Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.16258
Revised: July 9, 2014
Accepted: August 13, 2014
Published online: November 21, 2014
Processing time: 161 Days and 23.4 Hours
AIM: To examine the correlation of phosphatidylinositol 3-kinase (PIK3) CB expression with preoperative radiotherapy response in patients with stage II/III rectal adenocarcinoma.
METHODS: PIK3CB immunoexpression was retrospectively assessed in pretreatment biopsies from 208 patients with clinical stage II/III rectal adenocarcinoma, who underwent radical surgery after 30-Gy/10-fraction preoperative radiotherapy. The relation between PIK3CB expression and tumor regression grade, clinicopathological characteristics, and survival time was statistically analyzed. Western blotting and in vitro clonogenic formation assay were used to detect PIK3CB expression in four colorectal cancer cell lines (HCT116, HT29, LoVo, and LS174T) treated with 6-Gy ionizing radiation. Pharmacological assays were used to evaluate the therapeutic relevance of TGX-221 (a PIK3CB-specific inhibitor) in the four colorectal cancer cell lines.
RESULTS: Immunohistochemical staining indicated that PIK3CB was more abundant in rectal adenocarcinoma tissues with poor response to preoperative radiotherapy. High expression of PIK3CB was closely correlated with tumor height (P < 0.05), ypT stage (P < 0.05), and high-degree tumor regression grade (P < 0.001). High expression of PIK3CB was a potential prognostic factor for local recurrence-free survival (P < 0.05) and metastasis-free survival (P < 0.05). High expression of PIK3CB was also associated with poor therapeutic response and adverse outcomes in rectal adenocarcinoma patients treated with 30-Gy/10-fraction preoperative radiotherapy. In vitro, PIK3CB expression was upregulated in all four colorectal cancer cell lines concurrently treated with 6-Gy ionizing radiation, and the PIK3CB-specific inhibitor TGX-221 effectively inhibited the clonogenic formation of these four colorectal cancer cell lines.
CONCLUSION: PIK3CB is critically involved in response to preoperative radiotherapy and may serve as a novel target for therapeutic intervention.
Core tip: The response of rectal cancer cells to preoperative radiotherapy may be related to the dysregulation of phosphatidylinositol 3-kinase (PI3K) signaling molecules, in particular high expression of PIK3CB. Therefore, we evaluated whether PIK3CB upregulation was involved in poor response to preoperative radiotherapy in patients with rectal adenocarcinoma. We found that high expression of PIK3CB was indeed involved in poor response to preoperative radiotherapy, as well as being associated with adverse outcomes in rectal adenocarcinoma patients treated with 30-Gy/10-fraction preoperative radiotherapy. Using TGX-221 (PIK3CB-specific inhibitor), we showed that inhibition of PIK3CB expression enhanced the response of colorectal cancer cell lines to radiotherapy.
- Citation: Yu WD, Peng YF, Pan HD, Wang L, Li K, Gu J. Phosphatidylinositol 3-kinase CB association with preoperative radiotherapy response in rectal adenocarcinoma. World J Gastroenterol 2014; 20(43): 16258-16267
- URL: https://www.wjgnet.com/1007-9327/full/v20/i43/16258.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i43.16258
Colorectal carcinoma is one of the leading causes of cancer deaths worldwide, and rectal cancer accounts for 30%-35% of these cases[1,2]. According the new National Comprehensive Cancer Network (NCCN) guidelines for colon cancer (2012), neoadjuvant chemoradiotherapy followed by total mesorectal excision is the current standard treatment for advanced mid- and low-rectal cancer. This regimen can induce tumor regression and thereby facilitate subsequent resection, resulting in improved local control and survival[3]. However, this strategy does not provide benefit to all patients[4]. Analysis of intracellular signaling pathways, such as phosphatidylinositol 3-kinase (PI3K), in patients with locally advanced rectal cancer has identified an expression profile that may identify patients who will not respond to neoadjuvant chemoradiotherapy[4].
PI3K proteins are involved in the regulation of many fundamental cellular processes, such as proliferation, migration and survival[5,6]. Deregulation of PI3K-mediated pathways has been implicated in the development of many human tumors, including colorectal cancer[7-10], and has also been shown to affect chemoradiotherapy sensitization of these tumors[3,4,11,12].
The most thoroughly studied PI3Ks are the class IA PI3Ks, which are constitutive heterodimers composed of a p110 catalytic subunit complexed to one of several regulatory subunits, collectively called p85[13]. There are four members of class I PI3K catalytic subunits: PIK3CA, PIK3CB, PIK3CD and PIK3CG[14,15]. Potential oncogenic roles of PIK3CA mutation are involved in tumorigenesis and chemoradiotherapy resistance of colorectal cancer; however, the role of upregulation of PIK3CB remains largely unknown. In this study, we found that expression of PIK3CB is upregulated in cancer tissues and PIK3CB expression is closely correlated to ypT stage and tumor regression grade (TRG) using immunohistochemical staining. Treatment with radiation and/or a PIK3CB- specific inhibitor in vitro provided evidence to support a role of PIK3CB in radiation resistance. Higher expression of PIK3CB correlates well with worse response of rectal cancer to preoperative radiation. Identification of PIK3CB as a novel target of increasing radiosensitization may help to shed new light on colorectal cancer treatment.
This study was carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association. This study was granted ethical approval by the Peking University Cancer Hospital Institutional Review Board. All patients provided written informed consent.
Clinical data: Data were collected from 208 patients with resectable rectal carcinoma treated in our hospital between June 2004 and August 2010. Eligible patients were selected according to the following criteria: (1) resectable rectal cancer ≤ 10 cm from the anal verge; (2) evaluated by endorectal ultrasound (ERUS) or magnetic resonance imaging (MRI) before treatment; (3) histologically identified primary carcinoma of the rectum; (4) no clinical evidence of distant metastases; (5) having undergone transabdominal radical resection based on the principle of total mesorectal excision (TME); and (6) having undergone R0 resection. Exclusion criteria were as follows: (1) receipt of concurrent nRCT; (2) presence of synchronous tumors, or history of other malignant tumors within 5 years; (3) familial adenomatous polyposis and hereditary non-polyposis colorectal carcinoma; and (4) death due to complications or other non-cancer-related causes[16].
Pretreatment evaluation and surgery: All patients underwent ERUS or MRI to evaluate tumor size, invasion depth, and extent (T stage). All patients had involvement of pararectal lymph nodes and were diagnosed with clinical stage II/III rectal cancer. Serum carcinoembryonic antigen (CEA) was measured, and abdominal computed tomography (CT) and chest radiography were also routinely performed before treatment. Short-course neoadjuvant radiotherapy with a total dose of 30-Gy (30-Gy/10-fraction) was adopted, as recommended by the Chinese Anti-Cancer Association, based on high-level clinical evidence[13-16]. Surgical resection was performed 2-4 wk after full-dose radiation. All included patients underwent radical resection strictly in accordance with the principles of TME[17], both for abdominoperineal resection and low anterior resection.
Standard pathological tumor staging of the resected specimen was performed in accordance with the guidelines of the American Joint Committee on Cancer. Evidence of ypCR was defined as absence of viable adenocarcinoma in the surgical specimen or the presence of lakes of mucus without tumor cells. The histology of all surgical specimens was reviewed and confirmed by an independent element and was classified based on the Mandard TRG system[18], as follows: (1) complete regression (fibrosis without detectable tissue of tumor); (2) fibrosis with scattered tumor cells; (3) fibrosis and tumor cells with preponderance of fibrosis; (4) fibrosis and tumor cells with preponderance of tumor cells; and (5) tumor tissue lacking changes related to regression.
Tissue microarrays were constructed as follows. Tumor and normal tissues were embedded in paraffin, and 5 μm sections stained with hematoxylin and eosin (HE) were generated to select representative areas for biopsies. Core tissue biopsy specimens (1.0 mm in diameter) were taken from these areas of individual donor paraffin blocks and precisely arrayed into a new recipient paraffin block with a custom-built instrument (Tissue Arrayer MiniCore; Alphelys, Plaisir, France). Two-hundred-and-eight pairs of rectal adenocarcinoma and matched adjuvant normal rectal mucosa specimens were arranged in seven recipient paraffin blocks. Three core tissue biopsies from adenocarcinomas and two from normal mucosa were obtained from each specimen. The presence of tumor tissue on the arrayed samples was verified on the HE-stained sections[19,20].
Immunohistochemistry was performed as follows[19]. TMA sections were deparaffinized in xylene and then rehydrated in a decreasing ethanol series. Endogenous peroxidase was blocked by incubating with 3% hydrogen peroxide for 15 min and nonspecific binding was blocked by incubating with 5% normal goat serum (Zymed Laboratories, San Francisco, CA, United States) for 30 min. Sections were then incubated with rabbit-anti PIK3CB monoclonal antibody (1:100 dilution; Epitomics, Burlingame, CA, United States) at 4 °C overnight, followed by a second-step incubation with ChemMate EnVision/horseradish peroxidase (HRP)-conjugated anti-mouse reagent, according to the manufacturer’s instructions (Gene Tech, Shanghai, China). The sections were stained with diaminobenzidine, rinsed gently, and counterstained with hematoxylin. The primary antibody was discarded, or had been replaced by phosphate-buffered saline (PBS) as a negative control. Two pathologists, who were blind to patient outcome, independently examined and scored the sections. Digital images were acquired using a Leica DM4000B digital camera microscope (Leica, Leipzig, Germany).
A modified semiquantitative scoring system was used to evaluate cytoplasmic PIK3CB[19,20]. The staining was evaluated by a previously described method for: (1) percentage of positive cells (0% = negative; 1 = 0%-10%; 2 = 10%-50%; 3 = 51%-80%; 4 = 81%-100%); and (2) staining intensity (0 = negative; 1 = low; 2 = moderate; 3 = strong). The final total score was obtained by multiplying the percentage of positive cells score by the staining intensity score, yielding a total score between 0 and 12. Based on this total score, tissues were classified as either PIK3CB low expression (scores 0-5) or PIK3CB high expression (scores 6-12).
All patients in the PRT group were given adjuvant chemotherapy for 6-8 cycles, using the standard regimens based on 5-fluorouracil or capecitabine, such as FOLFOX, CapeOX, or capecitabine alone[21].
Patients were followed at 3-mo intervals for the first 2 years and then at 6-mo intervals for the next 3 years. Evaluations consisted of physical examination, serum CEA measurement, complete blood count, and blood chemical analysis. Proctoscopy, abdominal ultrasonography, CT of the abdomen and pelvis, and chest radiography were also routinely performed every 6 to 12 mo.
Endpoints of this research were overall survival (OS), disease-specific survival (DSS), local recurrences-free survival (LRFS), and metastasis-free survival (MeFS).
HCT116, HT29, LoVo and LS174T colorectal cell lines (American Type Culture Collection, Manassas, VA, United States) were cultured in Dulbecco’s Modified Eagle’s Medium supplemented with 10% fetal bovine serum, and 1% penicillin/streptomycin in a fully humidified incubator equilibrated with 5% carbon dioxide.
TGX-221 was purchased from Selleck Chemicals (Boston, MA, United States) and stored according to the manufacturer’s recommendations. TGX221 was dissolved in distilled water, and stored at 80 °C as a 100 μmol/L stock until just prior to use. For in vitro experiments, 100 nmol/L concentration was generated at the time of each experiment by dilution with sterile PBS.
Quantitative real-time reverse transcriptase polymerase chain reaction was performed using an OPTICON 2 real-time PCR detection system (Bio-Rad, Hercules, CA, United States) using human PIK3CB primers (forward: 5’-GGT AAT CGG AGG ATA GGG CAG T-3’; reverse: 5’-CGG CAG TAT GCT TCA AGG ATG AC-3’) and β-actin primers (forward: 5’-TCC TCC TGA GCG CAA GTA CTC-3’; reverse: 5’-CAT ACT CCT GCT TGC TGA TCC A-3’) combined with SYBR Green Master Mix (Applied Biosystems Inc., Foster City, CA, United States). Total RNA was extracted from cells with the RNeasy Mini Kit (Qiagen, Limburg, Netherlands) and cDNA was subsequently generated with Super-Script III (Invitrogen, Carlsbad, CA, United States). The PCR conditions were: 94 °C for 2 min, followed by 40 cycles of 94 °C for 20 s, 59 °C for 20 s, 72 °C for 30 s, and 74 °C for 1 s.
Western blotting was performed as previously described[22], with antibodies targeting β-actin (1:2000 dilution) (#CB100997; Proteintech, Chicago, IL, United States) and anti-rabbit PIK3CB (1:1000 dilution) from the PI3 Kinase Antibody Sampler Kit (#9655; Cell Signaling Technology, Danvers, MA, United States). The protease inhibitor cocktail was purchased from Sigma (St. Louis, MO, United States). Cells were lysed in lysis buffer (20 mmol/L HEPES (pH = 7.2), 150 mmol/L NaCl, 1% Triton X-100, and 10% glycerol). Total protein (150 μg each) samples were separated on SDS-PAGE, transferred to nitrocellulose membranes, and immunoblotted with appropriate antibodies. Membranes were then washed with Tris-buffered-saline with Tween and incubated with secondary antibodies conjugated to HRP (Jackson ImmunoResearch Laboratories, West Grove, PA, United States). Signals were detected using the SuperSignal West Pico Trial Kit (Thermo Scientific, Rockford, IL, United States) and images were acquired using a MXP102 X-ray film processor (Kodak, Rochester, NY, United States).
Cells were plated in 6-well plates at a density chosen to result in 50-100 surviving colonies. Drug treatment occurred 2 h before radiotherapy with a kilovoltage irradiator (XRAD320; Precision X-Ray, North Branford, CT, United States) at a dose of 6-Gy. Cells were rinsed and incubated in complete medium for 12 d. Surviving colonies (> 50 cells) were counted with the GelCount System (Oxford Optronix, Oxford, United Kingdom). Each experiment was performed in triplicate and with three repeats for each group.
Statistical analysis was performed using SPSS for Windows version 16.0 statistical software (SPSS Inc., Chicago, IL, United States). Comparison of PIK3CB expression between the subgroups of various clinicopathological parameters was evaluated by χ2 test or Fisher’s exact test. LRFS, MeFS, and DSS, calculated from the date of operation to the date of event, were the endpoints analyzed. Survival curves were plotted using the Kaplan-Meier method, and log-rank tests were performed to evaluate prognostic differences between groups. The Cox proportional hazards model was used for multivariate analysis. For all analyses, two-sided tests of significance were used, and P < 0.05 was considered significant[23].
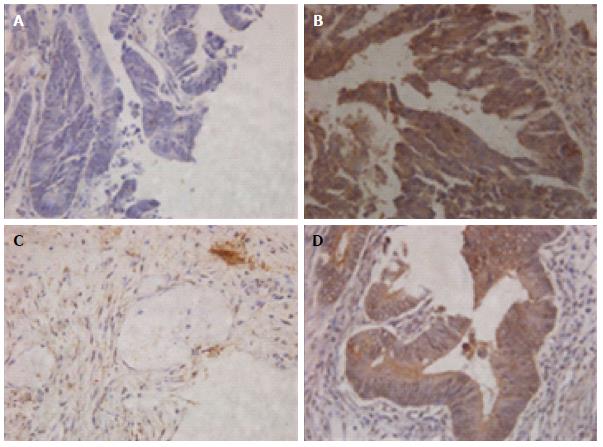
We first examined the expression of PIK3CB in clinical specimens of rectal cancer using immunohistochemistry. When detected in cell cytoplasm, PIK3CB immunoexpression was successfully scored in all 208 cases. Low expression of PIK3CB was detected in 67 (32.2%) cases (Figure 1A). PIK3CB was highly expressed in 141 (67.8%) cases that displayed moderate or strong nuclear immunostaining in > 50% of tumor cells (Figure 1B). As shown in Table 1, high expression of PIK3CB was correlated with tumor height (P < 0.05) and ypT (P < 0.05). Of note, the PIK3CB expression level was positively correlated with TRG (P < 0.000; Figure 1C and D), suggesting its role in resistance to preoperative radiotherapy. Otherwise, the PIK3CB expression status was not statistically related to other clinicopathological variables. Clinicopathological characteristics of all patients are summarized in Table 1.
| Parameter | No. of cases | PIK3CB expression | P value | ||
| Low | High | ||||
| Sex | Male | 120 | 37 | 83 | 0.619 |
| Female | 88 | 30 | 58 | ||
| Age in years | < 65 | 140 | 47 | 93 | 0.547 |
| ≥ 65 | 68 | 20 | 48 | ||
| TH in cm | < 5 | 74 | 14 | 60 | 0.002 |
| ≥ 5 | 134 | 53 | 81 | ||
| PreTNM | II | 29 | 9 | 20 | 0.945 |
| 1(18) | III | 161 | 51 | 110 | |
| BMI | 0 | 11 | 2 | 9 | 0.300 |
| 1 | 105 | 29 | 76 | ||
| 2 | 76 | 29 | 45 | ||
| 3 | 16 | 5 | 11 | ||
| LVI | Negative | 21 | 4 | 17 | 0.222 |
| Positive | 187 | 63 | 124 | ||
| Diff. | ypCR | 11 | 3 | 8 | 0.562 |
| 1(1) | G1-2 | 146 | 41 | 105 | |
| G3-4 | 50 | 18 | 32 | ||
| ypT | T0 | 8 | 6 | 2 | 0.043 |
| T1 | 11 | 4 | 7 | ||
| T2 | 55 | 20 | 35 | ||
| T3 | 134 | 37 | 97 | ||
| ypN | N0 | 122 | 36 | 86 | 0.608 |
| N1 | 55 | 20 | 35 | ||
| N2 | 31 | 11 | 20 | ||
| TRG | 1 (G1-2) | 32 | 29 | 3 | 0.000 |
| 2 (G3) | 79 | 26 | 53 | ||
| 3 (G4-5) | 97 | 12 | 85 | ||
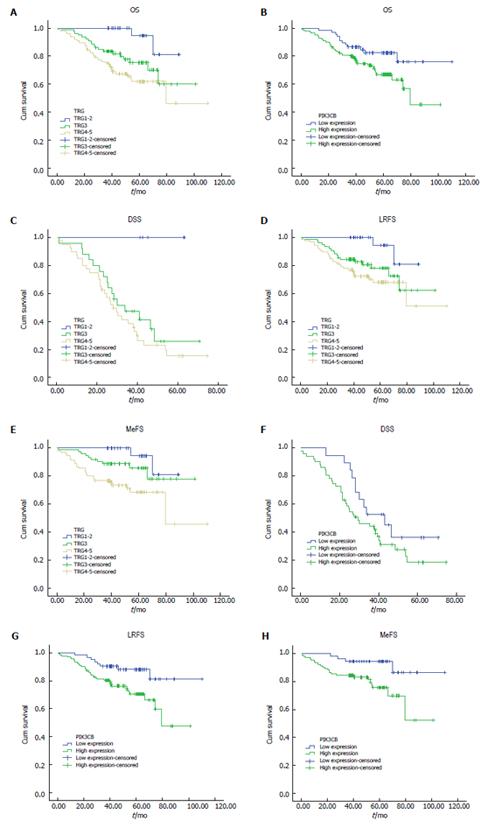
Next, we examined the correlation between PIK3CB expression and prognosis of rectal adenocarcinoma patients using a Kaplan-Meier analysis. Several clinicopathological parameters including tumor height, pre-TNM, body mass index (BMI), lymphovascular invasion, differentiation, ypT, ypN, and TRG (Figure 2A-E) were predictive of at least OS and one of the three endpoints of this study according to a univariate analysis (Table 2). Similarly, high expression of PIK3CB was associated with shorter durations of OS, LRFS, and MeFS (all P < 0.05; Figure 2F-H). However, high expression of PIK3CB was not an independent prognostic indicator for DSS, LRFS, and MeFS (Table 3) in multivariate analysis. In addition, TRG and ypN were found to have an independent prognostic impact on survival analysis.
| Parameter | DSS | LRFS | MeFS | ||||||
| HR | 95%CI | P value | HR | 95%CI | P value | HR | 95%CI | P value | |
| Sex | 1.482 | 0.654-3.356 | 0.346 | 0.970 | 0.483-1.945 | 0.931 | 0.586 | 0.219-1.570 | 0.288 |
| Age | 0.908 | 0.377-2.186 | 0.830 | 1.491 | 0.749-2.970 | 0.25602 | 1.947 | 0.808-4.693 | 0.138 |
| TH | 0.737 | 0.320-1.698 | 0.473 | 1.096 | 0.412-2.915 | 0.854 | 0.726 | 0.218-2.413 | 0.601 |
| PreTNM | 0.976 | 0.309-3.084 | 0.967 | 0.809 | 0.293-2.237 | 0.683 | 0.507 | 0.145-1.771 | 0.287 |
| BMI | 1.385 | 0.774-2.480 | 0.272 | 1.180 | 0.732-1.903 | 0.497 | 1.106 | 0.569-2.151 | 0.766 |
| LVI | 2.018 | 0.687-5.927 | 0.202 | 1.106 | 0.366-3.340 | 0.858 | 0.851 | 0.214-3.376 | 0.818 |
| Diff. | 1.065 | 0.470-2.415 | 0.880 | 0.460 | 0.203-1.041 | 0.062 | 0.438 | 0.128-1.494 | 0.187 |
| ypT | 1.200 | 0.546-2.640 | 0.650 | 0.971 | 0.537-1.756 | 0.923 | 1.067 | 0.488-2.332 | 0.872 |
| ypN | 0.839 | 0.498-1.414 | 0.510 | 2.926 | 1.816-4.713 | 0.000 | 2.442 | 1.275-4.674 | 0.007 |
| TRG | 2.337 | 1.064-5.136 | 0.035 | 1.981 | 1.012-3.877 | 0.046 | 2.755 | 1.052-7.215 | 0.039 |
| PIK3CB | 1.685 | 0.727-3.909 | 0.224 | 2.228 | 0.801-6.202 | 0.125 | 1.828 | 0.397-8.411 | 0.439 |
| Parameter | DSS | LRFS | MeFS | |||||
| No. c | No. e | P value | No. e | P value | No. e | P value | ||
| Sex | Male | 120 | 43 | 0.554 | 109 | 0.476 | 90 | 0.109 |
| Female | 88 | 27 | 86 | 64 | ||||
| Age in years | < 65 | 140 | 49 | 0.769 | 130 | 0.502 | 99 | 0.173 |
| ≥ 65 | 68 | 21 | 65 | 55 | ||||
| TH in cm | < 5 | 74 | 28 | 0.053 | 66 | 0.072 | 56 | 0.004 |
| ≥ 5 | 134 | 42 | 129 | 98 | ||||
| PreTNM | II | 29 | 7 | 0.544 | 27 | 0.959 | 23 | 0.437 |
| III | 161 | 581 (5) | 1481 (20) | 1151 (16) | ||||
| BMI | 0 | 11 | 5 | 0.008 | 11 | 0.628 | 6 | 0.906 |
| 1 | 105 | 29 | 96 | 84 | ||||
| 2 | 76 | 31 | 72 | 52 | ||||
| 3 | 16 | 5 | 16 | 13 | ||||
| LVI | Negative | 21 | 11 | 0.506 | 19 | 0.647 | 12 | 0.115 |
| Positive | 187 | 59 | 176 | 142 | ||||
| Diff. | ypCR | 11 | 11 (1) | 0.189 | 111 (1) | 0.974 | 10 | 0.951 |
| G1-2 | 146 | 48 | 139 | 108 | ||||
| G3-4 | 50 | 20 | 44 | 36 | ||||
| ypT | T0 | 8 | 1 | 0.359 | 8 | 0.374 | 8 | 0.544 |
| T1 | 11 | 4 | 11 | 8 | ||||
| T2 | 55 | 8 | 54 | 48 | ||||
| T3 | 134 | 57 | 122 | 90 | ||||
| ypN | 0 | 122 | 25 | 0.043 | 116 | 0.000 | 103 | 0.005 |
| 1 | 55 | 24 | 52 | 34 | ||||
| 2 | 31 | 21 | 27 | 17 | ||||
| TRG | 1 (G1-2) | 32 | 5 | 0.020 | 31 | 0.018 | 28 | 0.021 |
| 2 (G3) | 79 | 25 | 77 | 56 | ||||
| 3 (G4-5) | 97 | 40 | 87 | 70 | ||||
| PIK3CB | Low exp | 67 | 19 | 0.124 | 62 | 0.011 | 51 | 0.010 |
| High exp | 141 | 51 | 133 | 103 | ||||
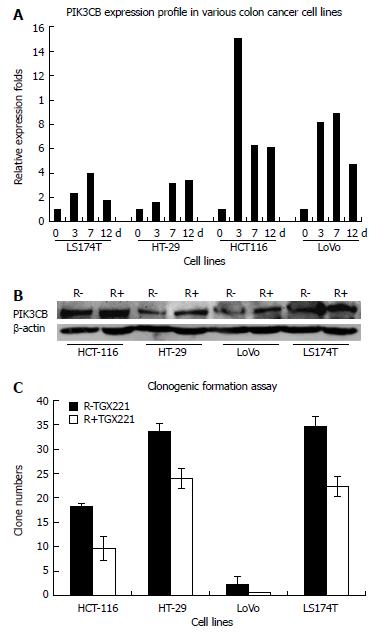
We investigated whether 6-Gy irradiation modulated PIK3CB expression and affected colorectal cancer cell growth and survival. TGX-221 functions as a PIK3CB-specific inhibitor[24]. Compared with the cells without irradiation, PIK3CB was upregulated in a time-dependent manner at both the mRNA and protein level (Figure 3A and B) followed by 6-Gy irradiation. In our colorectal cancer cell models, TGX-221 treatment decreased cell viability in a dose- and time-dependent manner, with substantial inhibition (> 50%) after 12 d at concentrations of 100 nmol/L in all four tested cell lines (Figure 3C).
PIK3CB does not show cancer-specific mutations. However, it is often differentially expressed in cancer. In contrast to p110α, wild-type PIK3CB is oncogenic when overexpressed in cell culture[25]. The poor prognostic effect of PIK3CB overexpression was demonstrated probably because it represents biological aggressiveness of human colorectal cancers[26].
To the best of our knowledge, this is the first study to examine the correlation between PIK3CB and patient survival in a large retrospective study of stage II/III colorectal cancer after 30-Gy/10-fraction preoperative radiotherapy. We found that high expression of PIK3CB in rectal cancer was associated with a high degree of TRG, OS, LRFS and MeFS, suggesting that PIK3CB is a biomarker to identify patients with better outcome, and a possible therapeutic target. Furthermore, PIK3CB is also activated in response to preoperative radiotherapy and represents a potential predictor for radiotherapy.
TGX-221 functions as a PIK3CB-specific inhibitor[24]. In our cell models, we confirmed that PIK3CB reduces colorectal cancer cell clonogenic formation. This indicates the invasive potential of PIK3CB expression. These aggressive biological phenotypes of the elevated expression of PIK3CB may contribute to the poor response to radiotherapy, and therefore are clinically correlated with advanced ypT. A high degree of TRG, representing a worse response after preoperative radiotherapy for rectal cancer, is closely related to poor survival and high local recurrence[27,28]. High expression of PIK3CB corresponded to higher TRG in our study. This also demonstrates the strong correlation between PIK3CB expression and poor response after radiotherapy in rectal cancer patients. Moreover, high expression of PIK3CB was a prognostic factor for poor OS, LRFS and MeFS in the univariate analysis but unfortunately not an independent prognostic factor in the multivariate analysis.
Our study population was homogeneous for rectal adenocarcinoma patients who received the same protocol of preoperative radiotherapy. Our results provide the first evidence that high expression of PIK3CB predicts poor sensitivity to radiotherapy, and is a prognostic factor for rectal cancer patients with the modified 30-Gy protocol for preoperative radiotherapy.
There were some limitations in our study. First, the histology of all surgical specimens was reviewed retrospectively. We did not compare the expression level of PIK3CB in radiation-pretreated rectal adenocarcinoma tissues with that in post-irradiation tissues. Instead, we studied PIK3CB expression in four colorectal cancer cell lines (HCT-116, HT-29, LoVo and LS174T)[29] and their response to 6-Gy irradiation. The fact that the PIK3CB mRNA and protein expression were all continuously upregulated in tested colorectal cell lines after 6-Gy irradiation, and the ability of TGX-221 to inhibit clonogenic formation, indicates that PIK3CB is involved in the response of colorectal cancer cells to radiotherapy, and functions as a potential target to assist the radiotherapy. Second, the redundant roles of different PI3K subunits, crosstalk between Ras, Src and PI3K, PI3K-PTEN balance, and some upstream or downstream signaling molecules need to be further analyzed. Finally, distinct biological functions other than clonogenic formation ability related to radioresistance may exist in rectal cancers, and further studies are warranted. Although further validation is necessary, these adverse phenotypes of PIK3CB to overcome cytotoxicity of radiotherapy are also important in addressing the poor survival of rectal cancer patients in our study.
In conclusion, we found for the first time that high expression of PIK3CB was correlated with poor response and prognosis of rectal cancer patients who received preoperative radiotherapy using a modified 30-Gy protocol. Suppression of PIK3CB expression by TGX-221 attenuated the clonogenic formation of colorectal cancer cells in the presence of concurrent irradiation. Our results suggest that high expression of PIK3CB correlates with poor prognosis and resistance of radiotherapy, and provide fundamental evidence of PIK3CB inhibition applied to preoperative radiotherapy protocols in rectal cancer.
Preoperative radiotherapy followed by total mesorectal excision does not induce tumor regression and improves local control and survival in all patients. Analysis of intracellular signaling pathways, such as that involving the phosphatidylinositol 3-kinase (PIK3)CB, in patients with locally advanced rectal cancer may identify patients who will not respond to preoperative radiotherapy.
Despite the oncogenic role of PIK3CA mutations in tumorigenesis and chemoradiotherapy resistance of colorectal cancer, high expression of PIK3CB remains largely unknown. In this study, the authors found that PIK3CB is critically involved in response to preoperative radiotherapy and may serve as a novel target for therapeutic intervention.
This is believed to be the first study to show that high expression of PIK3CB is correlated with poor response and prognosis of rectal cancer patients who received preoperative radiotherapy. Furthermore, the in vitro studies demonstrated that high expression of PIK3CB correlates with poor prognosis and resistance of radiotherapy.
By understanding how PIK3CB is induced and by blocking its expression, this study may provide fundamental evidence for PIK3CB inhibition that can be applied to preoperative radiotherapy protocols in rectal cancer.
The PIK3CB protein (PI3K catalytic subunit beta) is part of the activation pathway in some solid tumors, including colorectal cancer, and is involved in tumorigenesis and chemoradiotherapy resistance. Such a mechanism is thought to be crucial in identifying patients who do not respond to preoperative radiotherapy.
In the present study, the authors showed that PIK3CB may function as a novel response-predictive gene and a potential therapeutic target for locally advanced rectal cancer after 30-Gy/10-fraction preoperative radiotherapy.
P- Reviewer: Kwon RS, Yuki S S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN
| 1. | Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3032] [Cited by in RCA: 3123] [Article Influence: 223.1] [Reference Citation Analysis (0)] |
| 2. | Li M, Gu J. Changing patterns of colorectal cancer in China over a period of 20 years. World J Gastroenterol. 2005;11:4685-4688. [PubMed] |
| 3. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1457] [Article Influence: 97.1] [Reference Citation Analysis (0)] |
| 4. | Mammano E, Galdi F, Pierobon M, Tessari E, Deng J, Pucciarelli S, Agostini M, De Marchi F, Canzonieri V, De Paoli A. Multiplexed protein signal pathway mapping identifies patients with rectal cancer that responds to neoadjuvant treatment. Clin Colorectal Cancer. 2012;11:268-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 6. | Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27:5497-5510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1330] [Cited by in RCA: 1446] [Article Influence: 85.1] [Reference Citation Analysis (0)] |
| 7. | Wang B, Wang H, Yang Z. MiR-122 inhibits cell proliferation and tumorigenesis of breast cancer by targeting IGF1R. PLoS One. 2012;7:e47053. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 95] [Cited by in RCA: 127] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 8. | Xu Q, Simpson SE, Scialla TJ, Bagg A, Carroll M. Survival of acute myeloid leukemia cells requires PI3 kinase activation. Blood. 2003;102:972-980. [PubMed] |
| 9. | Johnson SM, Gulhati P, Rampy BA, Han Y, Rychahou PG, Doan HQ, Weiss HL, Evers BM. Novel expression patterns of PI3K/Akt/mTOR signaling pathway components in colorectal cancer. J Am Coll Surg. 2010;210:767-776, 776-778. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 180] [Cited by in RCA: 201] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 10. | Huang XF, Chen JZ. Obesity, the PI3K/Akt signal pathway and colon cancer. Obes Rev. 2009;10:610-616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 159] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 11. | Zhan M, Han ZC. Phosphatidylinositide 3-kinase/AKT in radiation responses. Histol Histopathol. 2004;19:915-923. [PubMed] |
| 12. | Zhang T, Cui GB, Zhang J, Zhang F, Zhou YA, Jiang T, Li XF. Inhibition of PI3 kinases enhances the sensitivity of non-small cell lung cancer cells to ionizing radiation. Oncol Rep. 2010;24:1683-1689. [PubMed] |
| 13. | Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1109] [Article Influence: 69.3] [Reference Citation Analysis (2)] |
| 14. | Campa D, Vodicka P, Pardini B, Naccarati A, Carrai M, Vodickova L, Novotny J, Hemminki K, Försti A, Barale R. A gene-wide investigation on polymorphisms in the taste receptor 2R14 (TAS2R14) and susceptibility to colorectal cancer. BMC Med Genet. 2010;11:88. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 15. | Cammà C, Giunta M, Fiorica F, Pagliaro L, Craxì A, Cottone M. Preoperative radiotherapy for resectable rectal cancer: A meta-analysis. JAMA. 2000;284:1008-1015. [PubMed] |
| 16. | Peng Y, Wang L, Du C, Gu J. Expression of vascular endothelial growth factor can predict distant metastasis and disease-free survival for clinical stage III rectal cancer following 30-Gy/10-f preoperative radiotherapy. Int J Colorectal Dis. 2012;27:1555-1560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479-1482. [PubMed] |
| 18. | Mandard AM, Dalibard F, Mandard JC, Marnay J, Henry-Amar M, Petiot JF, Roussel A, Jacob JH, Segol P, Samama G. Pathologic assessment of tumor regression after preoperative chemoradiotherapy of esophageal carcinoma. Clinicopathologic correlations. Cancer. 1994;73:2680-2686. [PubMed] |
| 19. | Cui M, Yu W, Dong J, Chen J, Zhang X, Liu Y. Downregulation of ABI1 expression affects the progression and prognosis of human gastric carcinoma. Med Oncol. 2010;27:632-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Hamm A, Veeck J, Bektas N, Wild PJ, Hartmann A, Heindrichs U, Kristiansen G, Werbowetski-Ogilvie T, Del Maestro R, Knuechel R. Frequent expression loss of Inter-alpha-trypsin inhibitor heavy chain (ITIH) genes in multiple human solid tumors: a systematic expression analysis. BMC Cancer. 2008;8:25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 127] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 21. | Wang L, Zhong XG, Peng YF, Li ZW, Gu J. Prognostic value of pretreatment level of carcinoembryonic antigen on tumour downstaging and early occurring metastasis in locally advanced rectal cancer following neoadjuvant radiotherapy (30 Gy in 10 fractions). Colorectal Dis. 2014;16:33-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Yu W, Sun X, Clough N, Cobos E, Tao Y, Dai Z. Abi1 gene silencing by short hairpin RNA impairs Bcr-Abl-induced cell adhesion and migration in vitro and leukemogenesis in vivo. Carcinogenesis. 2008;29:1717-1724. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 23. | Tian YF, Chen TJ, Lin CY, Chen LT, Lin LC, Hsing CH, Lee SW, Sheu MJ, Lee HH, Shiue YL. SKP2 overexpression is associated with a poor prognosis of rectal cancer treated with chemoradiotherapy and represents a therapeutic target with high potential. Tumour Biol. 2013;34:1107-1117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Gross C, Bassell GJ. Excess protein synthesis in FXS patient lymphoblastoid cells can be rescued with a p110β-selective inhibitor. Mol Med. 2012;18:336-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 25. | Zhao L, Vogt PK. Class I PI3K in oncogenic cellular transformation. Oncogene. 2008;27:5486-5496. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 473] [Cited by in RCA: 476] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 26. | Cui B, Tao J, Yang Y. Studies on the expression patterns of class I PI3K catalytic subunits and its prognostic significance in colorectal cancer. Cell Biochem Biophys. 2012;62:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 27. | Santos MD, Silva C, Rocha A, Matos E, Nogueira C, Lopes C. Tumor regression grades: can they influence rectal cancer therapy decision tree? Int J Surg Oncol. 2013;2013:572149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 13] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 28. | Abdul-Jalil KI, Sheehan KM, Kehoe J, Cummins R, O’Grady A, McNamara DA, Deasy J, Breathnach O, Grogan L, O’Neill BD. The prognostic value of tumour regression grade following neoadjuvant chemoradiation therapy for rectal cancer. Colorectal Dis. 2014;16:O16-O25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 29. | Ahmed D, Eide PW, Eilertsen IA, Danielsen SA, Eknæs M, Hektoen M, Lind GE, Lothe RA. Epigenetic and genetic features of 24 colon cancer cell lines. Oncogenesis. 2013;2:e71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 525] [Cited by in RCA: 674] [Article Influence: 56.2] [Reference Citation Analysis (0)] |