Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.16215
Revised: July 14, 2014
Accepted: July 24, 2014
Published online: November 21, 2014
Processing time: 162 Days and 14.6 Hours
AIM: To investigate the effects of a low fermentable, oligosaccharides, disaccharides, monosaccharides and polyols diet (LFD) and the probiotic Lactobacillus rhamnosus GG (LGG) in irritable bowel syndrome (IBS).
METHODS: Randomised, unblinded controlled trial on the effect of 6-wk treatment with LFD, LGG or a normal Danish/Western diet (ND) in patients with IBS fulfilling Rome III diagnostic criteria, recruited between November 2009 and April 2013. Patients were required to complete on a weekly basis the IBS severity score system (IBS-SSS) and IBS quality of life (IBS-QOL) questionnaires in a specially developed IBS web self-monitoring application. We investigated whether LFD or LGG could reduce IBS-SSS and improve QOL in IBS patients.
RESULTS: One hundred twenty-three patients (median age 37 years, range: 18-74 years), 90 (73%) females were randomised: 42 to LFD, 41 to LGG and 40 to ND. A significant reduction in mean ± SD of IBS-SSS from baseline to week 6 between LFD vs LGG vs ND was revealed: 133 ± 122 vs 68 ± 107, 133 ± 122 vs 34 ± 95, P < 0.01. Adjusted changes of IBS-SSS for baseline covariates showed statistically significant reduction of IBS-SSS in LFD group compared to ND (IBS-SSS score 75; 95%CI: 24-126, P < 0.01), but not in LGG compared to ND (IBS-SSS score 32; 95%CI: 18-80, P = 0.20). IBS-QOL was not altered significantly in any of the three groups: mean ± SD in LFD 8 ± 18 vs LGG 7 ± 17, LFD 8 ± 18 vs ND 0.1 ± 15, P = 0.13.
CONCLUSION: Both LFD and LGG are efficatious in patients with IBS.
Core tip: This is one of the first studies confirming the efficacy of a low fermentable, oligosaccharides, disaccharides, monosaccharides and polyols diet (LFD) in a Danish population with irritable bowel syndrome (IBS). The Lactobacillus rhamnosus GG (LGG) also has beneficial effects on IBS symptoms, particularly in diarrhoeal and alternating IBS subtypes. Web-based monitoring (such as at: http://www.ibs.constant-care.dk) of disease severity and quality of life appears to be feasible among patients with IBS. LFD and LGG should be recommended for patients with IBS. The web-based monitoring of disease severity is promising means and should be more widely implemented among patients with IBS.
-
Citation: Pedersen N, Andersen NN, Végh Z, Jensen L, Ankersen DV, Felding M, Simonsen MH, Burisch J, Munkholm P. Ehealth: Low FODMAP diet
vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World J Gastroenterol 2014; 20(43): 16215-16226 - URL: https://www.wjgnet.com/1007-9327/full/v20/i43/16215.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i43.16215
Pharmaceutical treatment with laxatives, antidiarrhoeals, antispasmodics or antidepressants in patients with irritable bowel syndrome (IBS) offers only a mild palliation of clinical symptoms[1-4]. Alternative treatment interventions such as cognitive behavioural[5], hypnotic[6], zone therapy[7], dietary interventions[8] and probiotics[9] have been suggested as potential treatment options for patients with IBS[10,11].
The low fermentable, oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet (LFD) was developed based on poor absorption of the short-chain carbohydrates in the small intestine, which in IBS patients can cause gas production and increase intestinal osmolarity[12-15]. The FODMAP components comprise fructose, lactose, fructo- and galacto-oligosaccharides (fructans, and galactans), and polyols (such sorbitol, mannitol, xylitol and maltitol) all of which putatively have three common functional properties: poorly absorbed in the small intestine, osmotically active molecules, and rapidly fermented by bacteria. The mechanism of LFD is assumed to be a decrease in the production of hydrogen and methane in the bowel upon minimizing the intake of these short-chain carbohydrates[16,17]. A recent study by Shepherd et al[14], revealed a reduction of global abdominal symptoms in up to 75% of patients with IBS, however, this result has not yet been replicated.
Probiotics containing the strain Lactobacillus rhamnosus GG (LGG), with six billion per capsule, has been proven to reduce symptoms in children with functional gastrointestinal disorders, but the efficacy in adult IBS patients remains uncertain[18-20].
Self-management and education in IBS patients have been shown to have a beneficial effect on the disease burden and economic burden[21-23]. Web-based concepts for treatment and follow-up of patients with inflammatory bowel disease have been successfully developed and shown to improve disease course, optimizing patients’ compliance, improving quality of life and generating economic benefits for the health care system[24-26].
The aim of this study was to conduct a randomised, controlled trial for 6-wk with patients randomised according to either treatment with LFD, LGG capsules, or a non-intervention control group following a normal Danish/Western diet (ND). Furthermore, we evaluated the feasibility of a web-application developed specifically for IBS patients and investigated whether this program had any effect on disease severity and disease-specific quality of life.
Between November 2009 and April 2013, all IBS patients from Herlev Hospital, University of Copenhagen, Denmark were assessed for eligibility. All patients fulfilled the Rome III diagnostic criteria for IBS, had negative outcome of colonoscopy, negative transglutaminase antibodies and lactose intolerance gene test prior to study enrolment[27]. Furthermore, all patients were subclassified in three different IBS subtypes depending on their stool habits, as either diarrhoea-predominant (IBS-D), constipation (IBS-C), or alternating periods of diarrhoea and constipation (IBS-A)[27].
Patients who had any alarm symptoms such as fever (> 38.5 °C), anaemia, unintended weight loss > 5 kg, familiar disposition to colorectal cancer or any other significant disease were excluded. Patients with a body mass index (BMI) below 18 were excluded from the dietary intervention group.
Patients were included in a 6-wk randomised unblinded controlled trial and allocated to one of three groups: LFD, LGG and a non-intervention control group (ND). Random allocation software at a 1:1:1 based on http://mahmoodsaghaei.tripod.com/Softwares/randalloc.html was used. A third person, not involved in the study, generated the random allocation sequence (randomization list) and numbered packing of the intervention. The randomization was not blinded for either the participants or the investigators.
All three groups were asked to register their symptoms weekly using the IBS-severity scoring system (IBS-SSS)[28,29] and IBS specific quality of life (IBS-QOL)[30,31] questionnaires on the web-application and were allowed to continue their regular IBS medication.
The primary endpoint of this study was to evaluate the reduction of IBS-SSS in the treatment group compared to a non-intervention control group.
Based on the primary endpoint an estimate of the required sample size (40 patients in each group) was calculated: (Type I error 5%, Type II error 20%, power 80%).
n1 = n1≥ [2(Z1-α/2 + Z1-β)2*SD2]/d2 = [2(1.96 + 0.84)2*802]/502 = 40
LFD: Each patient allocated to the LFD was instructed in the diet during a one hour session by nutritionists or dietitians with a special interest in IBS and the LFD. All patients were requested not to eat food components with a high content of FODMAPs and a list of these was provided at the session with the dietitian. All patients were encouraged to contact the dietitian in case of any uncertainties regarding the diet. All patients that underwent 6-wk LFD in our study were reintroduced by our dietitians/nutritionists to some of the restricted diet products, however, adjusting the quantity of these products. Afterwards patients were connected to the dietitian/nutritionists for a longer time. The lists of food not allowed in patients on low FODMAP diet used in our study are based on Australian lists provided by Gibson et al[16,32].
Control group: Patients allocated to control group (LGG and ND) were requested to follow an unchanged normal (Danish/Western) diet during the 6-wk study. At the end of the study, all ND patients were introduced to a LFD and thereafter guided by dietitians/nutritionists.
LGG in the form of Dicoflor60 capsules (Pharmaforce, Hvalsø, Denmark), containing the strain LGG six billion per capsule, was administered for 6-wk in the LGG group. Patients receiving probiotics intervention ingested two capsules of LGG daily. Side effects, if any, were registered every week.
Disease severity of IBS was measured by using the IBS-SSS questionnaire, which includes 5 items on a 0-100-mm visual analogue scale (VAS) with total scores ranging from 0 to 500 mm: severity of abdominal pain (Question 1), frequency of abdominal pain (Question 2), severity of abdominal distension (Question 3), dissatisfaction with bowel habits (Question 4), and interference with quality of life (Question 5). A cut-off IBS-SSS reduction level of 50 points was considered as an improvement[28].
Changes in quality of life during the study were measured by IBS-QOL questionnaire. The questionnaire consists of 34 items, each with a five-point response scale. The 34 items are based on the following eight variables; dysphoria, interference with activity, body image, health worry, food avoidance, social reactions, sexual and relationships[30].
The individual responses to the 34 items are summed (max score 170), averaged for a total score and then transformed to a 0-100 scale for easy interpretation, with higher scores indicating better QOL. The transformation formula used for the IBS-QOL total and scale scores is: Score = (The sum of the items - lowest possible score)/possible raw score range) × 100.
All patients completed both questionnaires via the web-application upon entry to the study and then weekly for the study’s duration.
In order to measure patients’ anxiety and depression the hospital anxiety and depression scale (HADS) was used[33]. HADS is a self-reported rating scale of 14 items used to identify and quantify depression and anxiety (seven items for each subscale). Both measures were administered at baseline and week 6. The scoring results are expressed as: 0-7 normal, 8-10 borderline, ≥ 11 anxious and/or depressed. Furthermore a satisfaction questionnaire (SQ) to evaluate patients’ satisfaction with the application was developed[24]. SQ consisted from seven items on a 0-100-mm VAS scale registered at the end of the study, covering satisfaction with the application, educational component, and the impact of the application on IBS. A mean score above ≥ 50-mm on the VAS was considered a satisfactory result.
Additional baseline characteristics regarding abdominal surgery prior to the study and any IBS medication such as laxatives, opstipantia, antispasmodics, analeptics and antidepressives were recorded at the entry of the study.
The web-application http://www.ibs.constant-care.dk was developed specifically for IBS patients. Access required a personal login provided by the responsible doctor. Via the website patients had access to information about IBS, an e-learning program, how to contact the study doctor, and treatment options. All patients were individually by a doctor educated in the use of the program and in IBS generally prior to entering the study and were required to complete the two questionnaires, IBS-SSS and IBS-QOL, once a week during the 6-wk trial. The total score of the questionnaires accumulates automatically after the patient’s insertion of values and allowed the patients to follow their symptoms severity in a traffic light chart: green = inactive-mild IBS severity (IBS-SSS < 175), yellow = moderate (IBS-SSS 175-300), and red = severe IBS (IBS-SSS > 300)[28].
The web-application is fully secured based on the net code principles. Each user requires a username and a password in order to access the web-application. Beside the patients, only certain care providers have access to patients’ data, allowing continuous monitoring of patients with regards to disease activity if necessary, to avoid complications. For the research purpose, registered data in the web-database were automatically linked to the Excel export function, allowing statistical analysis. The link between data and the patient was performed via a consequent personal and anonymous patient number.
The study was approved by the Ethical Committee, Denmark (protocol number H-2-2009-095). All patients included in this study signed an informed consent form.
Standard descriptive statistics were performed, including frequency distributions for categorical data and calculation of mean ± SD for continuous variables. Kruskal-Wallis 1-way ANOVA test was used to determine statistical differences among the three treatment groups and the Mann-Witney U two-sample test when analysing for statistical differences between the individual intervention groups and the ND group. The Wilcoxon matched pair test was used in order to assess changes in IBS-SSS and IBS-QOL in the dependent samples during the 6-wk. Linear analysis of covariance (ANCOVA) of changes in IBS-SSS/QOL from baseline to week 6 were used in controlling the effects of following baseline covariates: gender, age at diagnosis, smoking status, IBS type, surgery, IBS medication, IBS duration, BMI and treatment group. Correlations analysis between IBS-SSS and QOL were done using a non-parametric Spearman’s rank correlation test. A P-value of < 0.05 was considered statistically significant. All data were processed in SPSS software Version 20.0 for Windows (SPSS Inc., Chicago, IL) and SAS software version 9.3 (SAS Institute Inc. Care, NC, United States).
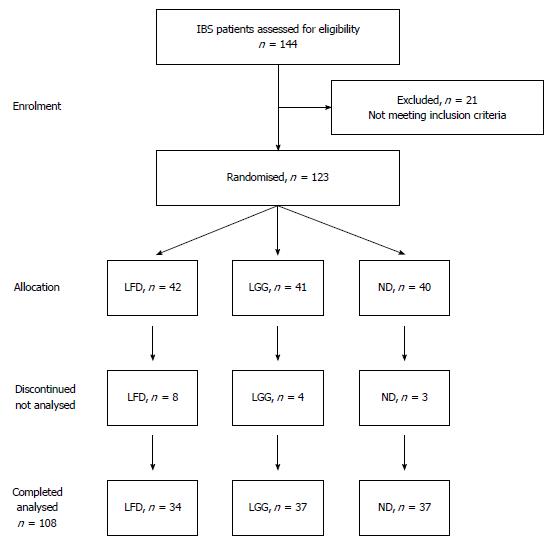
A total of 123 patients with IBS were recruited for the study. Enrolment and a progress diagram of patients with IBS through the study period are illustrated in Figure 1. The majority of patients were females, 90 (73%) and the median age was 37 years (range: 18-74 years). Forty-two (34%) patients were randomized to LFD, 41 (33%) to LGG and 40 (33%) to the ND group. Patients and disease characteristics by treatment group are shown in Table 1.
| Characteristics | LFD, n = 42 | LGG, n = 41 | ND, n = 40 | P = 0.23 |
| Male/Female | 8/34 | 14/27 | 11/29 | 0.30 |
| Age (yr, range) | 37 (18-71) | 43 (20-74) | 32 (18-73) | 0.08 |
| BMI (range) | 23 (19-38) | 22 (18-34) | 23 (17-34) | 0.50 |
| Smoking | 0.92 | |||
| Current | 5 (12) | 6 (15) | 6 (15) | |
| Former | 8 (19) | 11 (27) | 11 (27) | |
| Never | 24 (57) | 22 (54) | 22 (55) | |
| Missing | 5 | 2 | 1 | |
| Disease duration (yr, range) | 2.6 (0.7-13) | 1.8 (0.7-15) | 2 (0.5-9) | 0.20 |
| Diagnosis subtypes | 0.24 | |||
| IBS-A, mixed | 14 (33) | 19 (46) | 14 (35) | |
| IBS-C, constipation | 5 (12) | 7 (17) | 7 (17) | |
| IBS-D, diarrhoeal | 19 (45) | 13 (32) | 18 (35) | |
| Unknown | 4 | 2 | 1 | |
| Abdominal surgery | 7 (17) | 9 (22) | 6 (15) | 0.40 |
| IBS related medication | 16 (38) | 15 (37) | 18 (45) | 0.40 |
| IBS-SSS | 327 ± 94 | 279 ± 84 | 319 ± 83 | 0.17 |
| IBS-QOL | 56 ± 21 | 64 ± 15 | 57 ± 22 | 0.24 |
Fifteen patients discontinued participation in the study one week after enrolment: eight patients from LFD, four from LGG and three in the ND group. The reason for the drop outs in LFD group was difficulty with the diet, while it was lack of compliance with the web-application in the LGG and ND groups. Thus the final group for analysis comprised 108 IBS patients in three different groups: 34 LFD vs 37 LGG vs 37 ND.
Overall there was a significant reduction of IBS-SSS mean ± SD in all patients from baseline to week 6, mean IBS-SSS score 77 ± 104, P < 0.01, as well as in each treatment group (LFD, P < 0.001, LGG, P < 0.01 and ND, P = 0.03).
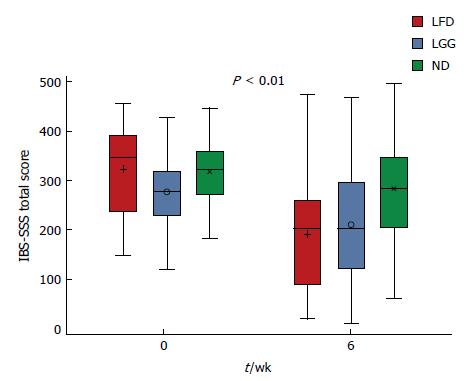
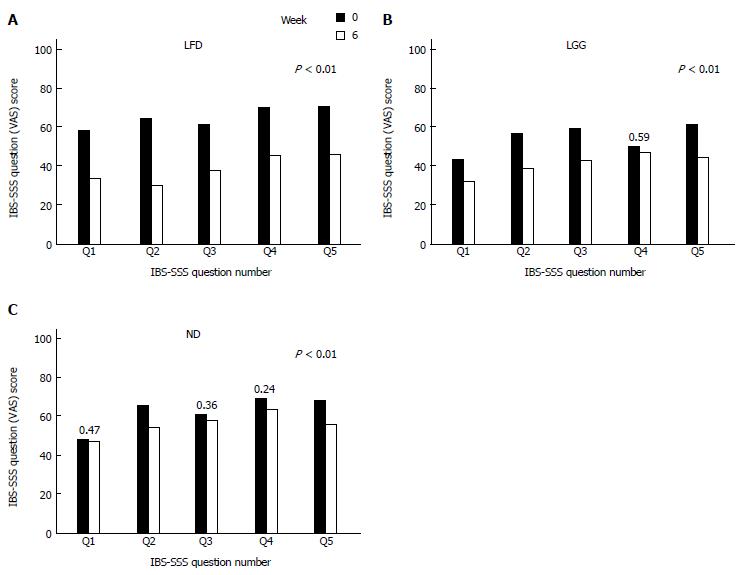
At week 6, comparing mean IBS-SSS between all three groups, a statistically significant reduction in the IBS-SSS was observed in LFD and LGG groups compared to the ND group, mean IBS-SSS 133 ± 122 vs 68 ± 107, 133 ± 122 vs 34 ± 95, P < 0.01 (Figure 2). Furthermore, comparing each question’s (Q) mean score of IBS-SSS between week 0 and 6, there was a significant reduction in all Qs (Q1-Q5) in LFD group (Figure 3A), in all Qs (apart from Q4) in LGG group (Figure 3B) and only in two Qs (Q2 and Q5) for ND group, (Figure 3C) (P < 0.01).
When dividing IBS patients into subtypes, patients with IBS-D in LFD experienced significant reduction in mean IBS-SSS from baseline to week 6, P < 0.01 and as well in LGG and ND groups (P = 0.01). In patients with IBS-A, a significant reduction in mean IBS-SSS was found in the LFD group (P = 0.01) and in the LGG (P = 0.04), but not in ND (P = 0.12). No significant reduction of IBS-SSS was found in patients with IBS-C type in any treatment group (Table 2).
The analyses of the covariance showed that patients taking IBS medication, having higher IBS-SSS at baseline and treated with LFD, significantly improved IBS-SSS at the end of the study (IBS-SSS = 55; 95%CI: 11-98, P = 0.01), (IBS-SSS = 0.41; 95%CI: 0.15-0.65, P = 0.002) and (IBS-SSS = 75; 95%CI: 23-123, P = 0.005). In contrast, no significant difference was observed in LFD when compared to the LGG group (IBS-SSS = 43; 95%CI: 8.07-95, P = 0.09. However, current and former smoking worsened the IBS-SSS (IBS-SSS = 51; 95%CI: 3.8-99, P = 0.03) and (IBS-SSS = 66; 95%CI: 1.08-131, P = 0.046) (Table 3).
| Effect | Contrast | Diff SSS | Lower | Upper | P value |
| Age | Below 40 yr vs above | 38.0246 | -9.4334 | 85.4826 | 0.11 |
| BMI | kg/m2 | 0.1812 | -5.1063 | 5.4687 | 0.94 |
| Gender | Females vs males | 8.4693 | -45.6473 | 62.5859 | 0.75 |
| IBS subtype | IBS-A vs IBS-D | 46.1096 | 0.6579 | 91.5613 | 0.041 |
| IBS subtype | IBS-C vs IBS-D | 50.3197 | -7.1618 | 107.80 | 0.08 |
| IBS medication | Yes vs no | -54.8795 | -98.4657 | -11.2933 | 0.011 |
| Prior surgery | Yes vs no | -11.6029 | -62.1065 | 38.9007 | 0.64 |
| IBS-SSS | Per baseline SSS point | -0.4082 | -0.6566 | -0.1598 | 0.0011 |
| Smoking | Current vs never | 66.0478 | 1.0815 | 131.01 | 0.041 |
| Smoking | Former vs never | 51.6542 | 3.8682 | 99.4401 | 0.031 |
| IBS duration | Per year | 4.7145 | -3.0897 | 12.5188 | 0.23 |
| Treatment group | LGG vs ND | -31.5190 | -80.7763 | 17.7384 | 0.20 |
| Treatment group | LFD vs ND | -75.3656 | -126.81 | -23.9216 | 0.0041 |
| Treatment group | LGG vs LFD | 43.8466 | -8.0787 | 95.7720 | 0.09 |
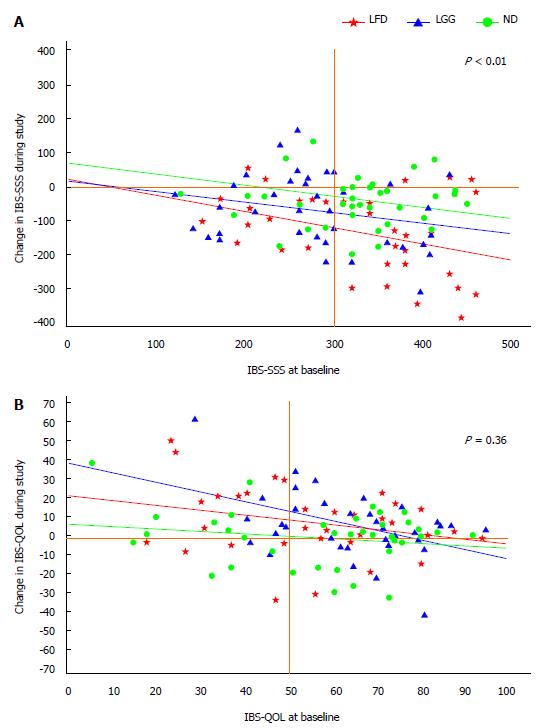
Adjusted linear regression analysis of changes of IBS-SSS from baseline toward the study period of 6-wk in all three groups showed a statistically significant improvement of IBS-SSS in LFD group vs ND, (IBS-SSS = 75; 95%CI: 126-24, P < 0.01), but not in LGG vs ND, (IBS-SSS = 32; 95%CI: 80-18, P = 0.20) (Table 3, Figure 4A).
Overall, there was a statistically significant improvement of IBS-QOL mean ± SD in all patients from baseline to week 6, mean IBS-QOL 5.0 ± 17, P < 0.01.
At week 6, no statistically significant improvement in IBS-QOL was observed in LFD and LGG groups as compared to the ND group, mean IBS-QOL 8 ± 18 vs 7 ± 17 vs 0.1 ± 15, P = 0.13.
Among the patients with IBS-D there was a statistically significant improvement of IBS-QOL in LFD (P = 0.02), but not for the LGG (P = 0.06), or ND group (P = 0.53). In patients with IBS-A and IBS-C, no statistically significant improvement was found in any group (P > 0.05).
The analyses of the covariance showed that patients with higher IBS-QOL at baseline, experienced no significantly improved quality of life (IBS-QOL = 0.29; 95%CI: 0.12-0.47, P = 0.001) by the end of the study. Patients taking IBS medication saw a significant improvement in IBS-QOL (IBS-QOL = 7.9; 95%CI: 1.02-14.9, P = 0.03) (Table 3).
Adjusted changes of IBS-QOL from baseline toward the study period of 6-wk by baseline covariates showed no statistically significant improvement of IBS-QOL in LFD compared to ND (IBS-QOL = 3.7; 95%CI: 4-12, P = 0.36) (Table 4, Figure 4B).
| Effect | Contrast | Diff QOL | Lower | Upper | P value |
| Age | Below 40 yr vs above | -6.3823 | -13.6223 | 0.8577 | 0.08 |
| BMI | Per kg/m2 | -0.2785 | -1.1185 | 0.5615 | 0.51 |
| Gender | Females vs males | 2.2012 | -6.3816 | 10.7840 | 0.61 |
| IBS subtype | IBS-A vs IBS-D | -6.4508 | -13.7027 | 0.8012 | 0.08 |
| IBS subtype | IBS-C vs IBS-D | -4.3839 | -13.5478 | 4.7800 | 0.34 |
| IBS medication | Yes vs no | 7.9708 | 1.0208 | 14.9209 | 0.021 |
| Prior surgery | Yes vs no | 6.1371 | -1.9592 | 14.2335 | 0.13 |
| IBS-QOL | Per baseline QOL point | -0.2972 | -0.4739 | -0.1205 | 0.0011 |
| Smoking | Current vs never | -6.2665 | -16.7074 | 4.1743 | 0.23 |
| Smoking | Former vs never | -6.9729 | -14.6297 | 0.6839 | 0.07 |
| Time from diagnosis | Per year | -0.1678 | -1.4072 | 1.0716 | 0.78 |
| Treatment group | LGG vs ND | 7.2158 | -0.5844 | 15.0160 | 0.06 |
| Treatment group | LFD vs ND | 3.7883 | -4.4003 | 11.9769 | 0.36 |
| Treatment group | LGG vs LFD | 3.4275 | -4.8844 | 11.7395 | 0.41 |
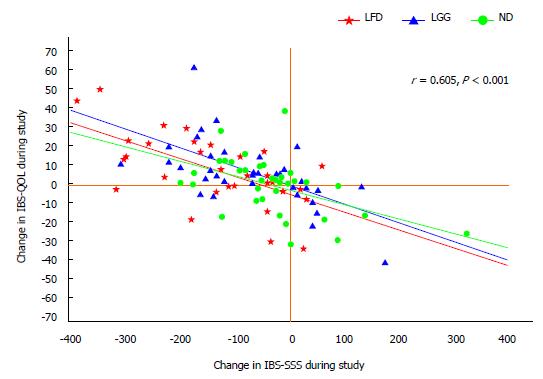
The changes in IBS-QOL correlated well with the changes in IBS-SSS, r = 0.6, P < 0.001, Figure 5.
Comparison of mean HADS at the baseline and at the end of the study revealed no statistically significant differences concerning either anxiety or depression in any of the three groups {Anxiety (median, range) at week 0 vs week 6 [LFD: 7 (2-15) vs 6 (2-15), P = 0.74; LGG: 5 (3-13) vs 5 (2-11), P = 0.14; ND: 7 (3-16) vs 7 (3-17), P = 0.93]. Depression (median, range) at week 0 vs week 6 [LFD 7 (2-17) vs 7 (3-15), P = 0.45; LGG: 7 (2-15) vs 6 (2-13), P = 0.53; ND: 8 (4-18) vs 7 (3-9), P = 0.22]}.
There were nine incidents of the web-application malfunction due to programming errors. The program administrator repaired these within six hours each time. There were no cases of patients who did not have access to the internet at their home and/or work. The age range of the patients was 18-74 years and no user-oriented problems were reported in any age group. Completing the questionnaires once a week was a problem for 11/108 (10%). There were a higher number of consultations in the LFD group (45%), mostly due to the questions regarding the diet.
Satisfaction with the web-application evaluated by the VAS questionnaire showed no statistically significant difference between intervention groups and the ND group, P = 0.2.
The present study is the first randomised controlled study addressing the efficacy of LFD and LGG vs a ND group in IBS in a European population using web-based symptom registration on a web-application. Overall, significant reduction of IBS symptoms were found in all three treatment groups: LFD, LGG and ND. Taking the different IBS types into account, a significant reduction in IBS-SSS from baseline to week 6 were found in patients with IBS-D and IBS-A when treated with LFD and LGG. This indicates that the effect of the diet is dependent upon the IBS subtype - being most effective in patients with the IBS diarrhoeal type, and this should be taken into account when recommending the diet to IBS patients. The reason of no effect of LFD on IBS-C subtype was observed in our study could be because of lack of power. The reduction of symptoms in our study was more evident at the end of the study (week 6), thus suggesting the diet should be adhered to for a minimum of 6-wk period[17].
Different diets for IBS patients have been proposed and their effects approved[34,35]. Food options, including LFD and, potentially, other dietary strategies are currently becoming treatment options for IBS patients[8,17,36,37]. In a recent controlled study in patients with IBS-D and HLA-DQ2/8, treatment with a gluten-free diet was associated with bowel function improvement when compared with a gluten-containing diet[35].
A previous retrospective trial by Gibson et al[16] along with other controlled prospective studies[15,38,39], have shown LFD to be effective in reducing abdominal symptoms (abdominal pain, bloating, flatulence and diarrhoea) in patients with IBS in an Australian population and in a New Zealand population. In our study, we described the effect of LFD on IBS-SSS, which is used for a detailed IBS symptom assessment in IBS[40].
The diet should be followed by patients for at least 6 wk. There is currently no data available indicating a lower symptom score after discontinued diet intervention, however, further studies on this are ongoing at Herlev University Hospital as a follow-up study. This follow up study will indicate whether or not IBS patient have discontinued the diet after the intervention period and the consequences hereof. In addition, Gibson et al from Australia are currently investigating the long term (side) effect of the low FODMAP diet in particular the effect of gut microbiota and immunology[36,41,42].
Higher number of consultations and high percentage of patients that dropped out of the LFD group elucidate the difficulty with this kind of the diet. The difficult to follow/adhere to LFD in our study was firstly due to lots of restriction on foods. In addition the feedbacks we have had from the patients are that the LFD is also expensive, difficult to find the products in the stores and requires more time for preparing the daily meals.
In our study former and current smoking was found to worsen the IBS-SSS symptoms. This is in agreement with previous results showing that smoking and other life style factors such as alcohol consumption and low levels of exercise have negative impact on IBS severity[43].
Regarding the weight and BMI, all patients in our study had a normal BMI with no impact on IBS symptoms in all three groups. Interestingly overweight has previously been shown to be protective against the onset or worsening of IBS[44].
In regard to IBS medication, a little more than one third of patients in our study received conventional IBS medication such as laxatives, antispasmodic and antidiarrheal agents and antidepressants. Patients being on IBS medication and LFD responded significantly better than patients on LFD without IBS medication[45].
The explanation of a better response on combined therapy in our study might be the complexity of IBS symptoms and natural disease course of IBS, requiring medications that might be effective against specific symptoms. Conventional IBS medication was shown to be more effective than placebo for treating IBS[46] in short-term studies. A combination of these agents, switch between the preparations or combination with the dietary options might be more effective[4]. There are limited data available supporting the long-term safety and effectiveness of these agents.
Probiotics like LGG are another potential treatment option in patients with IBS[20]. Our study revealed that patients treated with LGG improved their IBS-SSS significantly at the end of the study especially in IBS-D and IBS-A subtypes. Recent meta-analyses demonstrated the effect of LGG on abdominal pain related to functional disorders in children and on reduction of the duration of diarrhoea in children with acute gastroenteritis[19,47,48]. Contrary effects were shown in IBS patients treated with other probiotics (mixture of Lactobacillus and Bifidobacterium) during a six-month treatment period and compared with a placebo group, 52% vs 41% (P = 0.18) in primary care functional gastrointestinal disorders and in acute gastroenteritis in small children[9,49].
A significant reduction in disease severity during the 6-wk period was observed in the ND group, although this group did not receive any specific treatment. This result indicates that the web-application could potentially be used as a treatment option in IBS patients in the future. The web-application was an efficient way to collect data and also to give patients the opportunity to describe their own disease pattern, thereby hopefully aiding understanding and education about their condition. Previous studies have established the importance of offering education, information, disease recognition and general support in the management of IBS and a web-application could potentially be a simple way to provide these sources[22,50]. In this study we showed that the specially developed web-based application was a practical and user-friendly tool for patients with IBS, and in fact, had some beneficial effects on disease severity and quality of life. Overall the web-based, e-health approach to the management of IBS is new and little tested but a positive effect on both patients and health care providers is entirely possible and should be further substantiated. The goal of this work to ensure more satisfied patients, to ease the pressure on health care providers, and to lower the economic cost for health care systems.
The strength of this study is that this is one of the first randomised European studies on LFD and LGG in IBS where a specific web-application was used. The size of its sample population is also comparatively large, encouraging confidence in its results.
However, some limitations need to be taken into consideration. One such limitation was a lack of blinding, the fact that the ND group did not receive any placebo constitutes a weakness of the study, when referring to the large placebo responses in IBS revealed in other studies. Furthermore, a lack of blinded LFD patients means that ND patients could have been on a “semi LFD” due to accesses to information about LFD on the internet and other media. While a blinded dietary intervention is possible, this requires special facilities and is time consuming and expensive[38].
Other major limitation is that the conclusions of the results in interventional groups (LFD and LGG), are very hard to reach, due to lack of respective controls to each group and much more than for the “pure web” group. Moreover, the studied population could be compared with a historical one, followed for the same period length with no web assistance.
Finally, the results should be evaluated with some caution due to the fact that no evaluation carried out on individual adherence with diet in the LFD group. A new Food Frequency Questionnaire recently developed in order to semi-quantify the FODMAP intake could be useful in the screening of patients’ adherence in our study[51]. Moreover, the results could be influenced by the tight cooperation between LFD patients and dietitians/nutritionists as a placebo response.
In conclusion, both LFD and LGG are efficacious when treating IBS patients, especially in the IBS-D and IBS-A subtypes. Further evaluation of adherence to LFD, satisfaction with dietitians’ advice, and symptom improvement, short and long-term, is needed.
Support via the web-application appears to be a feasible option for patients with IBS. Further evaluation of the web-application is in progress and will reveal if there is any effect on disease course and whether it provides economic benefits for the health care system.
The authors wish to thank Henrik Wachmann (Larix A/S) for statistical analysis. ConstantMed Inc. provided the web development.
In recent randomized controlled studies, mainly using Australian and New Zealand populations, a diet low in fermentable, oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) has shown to relieve symptoms and improve quality of life for patients with irritable bowel syndrome (IBS). Efficacy of probiotics containing Lactobacillus rhamnosus GG (LGG) remains controversial.
FODMAP diet is diet low in carbohydrates which triggers increase gas production, abdominal bloating and stool fluid and has been prove to attenuate IBS symptoms.
This is one of the first studies confirming the efficacy of a low FODMAP diet (LFD) in a Danish population with IBS. LGG also has beneficial effects on IBS symptoms, particularly in IBS-diarrhoea and alternating periods of diarrhoea and constipation subtypes. Web-based monitoring (such as at: http://www.ibs.constant-care.dk) of disease severity and quality of life appears to be feasible among patients with IBS.
LFD and LGG should be recommended for patients with IBS. The web-application monitoring of disease severity is promising means and should be more widely implemented among patients with IBS.
FODMAPs are poorly absorbed in the small intestine, hence they are fermented by bacteria in the colon, causing symptoms like bloating, pain, altered bowel habits (diarrhoea, constipation or both), gas and more in sensitive individuals. LFD is a diet based on reduction of poor absorption of the short-chain carbohydrates in the small intestine, reducing gas production and increase intestinal permeability in IBS patients. LGG is a probiotic, containing six billion bacteria per capsule and the strain is characterised by good adherence to the intestinal wall and it is not decomposed by the bile salt or gastric acid and has been proven to reduce symptoms in children with infectious and functional GUT disorders. IBS-severity scoring symptom is an international validated used for the measuring of the disease severity in IBS patients.
The manuscript addresses an important issue in regards to the influence of diet on IBS symptoms. The authors compare a low FODMAP diet with a probiotic strain against diet. They address the limitations of a non-blinded study which would require further investigation and likely a different study design. The innovative use of web-application (online survey/diary) is an interesting approach. This is an excellent work, providing new and interesting insights for the management of IBS patients.
P- Reviewer: Carroccio A, Chiba T, Ducrotte P, Grundmann O, Krogsgaard LR, Quigley EMM, Sinagra E S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Dalton CB, Drossman DA. Diagnosis and treatment of irritable bowel syndrome. Drugs Today (Barc). 1998;34:585-592. [PubMed] |
| 2. | Eswaran S, Goel A, Chey WD. What role does wheat play in the symptoms of irritable bowel syndrome? Gastroenterol Hepatol (N Y). 2013;9:85-91. [PubMed] |
| 3. | Ford AC, Talley NJ. IBS in 2010: advances in pathophysiology, diagnosis and treatment. Nat Rev Gastroenterol Hepatol. 2011;8:76-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 14] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Brandt LJ, Chey WD, Foxx-Orenstein AE, Schiller LR, Schoenfeld PS, Spiegel BM, Talley NJ, Quigley EM. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol. 2009;104 Suppl 1:S1-S35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 182] [Cited by in RCA: 261] [Article Influence: 16.3] [Reference Citation Analysis (1)] |
| 5. | Jones M, Koloski N, Boyce P, Talley NJ. Pathways connecting cognitive behavioral therapy and change in bowel symptoms of IBS. J Psychosom Res. 2011;70:278-285. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 6. | Miller V, Whorwell PJ. Hypnotherapy for functional gastrointestinal disorders: a review. Int J Clin Exp Hypn. 2009;57:279-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 36] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Manheimer E, Cheng K, Wieland LS, Min LS, Shen X, Berman BM, Lao L. Acupuncture for treatment of irritable bowel syndrome. Cochrane Database Syst Rev. 2012;5:CD005111. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Talley NJ. Dietary modification as a treatment for irritable bowel syndrome. Gastroenterol Hepatol (N Y). 2012;8:552-554. [PubMed] |
| 9. | Hungin AP, Mulligan C, Pot B, Whorwell P, Agréus L, Fracasso P, Lionis C, Mendive J, Philippart de Foy JM, Rubin G, Winchester C, de Wit N. Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice -- an evidence-based international guide. Aliment Pharmacol Ther. 2013;38:864-886. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 138] [Cited by in RCA: 138] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 10. | Halland M, Talley NJ. New treatments for IBS. Nat Rev Gastroenterol Hepatol. 2013;10:13-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (1)] |
| 11. | Tack J. Functional diarrhea. Gastroenterol Clin North Am. 2012;41:629-637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Yao CK, Tan HL, van Langenberg DR, Barrett JS, Rose R, Liels K, Gibson PR, Muir JG. Dietary sorbitol and mannitol: food content and distinct absorption patterns between healthy individuals and patients with irritable bowel syndrome. J Hum Nutr Diet. 2014;27 Suppl 2:263-275. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 13. | Barrett JS, Gearry RB, Muir JG, Irving PM, Rose R, Rosella O, Haines ML, Shepherd SJ, Gibson PR. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31:874-882. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 122] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 14. | Shepherd SJ, Gibson PR. Fructose malabsorption and symptoms of irritable bowel syndrome: guidelines for effective dietary management. J Am Diet Assoc. 2006;106:1631-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 276] [Cited by in RCA: 260] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 15. | Shepherd SJ, Parker FC, Muir JG, Gibson PR. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: randomized placebo-controlled evidence. Clin Gastroenterol Hepatol. 2008;6:765-771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 385] [Cited by in RCA: 380] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 16. | Gibson PR, Shepherd SJ. Personal view: food for thought--western lifestyle and susceptibility to Crohn’s disease. The FODMAP hypothesis. Aliment Pharmacol Ther. 2005;21:1399-1409. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 242] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 17. | Barrett JS, Gibson PR. Fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) and nonallergic food intolerance: FODMAPs or food chemicals? Therap Adv Gastroenterol. 2012;5:261-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 101] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 18. | Moayyedi P, Ford AC, Talley NJ, Cremonini F, Foxx-Orenstein AE, Brandt LJ, Quigley EM. The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. Gut. 2010;59:325-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 470] [Cited by in RCA: 452] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 19. | Szajewska H, Skórka A, Ruszczyński M, Gieruszczak-Białek D. Meta-analysis: Lactobacillus GG for treating acute gastroenteritis in children--updated analysis of randomised controlled trials. Aliment Pharmacol Ther. 2013;38:467-476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 112] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 20. | McFarland LV, Dublin S. Meta-analysis of probiotics for the treatment of irritable bowel syndrome. World J Gastroenterol. 2008;14:2650-2661. [PubMed] |
| 21. | O’Sullivan MA, Mahmud N, Kelleher DP, Lovett E, O’Morain CA. Patient knowledge and educational needs in irritable bowel syndrome. Eur J Gastroenterol Hepatol. 2000;12:39-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 51] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Robinson A, Lee V, Kennedy A, Middleton L, Rogers A, Thompson DG, Reeves D. A randomised controlled trial of self-help interventions in patients with a primary care diagnosis of irritable bowel syndrome. Gut. 2006;55:643-648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 23. | Pedersen N, Vegh Z, Burisch J, Jensen L, Ankersen DV, Felding M, Andersen NN, Munkholm P. Ehealth monitoring in irritable bowel syndrome patients treated with low fermentable oligo-, di-, mono-saccharides and polyols diet. World J Gastroenterol. 2014;20:6680-6684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 45] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 24. | Pedersen N, Elkjaer M, Duricova D, Burisch J, Dobrzanski C, Andersen NN, Jess T, Bendtsen F, Langholz E, Leotta S. eHealth: individualisation of infliximab treatment and disease course via a self-managed web-based solution in Crohn’s disease. Aliment Pharmacol Ther. 2012;36:840-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 25. | Elkjaer M, Shuhaibar M, Burisch J, Bailey Y, Scherfig H, Laugesen B, Avnstrøm S, Langholz E, O’Morain C, Lynge E. E-health empowers patients with ulcerative colitis: a randomised controlled trial of the web-guided ‘Constant-care’ approach. Gut. 2010;59:1652-1661. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 245] [Cited by in RCA: 212] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 26. | Huang VW, Reich KM, Fedorak RN. Distance management of inflammatory bowel disease: systematic review and meta-analysis. World J Gastroenterol. 2014;20:829-842. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 61] [Cited by in RCA: 56] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 27. | Dorn SD, Morris CB, Hu Y, Toner BB, Diamant N, Whitehead WE, Bangdiwala SI, Drossman DA. Irritable bowel syndrome subtypes defined by Rome II and Rome III criteria are similar. J Clin Gastroenterol. 2009;43:214-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 28. | Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther. 1997;11:395-402. [PubMed] |
| 29. | Drossman DA, Whitehead WE, Toner BB, Diamant N, Hu YJ, Bangdiwala SI, Jia H. What determines severity among patients with painful functional bowel disorders? Am J Gastroenterol. 2000;95:974-980. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 30. | Patrick DL, Drossman DA, Frederick IO, DiCesare J, Puder KL. Quality of life in persons with irritable bowel syndrome: development and validation of a new measure. Dig Dis Sci. 1998;43:400-411. [PubMed] |
| 31. | Drossman DA, Patrick DL, Whitehead WE, Toner BB, Diamant NE, Hu Y, Jia H, Bangdiwala SI. Further validation of the IBS-QOL: a disease-specific quality-of-life questionnaire. Am J Gastroenterol. 2000;95:999-1007. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 32. | Gibson PR, Shepherd SJ. Evidence-based dietary management of functional gastrointestinal symptoms: The FODMAP approach. J Gastroenterol Hepatol. 2010;25:252-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 404] [Cited by in RCA: 397] [Article Influence: 26.5] [Reference Citation Analysis (0)] |
| 33. | Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens AE, Van Hemert AM. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychol Med. 1997;27:363-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1463] [Cited by in RCA: 1576] [Article Influence: 56.3] [Reference Citation Analysis (0)] |
| 34. | Eswaran S, Muir J, Chey WD. Fiber and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:718-727. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 294] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 35. | Vazquez-Roque MI, Camilleri M, Smyrk T, Murray JA, Marietta E, O’Neill J, Carlson P, Lamsam J, Janzow D, Eckert D. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology. 2013;144:903-911.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 323] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 36. | Shepherd SJ, Lomer MC, Gibson PR. Short-chain carbohydrates and functional gastrointestinal disorders. Am J Gastroenterol. 2013;108:707-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 183] [Cited by in RCA: 192] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 37. | Staudacher HM, Lomer MC, Anderson JL, Barrett JS, Muir JG, Irving PM, Whelan K. Fermentable carbohydrate restriction reduces luminal bifidobacteria and gastrointestinal symptoms in patients with irritable bowel syndrome. J Nutr. 2012;142:1510-1518. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 373] [Cited by in RCA: 379] [Article Influence: 29.2] [Reference Citation Analysis (0)] |
| 38. | Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A diet low in FODMAPs reduces symptoms of irritable bowel syndrome. Gastroenterology. 2014;146:67-75.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 810] [Cited by in RCA: 829] [Article Influence: 75.4] [Reference Citation Analysis (0)] |
| 39. | de Roest RH, Dobbs BR, Chapman BA, Batman B, O’Brien LA, Leeper JA, Hebblethwaite CR, Gearry RB. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. Int J Clin Pract. 2013;67:895-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 222] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 40. | Bijkerk CJ, de Wit NJ, Muris JW, Jones RH, Knottnerus JA, Hoes AW. Outcome measures in irritable bowel syndrome: comparison of psychometric and methodological characteristics. Am J Gastroenterol. 2003;98:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 94] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Gibson PR, Barrett JS, Muir JG. Functional bowel symptoms and diet. Intern Med J. 2013;43:1067-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 42. | Muscatello MR, Bruno A, Scimeca G, Pandolfo G, Zoccali RA. Role of negative affects in pathophysiology and clinical expression of irritable bowel syndrome. World J Gastroenterol. 2014;20:7570-7586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 31] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 43. | Liu L, Xiao QF, Zhang YL, Yao SK. A cross-sectional study of irritable bowel syndrome in nurses in China: prevalence and associated psychological and lifestyle factors. J Zhejiang Univ Sci B. 2014;15:590-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 44. | Carter D, Beer-Gabel M, Tzur D, Levy G, Derazne E, Novis B, Afek A. Predictive Factors for the Diagnosis of Irritable Bowel Syndrome in a Large Cohort of 440,822 Young Adults. J Clin Gastroenterol. 2014;Epub ahead of print. [PubMed] |
| 45. | Saha L. Irritable bowel syndrome: pathogenesis, diagnosis, treatment, and evidence-based medicine. World J Gastroenterol. 2014;20:6759-6773. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 318] [Cited by in RCA: 295] [Article Influence: 26.8] [Reference Citation Analysis (14)] |
| 46. | Ford AC, Talley NJ. Irritable bowel syndrome. BMJ. 2012;345:e5836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 47. | Horvath A, Dziechciarz P, Szajewska H. Meta-analysis: Lactobacillus rhamnosus GG for abdominal pain-related functional gastrointestinal disorders in childhood. Aliment Pharmacol Ther. 2011;33:1302-1310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 48. | Szajewska H, Wanke M, Patro B. Meta-analysis: the effects of Lactobacillus rhamnosus GG supplementation for the prevention of healthcare-associated diarrhoea in children. Aliment Pharmacol Ther. 2011;34:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 83] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 49. | Begtrup LM, de Muckadell OB, Kjeldsen J, Christensen RD, Jarbøl DE. Long-term treatment with probiotics in primary care patients with irritable bowel syndrome--a randomised, double-blind, placebo controlled trial. Scand J Gastroenterol. 2013;48:1127-1135. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 50. | Dorn SD. Systematic review: self-management support interventions for irritable bowel syndrome. Aliment Pharmacol Ther. 2010;32:513-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 51. | Barrett JS, Gibson PR. Development and validation of a comprehensive semi-quantitative food frequency questionnaire that includes FODMAP intake and glycemic index. J Am Diet Assoc. 2010;110:1469-1476. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |