Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.16275
Revised: July 21, 2014
Accepted: August 13, 2014
Published online: November 21, 2014
Processing time: 157 Days and 12.6 Hours
AIM: To report the outcome of patients with ruptured hepatocellular carcinoma (HCC) treated at a single center during a 5-year period.
METHODS: We retrospectively analyzed 32 patients who presented with ruptured HCC at Shandong Provincial Hospital Affiliated to Shandong University between 2008 and 2013.
RESULTS: The mean age of the patients was 53 years (range 39-71 years). Of these patients, 22 received surgical management, 10 underwent transarterial embolization (TAE) or transarterial chemoembolization (TACE), and 12 received sorafenib after surgery, TAE or TACE. Cumulative survival rates at 4, 8 and 12 mo were 72.9%, 50.0% and 33.3%, respectively, in the surgery only group and were 90.0%, 80.6% and 64.1%, respectively, in the surgery plus sorafenib group. Cumulative survival rates at 4, 8 and 12 mo were 68.4%, 43.6% and 19.4%, respectively, in the surgery only or TAE/TACE only groups, and were 91.7%, 75.0% and 60.2%, respectively, in the sorafenib combination groups (P = 0.04). No unexpected side effects due to sorafenib were observed. The most common side effect was hand-foot skin reaction. To date, 5 patients have died. Median follow-up from the start of sorafenib therapy for the remaining 7 patients is 12.7 mo (range 5.8-32.2 mo).
CONCLUSION: Sorafenib can be used in patients with ruptured HCC as it has interesting activity and is well tolerated; dose adjustment is generally not required. However, a larger prospective study is necessary to determine the efficacy of sorafenib in this group of patients.
Core tip: Spontaneous rupture of hepatocellular carcinoma (HCC) is a life-threatening condition. Currently, sorafenib is available for HCC patients with spontaneous rupture, although its efficacy and safety have not been reported. This study aims to report the outcome of patients with ruptured HCC in a single center during a 5-year period. We retrospectively analyzed 32 patients who presented with ruptured HCC at our institute between 2008 and 2013. We concluded that sorafenib can be used in patients with ruptured HCC as it has interesting activity and is well tolerated. However, a larger prospective study is necessary to determine the efficacy of sorafenib in this group of patients.
- Citation: Zheng SZ, Liu DJ, Sun P, Yu GS, Xu YT, Gong W, Liu J. Feasibility and safety of sorafenib treatment in hepatocellular carcinoma patients with spontaneous rupture. World J Gastroenterol 2014; 20(43): 16275-16281
- URL: https://www.wjgnet.com/1007-9327/full/v20/i43/16275.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i43.16275
Hepatocellular carcinoma (HCC) is the most common form of liver cancer, accounting for approximately 90% of primary malignant liver tumors in adults. It is the sixth most common cancer and the third most common cause of cancer-related death worldwide[1]. The incidence of HCC has increased dramatically in the past decades and is expected to continue to increase for the next two decades[2]. Spontaneous rupture of HCC is an uncommon and potentially life-threatening presentation with a varied incidence throughout the world. One report showed that its incidence was < 3% in Western countries and was > 10% in Asian countries[3-5]. The mortality rate due to spontaneous rupture of HCC is high and has been reported to range from 32%-100%[6-8].
The treatment of ruptured HCC includes emergency or staged liver resections, transarterial embolization (TAE), transarterial chemoembolization (TACE), and hepatic artery ligation[7,9]. Although the overall prognosis of patients with ruptured HCC is poor, some studies have reported a promising outcome following emergency liver resection.
Recently, sorafenib, a multi-targeted small molecule tyrosine kinase inhibitor, which inhibits tumor growth and angiogenesis by inhibiting intracellular RAF kinases (CRAF, BRAF and mutant BRAF) and cell surface kinase receptors (VEGFR-2, VEGFR-3, PDGFR-beta, cKIT and FLT-3), has become the standard treatment for advanced HCC based on two large randomized phase III trials[10,11].
However, the use of sorafenib in spontaneous rupture of HCC has not been studied and there is little information on this drug for patients with ruptured HCC. Therefore, we conducted a retrospective study to determine the efficacy and safety of sorafenib in patients with spontaneous rupture of HCC.
This was a retrospective study of patients with spontaneous rupture of HCC treated in Shandong Provincial Hospital Affiliated to Shandong University, Jinan, China, during the period 2008-2013. The aim of this study was to examine the survival of the patients with spontaneous rupture of HCC treated with sorafenib and its side effects. Between January 2008 and August 2013, 32 patients were clinically diagnosed with spontaneously ruptured HCC. Of these patients, 22 received surgical management (emergency or staged liver resections), 10 received TAE or TACE, and 12 received sorafenib after surgery, TAE or TACE. None of the patients had recently received HCC treatment such as surgery or TACE within one month of the diagnosis of HCC rupture. HCC was diagnosed according to the diagnostic guidelines issued by the American Association for the Study of Liver Diseases[12]. HCC rupture was defined as disruption of the peritumoral liver capsule with enhanced fluid collection in the perihepatic area adjacent to the HCC by dynamic liver computed tomography (CT)[13], and when abdominal paracentesis showed an ascitic red blood cell count of > 50000 mm3/mL in bloody fluid[14]. Details of patient demographics, clinical features, and medical history, including hepatitis status and histologic evidence of cirrhosis, were recorded. The severity of liver disease was classified according to Child-Pugh criteria. Patient demographics, prior therapy, best response, survival data and relevant toxicities were obtained and recorded in a database. All patients were started on sorafenib 200 mg b.i.d. and titrated up to a full dose of 400 mg b.i.d. after 5-7 d if no toxicity was observed. The primary outcome measure was patient survival. Complete data, with a median follow-up of 12.7 mo, were available.
Statistical analyses were performed using SPSS 13 for Windows software. A P value < 0.05 was considered statistically significant. Categorical variables were summarized as frequencies and percentages. Quantitative variables were summarized as medians and ranges. Overall survival from the start of sorafenib was summarized using the method of Kaplan and Meier.
The baseline characteristics of the 32 patients are summarized in Table 1. The patients (25 male and 7 female) were classified as Child-Pugh A, Child-Pugh B or Child-Pugh C, and had a median age of 53 years. The etiology of liver disease was hepatitis B infection in 25, hepatitis C in 2, alcohol in 2, and unknown in the remaining 3 patients. Fifteen of the 32 patients had a single HCC and 17 patients had multiple HCCs. Median tumor size was 8.3 cm (range, 2.9-25.5 cm), and only 3 patients had HCCs within the Milan criteria. Before treatment, 0, 3, 11, 12 and 6 patients were found to have Barcelona Clinical Liver Cancer stage 0, A, B, C, or D stage HCC, respectively. Median alpha-fetoprotein concentration at diagnosis of HCC rupture was 864 ng/mL (range, 6-54 000 ng/mL). Seven patients required a vasopressor due to shock at presentation. Ruptured HCC was located on the surface of the liver in all patients.
| Variable | Total (n = 32) |
| Age | |
| Mean (range), yr | 53 (39-71) |
| Gender (male/female) | 25/7 |
| Etiology | |
| HBV/HCV/alcohol/others | 25 (78.1)/2 (6.3)/2 (6.3)/3 (9.4) |
| Child-Pugh classification | |
| A/B/C | 3 (9.4)/12 (37.5)/10 (31.3) |
| Extra-liver sites of metastatic disease | 4 (12.5) |
| Tumor size (cm) | 8.3 (2.9-25.5) |
| Tumor number | |
| Single/multiple | 8 (25.0)/24 (75.0) |
| Within Milan criteria | 3 (9.4) |
| BCLC stage | |
| 0/A/B/C/D | 0 (0.0)/3 (9.4)/11 (34.4)/ 12 (37.5)/6 (18.8) |
| Alpha-fetoprotein (ng/mL) | |
| mean (range) | 864 (6-54000) |
| Treatment Resection/TAE(TACE) | 22 (68.8)/10 (31.2) |
| Sorafenib | 12 (37.5) |
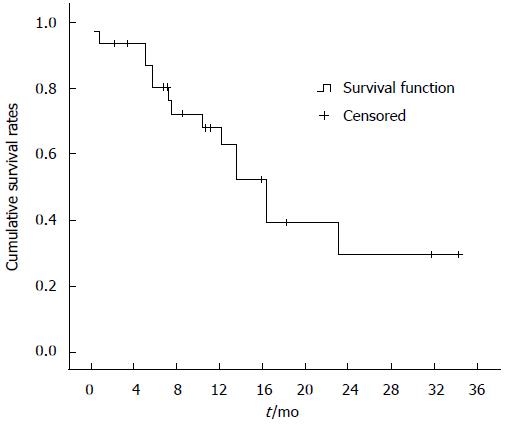
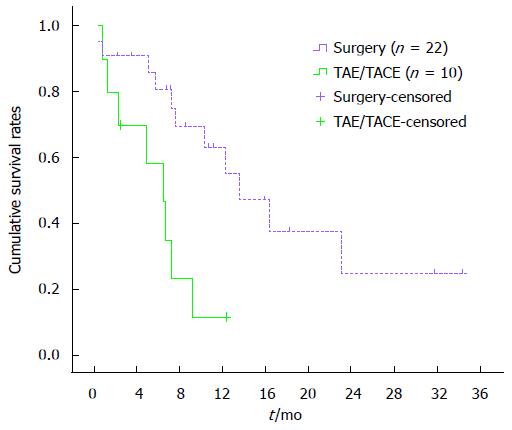
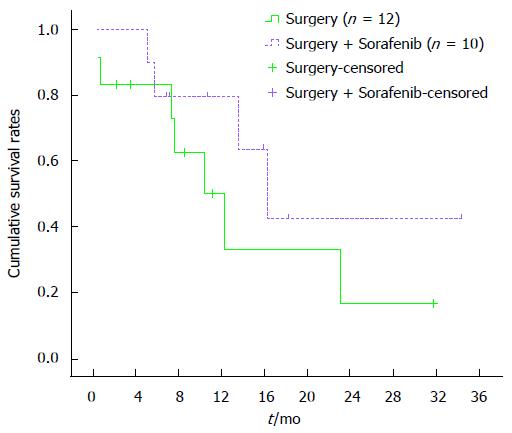
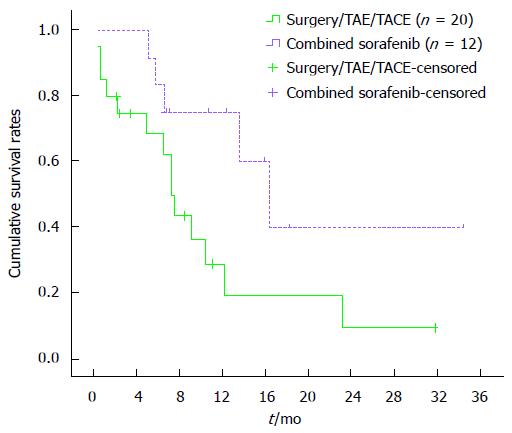
One-month overall cumulative mortality for the 32 study subjects was 12.5% (Figure 1). Cumulative survival rates at 4, 8 and 12 mo were 85.9%, 69.3% and 55.1%, respectively, in the surgical group and 70.3%, 23.3% and 11.7%, respectively, in the TAE/TACE group. Cumulative survival rates at 4, 8 and 12 mo were higher in the surgical group than in the TAE/TACE group (P < 0.01) (Figure 2). Cumulative survival rates at 4, 8 and 12 mo were 72.9%, 50.0% and 33.3%, respectively, in the surgery only group and were 90.0%, 80.6% and 64.1%, respectively in the surgery plus sorafenib group. Cumulative survival rates at 4, 8 and 12 mo tended to be higher in the surgery plus sorafenib group, although the difference was statistically insignificant (P = 0.18) (Figure 3). Cumulative survival rates at 4, 8 and 12 mo were 68.4%, 43.6% and 19.4%, respectively, in the groups treated with surgery or TAE/TACE only, and were 91.7%, 75.0% and 60.2%, respectively, in the surgery or TAE/TACE plus sorafenib groups (P = 0.04) (Figure 4).
Three patients discontinued sorafenib and 9 continue with sorafenib treatment. To date, patients have been treated for a median of 12.8 mo (range 4.1-32.2 mo). Table 2 summarizes sorafenib-induced toxicity observed thus far. All patients were started on sorafenib 200 mg b.i.d. and titrated up to a full dose of 400 mg b.i.d. after 5-7 d if there was no toxicity noted; however, 3 patients required temporary dose reduction due to hand-foot skin reaction and diarrhea. Most of the toxicities seen in this group of patients occurred at the dose of 400 mg b.i.d. and were expected side effects of sorafenib. Hand-foot skin reaction was the most common side effect.
| Adverse events | Grade 1/2 | Grade 3/4 |
| Hand-foot skin reaction | 3 | 1 |
| Diarrhea | 2 | 2 |
| Hypertension | 1 | 0 |
| Skin rash | 2 | 0 |
| Anorexia | 2 | 0 |
| Mucositis | 1 | 0 |
| Agrypnia | 1 | 0 |
To date, 2 patients have achieved a complete radiographic response. Three patients achieved disease stabilization as their best response. Seven patients have progressed, and 5 patients have died. Median follow-up from the start of sorafenib therapy in the remaining 6 patients is 12.7 mo (range 5.8-32.2 mo). None of the patients discontinued sorafenib due to toxicity.
Hepatocellular carcinoma is a common cause of death, with 100000 lives lost annually in China[4]. Spontaneous rupture of HCC is an uncommon, but lethal complication with an incidence of approximately 15% in Asia[15]. The mechanism of spontaneous rupture of HCC is unclear, but has been attributed to central necrosis in the rapidly growing HCC, hemorrhage and venous congestion inside the tumor, coagulopathy due to underlying cirrhosis, and minor trauma causing a sudden increase in pressure within the tumor[9,16,17]. The primary objective of initial management is to achieve hemostasis by either TAE/TACE or surgery. Several studies have proposed that selective hepatic artery ligation or emergency hepatectomy are the treatments of choice in patients with limited tumor and preserved liver function (Child-Pugh grade A or B), with TAE/TACE as the next-best choice[16,18]. TAE/TACE is effective in controlling bleeding in HCCs, with a lower in-hospital mortality rate than surgery; however, the outcome is still dependent on liver function. In addition to poor liver function, the multifocal nature of the tumor and large tumor size may also help explain the risk of decompensation and poor outcome. It has been shown that early mortality is not dependent on the modality of treatment, but on the patient’s pre-rupture disease state, history of cirrhosis, liver function at the time of rupture, and the degree of shock[19,20].
Previous reports demonstrated that patients who underwent emergency hepatectomy had significantly better survival rates. This may have been due to the fact that the majority of patients who underwent emergency hepatectomy were non-cirrhotic and had reasonable liver function. Emergency or early hepatectomy in such patients will facilitate restoration of hepatic perfusion and preservation of liver function[8,21]. A comparable survival rate was also noted in patients who underwent elective hepatectomy after initial control of bleeding by surgical perihepatic packing. Although surgical treatment of peritoneal dissemination of HCC is not curative, surgery may improve survival and provide a good quality of life in selected patients[22]. Yang et al[23] concluded that improvements in surgical technique and perioperative care have made partial hepatectomy a safe and effective treatment in the event of spontaneous HCC rupture. Yoshida et al[16] reported that cumulative survival rates in the ruptured group at 1, 5 and 10 years were 90.0%, 67.5% and 20.3%, respectively, and concluded that survival rates after elective hepatectomy in patients with ruptured HCC are good, and that TAE followed by elective hepatectomy is considered an effective strategy for patients with ruptured HCC[6].
In our study, cumulative survival rates at 4, 8 and 12 mo were higher in the surgical group than in the TAE/TACE group (P < 0.01). Thus, surgical management was superior to TAE/TACE in treating ruptured HCC. We observed an overall 30-d mortality rate of 12.5%, while the mortality rate was over 30% in other similar reports. This may be because our institute focuses on surgical treatment and patients with severe rupture of HCC and poor liver function have an extremely guarded prognosis and cannot tolerate surgical hepatic resection. Thus, TAE/TACE should be suggested in patients who cannot tolerate liver resection, especially in those with poor liver function.
Treatment of ruptured HCC is challenging. Recently, sorafenib, an oral multikinase inhibitor with activity against Raf-1, B-Raf, VEGFR2, PDGFR and c-Kit receptors, was shown to have potent antiangiogenic and proapoptotic activity and therefore has a marked antitumoral effect[24]. The improved survival in patients treated with sorafenib is supported by the highest level of evidence, and sorafenib is considered the standard systemic therapy for HCC in patients with well-preserved liver function and advanced-stage HCC or in patients with HCC progressing after locoregional therapies[25,26]. Rupture of HCC can lead to peritoneal dissemination and recurrence, and the tumor-node-metastasis classification of the International Union Against Cancer classifies ruptured HCC as T4[27]. Sorafenib should be available for HCC patients with spontaneous rupture. However, thus far only limited data are available with regard to the safety and efficacy of sorafenib in HCC patients with spontaneous rupture. Therefore, it is important that more data on the use of sorafenib in HCC patients with spontaneous rupture become available.
Our single-institution data clearly showed that it is feasible to use sorafenib in patients with ruptured HCC. In terms of efficacy, thus far, 2 patients have achieved a complete radiographic response. Three patients have achieved disease stabilization as their best response. Seven patients have progressed and 5 patients have died. Median follow-up from the start of sorafenib therapy for the remaining 6 patients is 12.7 mo (range 5.8-32.2 mo). Cumulative survival rates at 4, 8 and 12 mo tended to be higher in the surgery plus sorafenib group than in the surgery only group, although the difference was statistically insignificant (P = 0.18). Cumulative survival rates at 4, 8 and 12 mo were higher in the groups treated with surgery or TAE/TACE plus sorafenib than in the groups treated with surgery or TAE/TACE only (P = 0.04). Our results showed that sorafenib is effective in the treatment of ruptured HCC.
To date, two studies have reported the clinical outcomes of patients on sorafenib treatments and have addressed the management of sorafenib-related adverse events (AEs) in daily clinical practice: the GIDEON study (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib), conducted on a very large population of HCC patients in more than 40 countries[28], and the SOFIA (SOraFenib Italian Assessment) study, conducted in Italy[29]. Sorafenib appeared to be well-tolerated in both the GIDEON and the SOFIA studies; however, a number of AEs were reported in both studies. The most frequent sorafenib-associated AEs were dermatological lesions, cancer-related fatigue and diarrhea. Grade 3/4 liver dysfunction has also been reported in 3% of patients treated with sorafenib compared with placebo in the SHARP trial (supplementary appendices)[10] and in 1% of patients treated with sorafenib in the GIDEON study[30]. The safety profile of sorafenib was similar, although a slightly higher rate of AEs and the need for dose reduction was reported when compared with the landmark phase III trials. Proper management of sorafenib-associated AEs reduces the risk of dose reductions and/or treatment interruptions, thus optimizing the clinical benefits associated with this agent[31].
In our study, all of the patients were started on a reduced sorafenib dose of 200 mg b.i.d. and titrated up to the full dose of 400 mg b.i.d. after 5-7 d without any problems. Toxicities seen at the higher dose were mostly the expected side effects of sorafenib. None of the patients needed permanent dose reduction; however, 3 patients required temporary dose reduction due to hand-foot skin reaction and diarrhea. None of the patients discontinued sorafenib due to toxicity. Recently, sorafenib has been used in the treatment of patients with advanced HCC, even in patients with recurrent HCC after liver transplantation; however, dose adjustment may be required[32]. Our study showed that sorafenib dose adjustment was not required in the treatment of ruptured HCC. In our study, almost all toxicities were expected and no new serious AEs were observed.
To our knowledge, our series includes one of the largest groups of patients with spontaneous rupture of HCC who received sorafenib in a single institution. Our series has shown that it is feasible to use sorafenib in conjunction with other therapeutic regimens such as surgery, TAE and TACE in patients with rupture of HCC and dose adjustment is generally not required.
The limitations of the present study are as follows: the retrospective design and small sample size are two main limitations of this study. Nevertheless, our study is the first to provide evidence of the outcome of sorafenib treatment and its toxicity in patients with rupture of HCC.
In conclusion, we confirm that sorafenib has interesting activity and acceptable tolerability in patients with HCC rupture. However, future trials are required to confirm the efficacy and safety of this drug in patients with rupture of HCC.
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most common cause of cancer-related death worldwide. Spontaneous rupture of HCC is an uncommon and potentially life-threatening presentation. The reported high mortality rate due to spontaneous rupture of HCC ranges from 32%-100%. Recently, sorafenib has become the standard treatment for advanced HCC. However, the use of sorafenib in spontaneous rupture of HCC has not been studied and there is little information on this drug in HCC patients with spontaneous rupture. Therefore, the authors conducted a retrospective study to determine the efficacy and safety of sorafenib in patients with spontaneous rupture of HCC.
Sorafenib is a multi-targeted small molecule tyrosine kinase inhibitor which inhibits tumor growth and angiogenesis by inhibiting intracellular RAF kinases (CRAF, BRAF, and mutant BRAF) and cell surface kinase receptors (VEGFR-2, VEGFR-3, PDGFR-beta, cKIT, and FLT-3). Sorafenib has become the standard treatment for advanced HCC. It demonstrated a modest survival benefit over placebo in 2 phase III trials, especially in patients with Child-Pugh A liver disease. However, there is little information on this drug in HCC patients with spontaneous rupture.
Sorafenib is a multi-targeted small molecule tyrosine kinase inhibitor which inhibits tumor growth and angiogenesis by inhibiting intracellular RAF kinases (CRAF, BRAF, and mutant BRAF) and cell surface kinase receptors (VEGFR-2, VEGFR-3, PDGFR-beta, cKIT, and FLT-3), and it has become the standard treatment for advanced HCC based on two large randomized phase III trials. However, the use of sorafenib in spontaneous rupture of HCC has not been studied and there is little information on this drug in HCC patients with spontaneous rupture. The authors conducted a retrospective study to determine the efficacy and safety of sorafenib in patients with spontaneous rupture of HCC.
The study results suggest that sorafenib can be used in patients with ruptured HCC as it has interesting activity and is well tolerated; dose adjustment is generally not required.
Sorafenib: Sorafenib is an oral multi-kinase inhibitor which blocks tumor-cell proliferation and increases the rate of apoptosis. Sorafenib is the only agent with proven survival benefit in the treatment of advanced hepatocellular carcinoma.
This is a good retrospective study in which authors analyze the feasibility and safety of using sorafenib in hepatocellular carcinoma patients with spontaneous rupture. The results are interesting and suggest that sorafenib can be used in ruptured HCC patients with an interesting activity and tolerable toxicity.
P- Reviewer: Guanabens N, Vela S S- Editor: Qi Y L- Editor: Logan S E- Editor: Ma S
| 1. | Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13286] [Cited by in RCA: 13558] [Article Influence: 677.9] [Reference Citation Analysis (1)] |
| 2. | Padma S, Martinie JB, Iannitti DA. Liver tumor ablation: percutaneous and open approaches. J Surg Oncol. 2009;100:619-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 72] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 3. | Chearanai O, Plengvanit U, Asavanich C, Damrongsak D, Sindhvananda K, Boonyapisit S. Spontaneous rupture of primary hepatoma: report of 63 cases with particular reference to the pathogenesis and rationale treatment by hepatic artery ligation. Cancer. 1983;51:1532-1536. [PubMed] |
| 4. | Battula N, Madanur M, Priest O, Srinivasan P, O’Grady J, Heneghan MA, Bowles M, Muiesan P, Heaton N, Rela M. Spontaneous rupture of hepatocellular carcinoma: a Western experience. Am J Surg. 2009;197:164-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 61] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 5. | Miyamoto M, Sudo T, Kuyama T. Spontaneous rupture of hepatocellular carcinoma: a review of 172 Japanese cases. Am J Gastroenterol. 1991;86:67-71. [PubMed] |
| 6. | Kirikoshi H, Saito S, Yoneda M, Fujita K, Mawatari H, Uchiyama T, Higurashi T, Imajo K, Sakaguchi T, Atsukawa K. Outcomes and factors influencing survival in cirrhotic cases with spontaneous rupture of hepatocellular carcinoma: a multicenter study. BMC Gastroenterol. 2009;9:29. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 63] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 7. | Zhu Q, Li J, Yan JJ, Huang L, Wu MC, Yan YQ. Predictors and clinical outcomes for spontaneous rupture of hepatocellular carcinoma. World J Gastroenterol. 2012;18:7302-7307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 66] [Cited by in RCA: 79] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 8. | Vergara V, Muratore A, Bouzari H, Polastri R, Ferrero A, Galatola G, Capussotti L. Spontaneous rupture of hepatocelluar carcinoma: surgical resection and long-term survival. Eur J Surg Oncol. 2000;26:770-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 85] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 9. | Lai EC, Lau WY. Spontaneous rupture of hepatocellular carcinoma: a systematic review. Arch Surg. 2006;141:191-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 168] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 10. | Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378-390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9016] [Cited by in RCA: 10272] [Article Influence: 604.2] [Reference Citation Analysis (2)] |
| 11. | Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3854] [Cited by in RCA: 4653] [Article Influence: 273.7] [Reference Citation Analysis (0)] |
| 12. | Bruix J, Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020-1022. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5972] [Cited by in RCA: 6573] [Article Influence: 469.5] [Reference Citation Analysis (1)] |
| 13. | Pombo F, Arrojo L, Perez-Fontan J. Haemoperitoneum secondary to spontaneous rupture of hepatocellular carcinoma: CT diagnosis. Clin Radiol. 1991;43:321-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 14. | Kim JY, Lee JS, Oh DH, Yim YH, Lee HK. Transcatheter arterial chemoembolization confers survival benefit in patients with a spontaneously ruptured hepatocellular carcinoma. Eur J Gastroenterol Hepatol. 2012;24:640-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 15. | Li WH, Cheuk EC, Kowk PC, Cheung MT. Survival after transarterial embolization for spontaneous ruptured hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2009;16:508-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 16. | Yoshida H, Mamada Y, Taniai N, Mizuguchi Y, Kakinuma D, Ishikawa Y, Kanda T, Matsumoto S, Bando K, Akimaru K. Long-term results of elective hepatectomy for the treatment of ruptured hepatocellular carcinoma. J Hepatobiliary Pancreat Surg. 2008;15:178-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 31] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Kondo S, Okusaka T, Ueno H, Ikeda M, Morizane C. Spontaneous regression of hepatocellular carcinoma. Int J Clin Oncol. 2006;11:407-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 18. | Cherqui D, Panis Y, Rotman N, Fagniez PL. Emergency liver resection for spontaneous rupture of hepatocellular carcinoma complicating cirrhosis. Br J Surg. 1993;80:747-749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 58] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 19. | Liu CL, Fan ST, Lo CM, Tso WK, Poon RT, Lam CM, Wong J. Management of spontaneous rupture of hepatocellular carcinoma: single-center experience. J Clin Oncol. 2001;19:3725-3732. [PubMed] |
| 20. | Buczkowski AK, Kim PT, Ho SG, Schaeffer DF, Lee SI, Owen DA, Weiss AH, Chung SW, Scudamore CH. Multidisciplinary management of ruptured hepatocellular carcinoma. J Gastrointest Surg. 2006;10:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Recordare A, Bonariol L, Caratozzolo E, Callegari F, Bruno G, Di Paola F, Bassi N. Management of spontaneous bleeding due to hepatocellular carcinoma. Minerva Chir. 2002;57:347-356. [PubMed] |
| 22. | Yoshida H, Onda M, Tajiri T, Akimaru K, Takasaki H, Mamada Y, Taniai N, Nakamura Y, Kawano Y, Takahashi T. Successful surgical treatment of peritoneal dissemination of hepatocellular carcinoma. Hepatogastroenterology. 2002;49:1663-1665. [PubMed] |
| 23. | Yang T, Sun YF, Zhang J, Lau WY, Lai EC, Lu JH, Shen F, Wu MC. Partial hepatectomy for ruptured hepatocellular carcinoma. Br J Surg. 2013;100:1071-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 24. | Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245-1255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3249] [Cited by in RCA: 3597] [Article Influence: 276.7] [Reference Citation Analysis (4)] |
| 25. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4521] [Article Influence: 347.8] [Reference Citation Analysis (2)] |
| 26. | Verslype C, Rosmorduc O, Rougier P. Hepatocellular carcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii41-vii48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 282] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 27. | Sobin LH, Wittekind CH. TNM classification of malignant tumours. 6th ed. New York: Wiley 2002; 81-83. |
| 28. | Lencioni R, Marrero J, Venook A, Ye SL, Kudo M. Design and rationale for the non-interventional Global Investigation of Therapeutic DEcisions in Hepatocellular Carcinoma and Of its Treatment with Sorafenib (GIDEON) study. Int J Clin Pract. 2010;64:1034-1041. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 29. | Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, Cammà C, Colombo M. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54:2055-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 287] [Cited by in RCA: 299] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 30. | Lencioni R, Kudo M, Ye SL, Bronowicki JP, Chen XP, Dagher L, Furuse J, Geschwind JF, de Guevara LL, Papandreou C. GIDEON (Global Investigation of therapeutic DEcisions in hepatocellular carcinoma and Of its treatment with sorafeNib): second interim analysis. Int J Clin Pract. 2014;68:609-617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 181] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 31. | Di Marco V, De Vita F, Koskinas J, Semela D, Toniutto P, Verslype C. Sorafenib: from literature to clinical practice. Ann Oncol. 2013;24 Suppl 2:ii30-ii37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Kim R, El-Gazzaz G, Tan A, Elson P, Byrne M, Chang YD, Aucejo F. Safety and feasibility of using sorafenib in recurrent hepatocellular carcinoma after orthotopic liver transplantation. Oncology. 2010;79:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |