seen in AIP patients is distinct from Sjögren’s syndrome.
The level of serum markers is usually correlated with the autoimmune activity and a large number of systemic lesions are more often recognized in type 1 AIP with high levels of serum markers (IgG4, soluble IL2 receptor, etc.)[51,52]. Relapse is also often recognized in cases with elevated levels of serum IgG[33] or IgG4[34]. Hence, these serum markers are also applicable to the clinical follow up of patients with type 1 AIP.
Extrapancreatic lesions are often associated with type 1 AIP and are correlated with disease activity. The most common extrapancreatic lesion seen in type 1 AIP is sclerosing cholangitis (bile duct), with other typical lesions including dacryoadenitis (lachrymal gland), sialadenitis (salivary gland), interstitial pneumonitis (lung), tubulointerstitial nephritis (kidney), retroperitoneal fibrosis (retroperitoneum), and lymph node lesions at the hepatic hilar portion. Many of reported extrapancreatic lesions are summarized in Table 5 and classified as having close association or possible association with AIP. Representative extrapancreatic lesions have been reported as showing pathological findings similar to the pancreas, including massive lymphoplasmacytic infiltration and fibrosis, obliterating phlebitis, and presence of prominent IgG4 positive plasma cells[7]. These lesions can be detected incidentally in cross-sectional images and whole body imaging such as 18F-Fluoro-deoxyglucose positron emission tomography (PET)[53,54] and Gallium scintigraphy[55]. These extrapancreatic lesions sometimes confuse the diagnosis; i.e., type 1 AIP is sometimes accompanied by pseudotumor of the liver or lung, mimicking metastases from PC[56]. The occurrence of OOI in AIP patients sometimes causes serious physical conditions, such as loss of consciousness due to swelling of the pituitary gland[57] or hemorrhagic risk due to the decreased platelet numbers caused by autoimmune thrombocytopenic purpura in cases with anticoagulant intake[58].
The pancreatic lesion of type 1 AIP histologically shows LPSP with 3 essential features: (1) a lymphoplasmacytic infiltrate surrounding small-sized interlobular pancreatic ducts that does not destroy the pancreatic ductal epithelium; (2) a swirling fibrosis centered around ducts and veins (storiform fibrosis); and (3) obliterative phlebitis wherein the infiltrate surrounds and obliterates pancreatic veins. Destructive changes to the ducts and acini caused by infiltrating granulocytes are typically absent. Immunostaining reveals abundant IgG4-positive cells (> 10 cells/HPF)[27,31].
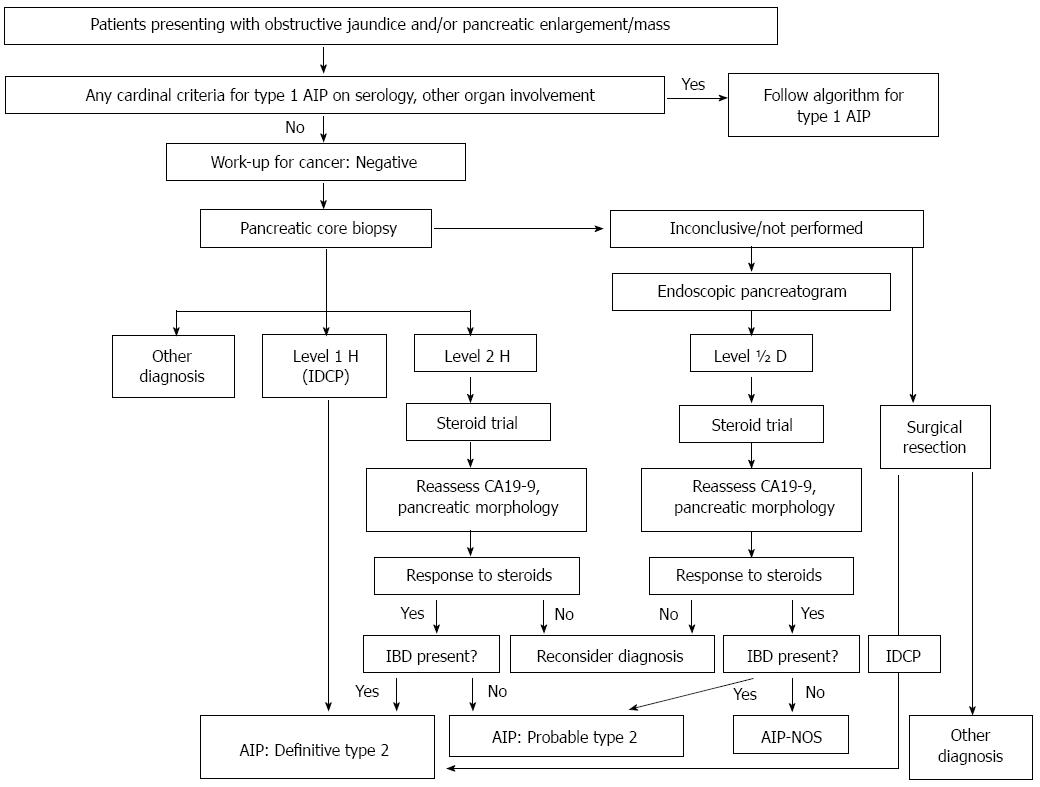
Type 2 AIP histology typically shows IDCP (AIP with GELs)[21,27,31], which is a distinct histological pattern from that of LPSP. The predominant interlobular stroma composed of lymphocytes plasma cells and reactive fibroblasts/myofibroblasts seen in type 1 AIP is replaced by the presence of GELs as the most distinctive feature of IDCP. These changes may lead to the destruction and obliteration of the duct lumen, seen in the medium to small-sized ducts and also in the acini. Infiltrates of IgG4-positive plasma cells are scant or absent in IDCP[27,31]. Currently, a definitive diagnosis of type 2 AIP requires histology (Table 3 and Figure 3). This unique histological subtype could be distinguished from type 1 AIP by expert pathologists with high diagnostic ratio (concordances: 60%-100%, multirater kappa: 0.54) using the international consensus histopathological diagnostic criteria[28].
The feasibility of arriving at a histological diagnosis for AIP using endoscopically obtained tissue samples has been argued[59-62]. Several studies demonstrated that tissue samples obtained by endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) enabled histological diagnosis of both type 1[60-62] and type 2[63,64] AIP.
Exclusion of pancreatobiliary malignancies is necessary for the diagnosis of AIP, especially in atypical cases. Today, the diagnosis of pancreatic mass lesions by EUS-FNA provides a sensitivity for detecting PC tissue that exceeds 90% (91%-93%)[59,65,66], making EUS-FNA the most effective tool for excluding pancreatic malignancies. However, core biopsy using a large-caliber needle[60,61,67] may increase the chance of a definitive histological diagnosis of AIP. A Japanese nationwide survey published in 2012[68] reported that histological confirmation was obtained in about 40% of AIP cases by EUS-guided tissue sampling, in 22% by resection, and in 18% by percutaneous biopsy. The choice of suitable modalities for histological evaluation can therefore eliminate non-necessary surgery in a large number of cases.
AIP is often associated with sclerosing cholangitis, which needs differential diagnosis from bile duct cancer. In this sense, periampullary forceps biopsy (and cytology) should be added in cases with biliary stricture, as this method has high sensitivity for confirming cancer tissue in the biliary cancer cases (77%[69,70]-92%[71]).
Steroid response is seen in 97%-98% of both type 1 and type 2 AIP cases[33,34]; hence, it is considered a useful diagnostic tool. Moon et al[72] performed a 2 wk steroid trial on 22 consecutive patients with a pancreatic mass lesions atypical for AIP and used by CT and MRCP/ERCP to determine the steroid response. All 15 patients who responded to steroid were diagnosed with AIP, whereas all 7 patients who did not show a steroid response were confirmed as having PC[72]. We also used abdominal US to analyze the steroid response of the pancreatic lesion of AIP, and we recognized a steroid response (shrinkage of the pancreatic lesion) in 86% of the cases in 2 wk and in 97% after one month[33]. However, one case in this study showed no response by US and CT and required ERCP, which revealed an improvement in the narrowing of the MPD and the occurrence of hilar bile duct stenosis after the withdrawal of corticosteroid[33,48]. Similarly, some cases of AIP fulfill the diagnostic criteria after cessation of steroid[73], so that clinicians need to remain aware of this. Many diagnostic criteria including those for ICDC (Table 2) can include evaluation of a steroid response either in the pancreatic or extrapancreatic lesions[9,17,18,25], but the diagnosis is worrisome when the steroid response is seen only in the extrapancreatic lesions and not in the pancreas.
Today, a “response to steroid” is a commonly evaluated diagnostic item for AIP in almost all diagnostic criteria[9,16-20,25]. However, it had not been included in the previous Japanese diagnostic criteria (2006)[15] in order to avoid simplistic therapeutic diagnosis by a steroid response without exclusion of possible pancreatobiliary malignancies. Clinicians must be careful in making differential diagnoses, and when malignant conditions are difficult to differentiate, pathological examination by EUS-FNA is preferable.
AIP is a unique form of chronic pancreatitis consisting of two distinct subtypes and associated with various systemic disorders. An accurate diagnosis can only be obtained when clinicians have a good understanding well on this disease entity and need to make use of diagnostic items including clinical images for pancreatic parenchyma, pancreatography and extrapancreatic lesions, serum markers, histological examinations of the pancreatic lesion, and steroid responses.
P- Reviewer: Triantos S, Vidal S S- Editor: Ma YJ L- Editor: A E- Editor: Zhang DN
| 1. | Yoshida K, Toki F, Takeuchi T, Watanabe S, Shiratori K, Hayashi N. Chronic pancreatitis caused by an autoimmune abnormality. Proposal of the concept of autoimmune pancreatitis. Dig Dis Sci. 1995;40:1561-1568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1044] [Cited by in RCA: 925] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 2. | Mackay IR. Autoimmune disease. Med J Aust. 1969;1:696-699. [PubMed] |
| 3. | Hamano H, Kawa S, Horiuchi A, Unno H, Furuya N, Akamatsu T, Fukushima M, Nikaido T, Nakayama K, Usuda N. High serum IgG4 concentrations in patients with sclerosing pancreatitis. N Engl J Med. 2001;344:732-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2026] [Cited by in RCA: 1878] [Article Influence: 78.3] [Reference Citation Analysis (0)] |
| 4. | Okazaki K, Uchida K, Matsushita M, Takaoka M. How to diagnose autoimmune pancreatitis by the revised Japanese clinical criteria. J Gastroenterol. 2007;42 Suppl 18:32-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Kawa S, Okazaki K, Kamisawa T, Shimosegawa T, Tanaka M. Japanese consensus guidelines for management of autoimmune pancreatitis: II. Extrapancreatic lesions, differential diagnosis. J Gastroenterol. 2010;45:355-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 6. | Kamisawa T, Okazaki K, Kawa S, Shimosegawa T, Tanaka M. Japanese consensus guidelines for management of autoimmune pancreatitis: III. Treatment and prognosis of AIP. J Gastroenterol. 2010;45:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 181] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 7. | Kamisawa T, Nakajima H, Egawa N, Funata N, Tsuruta K, Okamoto A. IgG4-related sclerosing disease incorporating sclerosing pancreatitis, cholangitis, sialadenitis and retroperitoneal fibrosis with lymphadenopathy. Pancreatology. 2006;6:132-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Kamisawa T, Chari ST, Lerch MM, Kim MH, Gress TM, Shimosegawa T. Recent advances in autoimmune pancreatitis: type 1 and type 2. Gut. 2013;62:1373-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 115] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 9. | Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Zhang L, Clain JE, Pearson RK, Petersen BT, Vege SS. Diagnosis of autoimmune pancreatitis: the Mayo Clinic experience. Clin Gastroenterol Hepatol. 2006;4:1010-106; quiz 934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 722] [Cited by in RCA: 657] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 10. | Matsubayashi H, Imai K, Kusumoto K, Ono H. Suspected autoimmune pancreatitis--an indication for steroid treatment? Dig Liver Dis. 2010;42:525-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Matsubayashi H, Sasaki K, Nagata K, Kanemoto H, Kiuchi R, Ono H. Pancreatic carcinoma mimicking diffuse-type autoimmune pancreatitis: important diagnostic role of pancreatic juice cytology using endoscopic naso-pancreatic drainage. J Dig Dis. 2012;13:287-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 12. | Matsubayashi H, Matsunaga K, Uesaka K, Fukutomi A, Sasaki A, Furukawa H, Ono H. A case of pancreatic carcinoma with suspected autoimmune pancreatitis. Clin J Gastroenterol. 2009;2:59-63. [RCA] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Matsubayashi H, Takagaki S, Otsubo T, Iiri T, Kobayashi Y, Yokota T, Shichijo K, Iwafuchi M, Kijima H. Pancreatic T-cell lymphoma with high level of soluble interleukin-2 receptor. J Gastroenterol. 2002;37:863-867. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Members of the Criteria Committee for Autoimmune Pancreatitis JPS. Diagnostic criteria for autoimmune pancreatitis. Suizo. 2002;17:585. |
| 15. | Okazaki K, Kawa S, Kamisawa T, Naruse S, Tanaka S, Nishimori I, Ohara H, Ito T, Kiriyama S, Inui K. Clinical diagnostic criteria of autoimmune pancreatitis: revised proposal. J Gastroenterol. 2006;41:626-631. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 439] [Cited by in RCA: 418] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 16. | Members of the Criteria Committee for Autoimmune Pancreatitis JPS. Diagnostic criteria for autoimmune pancreatitis (2011). Suizo. 2012;27:17-25. [RCA] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 17. | Ryu JK, Chung JB, Park SW, Lee JK, Lee KT, Lee WJ, Moon JH, Cho KB, Kang DW, Hwang JH. Review of 67 patients with autoimmune pancreatitis in Korea: a multicenter nationwide study. Pancreas. 2008;37:377-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 18. | Kim KP, Kim MH, Kim JC, Lee SS, Seo DW, Lee SK. Diagnostic criteria for autoimmune chronic pancreatitis revisited. World J Gastroenterol. 2006;12:2487-2496. [PubMed] |
| 19. | Schneider A, Löhr JM, Singer MV. The M-ANNHEIM classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. J Gastroenterol. 2007;42:101-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 311] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 20. | Frulloni L, Scattolini C, Falconi M, Zamboni G, Capelli P, Manfredi R, Graziani R, D’Onofrio M, Katsotourchi AM, Amodio A. Autoimmune pancreatitis: differences between the focal and diffuse forms in 87 patients. Am J Gastroenterol. 2009;104:2288-2294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 154] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 21. | Notohara K, Burgart LJ, Yadav D, Chari S, Smyrk TC. Idiopathic chronic pancreatitis with periductal lymphoplasmacytic infiltration: clinicopathologic features of 35 cases. Am J Surg Pathol. 2003;27:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 382] [Article Influence: 17.4] [Reference Citation Analysis (1)] |
| 22. | Zamboni G, Lüttges J, Capelli P, Frulloni L, Cavallini G, Pederzoli P, Leins A, Longnecker D, Klöppel G. Histopathological features of diagnostic and clinical relevance in autoimmune pancreatitis: a study on 53 resection specimens and 9 biopsy specimens. Virchows Arch. 2004;445:552-563. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 503] [Cited by in RCA: 440] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 23. | Umehara H, Okazaki K, Masaki Y, Kawano M, Yamamoto M, Saeki T, Matsui S, Yoshino T, Nakamura S, Kawa S. Comprehensive diagnostic criteria for IgG4-related disease (IgG4-RD), 2011. Mod Rheumatol. 2012;22:21-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1125] [Cited by in RCA: 1084] [Article Influence: 83.4] [Reference Citation Analysis (0)] |
| 24. | Otsuki M, Chung JB, Okazaki K, Kim MH, Kamisawa T, Kawa S, Park SW, Shimosegawa T, Lee K, Ito T. Asian diagnostic criteria for autoimmune pancreatitis: consensus of the Japan-Korea Symposium on Autoimmune Pancreatitis. J Gastroenterol. 2008;43:403-408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 361] [Cited by in RCA: 310] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 25. | Shimosegawa T, Chari ST, Frulloni L, Kamisawa T, Kawa S, Mino-Kenudson M, Kim MH, Klöppel G, Lerch MM, Löhr M. International consensus diagnostic criteria for autoimmune pancreatitis: guidelines of the International Association of Pancreatology. Pancreas. 2011;40:352-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1050] [Cited by in RCA: 1056] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 26. | Naitoh I, Nakazawa T, Hayashi K, Miyabe K, Shimizu S, Kondo H, Yoshida M, Yamashita H, Umemura S, Hori Y. Clinical evaluation of international consensus diagnostic criteria for type 1 autoimmune pancreatitis in comparison with Japanese diagnostic criteria 2011. Pancreas. 2013;42:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 27. | Chari ST, Kloeppel G, Zhang L, Notohara K, Lerch MM, Shimosegawa T. Histopathologic and clinical subtypes of autoimmune pancreatitis: the Honolulu consensus document. Pancreas. 2010;39:549-554. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 179] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 28. | Zhang L, Chari S, Smyrk TC, Deshpande V, Klöppel G, Kojima M, Liu X, Longnecker DS, Mino-Kenudson M, Notohara K. Autoimmune pancreatitis (AIP) type 1 and type 2: an international consensus study on histopathologic diagnostic criteria. Pancreas. 2011;40:1172-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 29. | Kamisawa T, Kim MH, Liao WC, Liu Q, Balakrishnan V, Okazaki K, Shimosegawa T, Chung JB, Lee KT, Wang HP. Clinical characteristics of 327 Asian patients with autoimmune pancreatitis based on Asian diagnostic criteria. Pancreas. 2011;40:200-205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 69] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 30. | Wu L, Li W, Huang X, Wang Z. Clinical features and comprehensive diagnosis of autoimmune pancreatitis in China. Digestion. 2013;88:128-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Deshpande V, Gupta R, Sainani N, Sahani DV, Virk R, Ferrone C, Khosroshahi A, Stone JH, Lauwers GY. Subclassification of autoimmune pancreatitis: a histologic classification with clinical significance. Am J Surg Pathol. 2011;35:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 133] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 32. | Kamisawa T, Funata N, Hayashi Y, Eishi Y, Koike M, Tsuruta K, Okamoto A, Egawa N, Nakajima H. A new clinicopathological entity of IgG4-related autoimmune disease. J Gastroenterol. 2003;38:982-984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1011] [Cited by in RCA: 984] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 33. | Matsubayashi H, Yoneyama M, Nanri K, Sugimoto S, Shinjo K, Kakushima N, Tanaka M, Ito S, Takao M, Ono H. Determination of steroid response by abdominal ultrasound in cases with autoimmune pancreatitis. Dig Liver Dis. 2013;45:1034-1040. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 34. | Kamisawa T, Shimosegawa T, Okazaki K, Nishino T, Watanabe H, Kanno A, Okumura F, Nishikawa T, Kobayashi K, Ichiya T. Standard steroid treatment for autoimmune pancreatitis. Gut. 2009;58:1504-1507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 500] [Cited by in RCA: 485] [Article Influence: 30.3] [Reference Citation Analysis (0)] |
| 35. | Klöppel G, Detlefsen S, Chari ST, Longnecker DS, Zamboni G. Autoimmune pancreatitis: the clinicopathological characteristics of the subtype with granulocytic epithelial lesions. J Gastroenterol. 2010;45:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 70] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 36. | Kamisawa T, Chari ST, Giday SA, Kim MH, Chung JB, Lee KT, Werner J, Bergmann F, Lerch MM, Mayerle J. Clinical profile of autoimmune pancreatitis and its histological subtypes: an international multicenter survey. Pancreas. 2011;40:809-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 37. | Sah RP, Chari ST, Pannala R, Sugumar A, Clain JE, Levy MJ, Pearson RK, Smyrk TC, Petersen BT, Topazian MD. Differences in clinical profile and relapse rate of type 1 versus type 2 autoimmune pancreatitis. Gastroenterology. 2010;139:140-18; quiz 140-18;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 299] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 38. | Detlefsen S, Bräsen JH, Zamboni G, Capelli P, Klöppel G. Deposition of complement C3c, immunoglobulin (Ig)G4 and IgG at the basement membrane of pancreatic ducts and acini in autoimmune pancreatitis. Histopathology. 2010;57:825-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 39. | Kamisawa T, Egawa N, Nakajima H, Tsuruta K, Okamoto A, Kamata N, Funata N. Comparison of radiological and histological findings in autoimmune pancreatitis. Hepatogastroenterology. 2006;53:953-956. [PubMed] |
| 40. | Matsubara H, Itoh A, Kawashima H, Kasugai T, Ohno E, Ishikawa T, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M. Dynamic quantitative evaluation of contrast-enhanced endoscopic ultrasonography in the diagnosis of pancreatic diseases. Pancreas. 2011;40:1073-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 91] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 41. | Imazu H, Kanazawa K, Mori N, Ikeda K, Kakutani H, Sumiyama K, Hino S, Ang TL, Omar S, Tajiri H. Novel quantitative perfusion analysis with contrast-enhanced harmonic EUS for differentiation of autoimmune pancreatitis from pancreatic carcinoma. Scand J Gastroenterol. 2012;47:853-860. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 42. | Irie H, Honda H, Baba S, Kuroiwa T, Yoshimitsu K, Tajima T, Jimi M, Sumii T, Masuda K. Autoimmune pancreatitis: CT and MR characteristics. AJR Am J Roentgenol. 1998;170:1323-1327. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 257] [Cited by in RCA: 217] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 43. | Okabe Y, Ishida Y, Kaji R, Sugiyama G, Yasumoto M, Naito Y, Toyonaga A, Tsuruta O, Sata M. Endoscopic ultrasonographic study of autoimmune pancreatitis and the effect of steroid therapy. J Hepatobiliary Pancreat Sci. 2012;19:266-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 44. | Itokawa F, Itoi T, Sofuni A, Kurihara T, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Umeda J, Tanaka R. EUS elastography combined with the strain ratio of tissue elasticity for diagnosis of solid pancreatic masses. J Gastroenterol. 2011;46:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 113] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 45. | Dietrich CF, Hirche TO, Ott M, Ignee A. Real-time tissue elastography in the diagnosis of autoimmune pancreatitis. Endoscopy. 2009;41:718-720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 81] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 46. | Nishino T, Oyama H, Toki F, Shiratori K. Differentiation between autoimmune pancreatitis and pancreatic carcinoma based on endoscopic retrograde cholangiopancreatography findings. J Gastroenterol. 2010;45:988-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 32] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Kamisawa T, Tu Y, Egawa N, Tsuruta K, Okamoto A, Kodama M, Kamata N. Can MRCP replace ERCP for the diagnosis of autoimmune pancreatitis? Abdom Imaging. 2009;34:381-384. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 48. | Matsubayashi H, Tomita H, Sugiura T, Sasaki K, Inui T, Takizawa K, Yamaguchi Y, Ono H. Autoimmune pancreatitis without a response to steroid therapy: a case which met criteria after withdrawal of steroid. Intern Med. 2009;48:2087-2091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 49. | Ghazale A, Chari ST, Smyrk TC, Levy MJ, Topazian MD, Takahashi N, Clain JE, Pearson RK, Pelaez-Luna M, Petersen BT. Value of serum IgG4 in the diagnosis of autoimmune pancreatitis and in distinguishing it from pancreatic cancer. Am J Gastroenterol. 2007;102:1646-1653. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 434] [Cited by in RCA: 362] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 50. | Okazaki K, Uchida K, Ohana M, Nakase H, Uose S, Inai M, Matsushima Y, Katamura K, Ohmori K, Chiba T. Autoimmune-related pancreatitis is associated with autoantibodies and a Th1/Th2-type cellular immune response. Gastroenterology. 2000;118:573-581. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 410] [Cited by in RCA: 377] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 51. | Matsubayashi H, Uesaka K, Kanemoto H, Asakura K, Kakushima N, Tanaka M, Kimura H, Ono H. Soluble IL-2 receptor, a new marker for autoimmune pancreatitis. Pancreas. 2012;41:493-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 52. | Hamano H, Arakura N, Muraki T, Ozaki Y, Kiyosawa K, Kawa S. Prevalence and distribution of extrapancreatic lesions complicating autoimmune pancreatitis. J Gastroenterol. 2006;41:1197-1205. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 227] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 53. | Matsubayashi H, Furukawa H, Maeda A, Matsunaga K, Kanemoto H, Uesaka K, Fukutomi A, Ono H. Usefulness of positron emission tomography in the evaluation of distribution and activity of systemic lesions associated with autoimmune pancreatitis. Pancreatology. 2009;9:694-699. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 55] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 54. | Ozaki Y, Oguchi K, Hamano H, Arakura N, Muraki T, Kiyosawa K, Momose M, Kadoya M, Miyata K, Aizawa T. Differentiation of autoimmune pancreatitis from suspected pancreatic cancer by fluorine-18 fluorodeoxyglucose positron emission tomography. J Gastroenterol. 2008;43:144-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 55. | Saegusa H, Momose M, Kawa S, Hamano H, Ochi Y, Takayama M, Kiyosawa K, Kadoya M. Hilar and pancreatic gallium-67 accumulation is characteristic feature of autoimmune pancreatitis. Pancreas. 2003;27:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 64] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 56. | Matsubayashi H, Furukawa H, Uesaka K, Sasaki K, Ono H, Hruban RH. Autoimmune pancreatitis accompanied by cholecystitis, periaortitis and pseudotumors of the liver. Case Rep Gastroenterol. 2008;2:155-161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 57. | Taniguchi T, Hamasaki A, Okamoto M. A case of suspected lymphocytic hypophysitis and organizing pneumonia during maintenance therapy for autoimmune pancreatitis associated with autoimmune thrombocytopenia. Endocr J. 2006;53:563-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 58. | Sawai H, Matsubayashi H, Tanaka M, Yamaguchi Y, Ono H. A case of autoimmune pancreatitis with metachronous appearance of idiopathic thrombocytopenic purpura. Clin J Gastroenterol. 2010;3:243-247. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 59. | Imai K, Matsubayashi H, Fukutomi A, Uesaka K, Sasaki K, Ono H. Endoscopic ultrasonography-guided fine needle aspiration biopsy using 22-gauge needle in diagnosis of autoimmune pancreatitis. Dig Liver Dis. 2011;43:869-874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 60. | Mizuno N, Bhatia V, Hosoda W, Sawaki A, Hoki N, Hara K, Takagi T, Ko SB, Yatabe Y, Goto H. Histological diagnosis of autoimmune pancreatitis using EUS-guided trucut biopsy: a comparison study with EUS-FNA. J Gastroenterol. 2009;44:742-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 131] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 61. | Iwashita T, Yasuda I, Doi S, Ando N, Nakashima M, Adachi S, Hirose Y, Mukai T, Iwata K, Tomita E. Use of samples from endoscopic ultrasound-guided 19-gauge fine-needle aspiration in diagnosis of autoimmune pancreatitis. Clin Gastroenterol Hepatol. 2012;10:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 119] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 62. | Kanno A, Ishida K, Hamada S, Fujishima F, Unno J, Kume K, Kikuta K, Hirota M, Masamune A, Satoh K. Diagnosis of autoimmune pancreatitis by EUS-FNA by using a 22-gauge needle based on the International Consensus Diagnostic Criteria. Gastrointest Endosc. 2012;76:594-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 63. | Detlefsen S, Mohr Drewes A, Vyberg M, Klöppel G. Diagnosis of autoimmune pancreatitis by core needle biopsy: application of six microscopic criteria. Virchows Arch. 2009;454:531-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 64. | Ishikawa T, Itoh A, Kawashima H, Ohno E, Matsubara H, Itoh Y, Nakamura Y, Hiramatsu T, Nakamura M, Miyahara R. Endoscopic ultrasound-guided fine needle aspiration in the differentiation of type 1 and type 2 autoimmune pancreatitis. World J Gastroenterol. 2012;18:3883-3888. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 65. | Hikichi T, Irisawa A, Bhutani MS, Takagi T, Shibukawa G, Yamamoto G, Wakatsuki T, Imamura H, Takahashi Y, Sato A. Endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses with rapid on-site cytological evaluation by endosonographers without attendance of cytopathologists. J Gastroenterol. 2009;44:322-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 66. | Haba S, Yamao K, Bhatia V, Mizuno N, Hara K, Hijioka S, Imaoka H, Niwa Y, Tajika M, Kondo S. Diagnostic ability and factors affecting accuracy of endoscopic ultrasound-guided fine needle aspiration for pancreatic solid lesions: Japanese large single center experience. J Gastroenterol. 2013;48:973-981. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 124] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 67. | Fujii LL, Chari ST, El-Youssef M, Takahashi N, Topazian MD, Zhang L, Levy MJ. Pediatric pancreatic EUS-guided trucut biopsy for evaluation of autoimmune pancreatitis. Gastrointest Endosc. 2013;77:824-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 68. | Kanno A, Nishimori I, Masamune A, Kikuta K, Hirota M, Kuriyama S, Tsuji I, Shimosegawa T. Nationwide epidemiological survey of autoimmune pancreatitis in Japan. Pancreas. 2012;41:835-839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 69. | Draganov PV, Chauhan S, Wagh MS, Gupte AR, Lin T, Hou W, Forsmark CE. Diagnostic accuracy of conventional and cholangioscopy-guided sampling of indeterminate biliary lesions at the time of ERCP: a prospective, long-term follow-up study. Gastrointest Endosc. 2012;75:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 167] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 70. | Kawashima H, Itoh A, Ohno E, Goto H, Hirooka Y. Transpapillary biliary forceps biopsy to distinguish benign biliary stricture from malignancy: how many tissue samples should be obtained? Dig Endosc. 2012;24 Suppl 1:22-27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 71. | Kimura H, Matsubayashi H, Sasaki K, Ito H, Hirosawa K, Uesaka K, Kanemoto H, Ono H. Factors affecting the yield of endoscopic transpapillary bile duct biopsy for the diagnosis of pancreatic head cancer. Pancreatology. 2013;13:524-529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 72. | Moon SH, Kim MH, Park DH, Hwang CY, Park SJ, Lee SS, Seo DW, Lee SK. Is a 2-week steroid trial after initial negative investigation for malignancy useful in differentiating autoimmune pancreatitis from pancreatic cancer? A prospective outcome study. Gut. 2008;57:1704-1712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 142] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 73. | Shimizu S, Naitoh I, Nakazawa T, Hayashi K, Okumura F, Miyabe K, Kondo H, Yoshida M, Yamashita H, Ohara H. A case of autoimmune pancreatitis showing narrowing of the main pancreatic duct after cessation of steroid therapy in the clinical course. Intern Med. 2012;51:2135-2140. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |