INTRODUCTION
Colorectal cancer remains a challenging clinical entity worldwide and in the United States it is the third most common cause of cancer-related mortality in both men and women. Fortunately, the incidence of colorectal cancer is declining, in large part due to more prevalent educational and screening programs designed to detect early cancers and their precursor polyps[1]. It is widely accepted that more than 95% of colorectal cancers arise from adenomatous polyps, which are generally defined as benign lesions with dysplastic epithelium that have variable potential for malignancy. This adenoma-carcinoma sequence is well described and is often an indolent process that can take many years to fully manifest after a stepwise accumulation of genetic alterations[2,3].
MALIGNANT COLON POLYPS
While adenomatous polyps can harbor high-grade dysplasia and other non-invasive histology, malignant polyps are defined by the invasion of adenocarcinoma through the muscularis mucosa but limited to the submucosa (pT1). These polyps account for up to 12% of polyps in polypectomy series and the incidence is increasing with more efficacious screening programs employing colonoscopy[2], which is a fundamental tool in the prevention of colorectal cancer and the treatment of some advanced polyps. Approximately 80%-90% of adenomas are less than 1 cm and, therefore, more easily amenable to complete excision with conventional snare polypectomy (especially pedunculated polyps). However, the treatment of larger lesions can be more challenging and require more advanced techniques, such as endoscopic mucosal resection (EMR) or endoscopic submucosal dissection (ESD), which are being used with increasing frequency in specialized centers. These techniques afford the opportunity for complete excision rather than a piecemeal approach. This is a critical initial step in the overall management of malignant polyps because a complete excision facilitates a more comprehensive histological examination. Unfortunately, this is not the typical presentation in routine clinical practice. More commonly, a patient presents for evaluation after a resected polyp, thought to have a benign appearance at endoscopy, is found to have an invasive focus of adenocarcinoma on final pathological review. This scenario can often become further complicated if the polypectomy site is not marked at the initial endoscopy. This limits endoscopic re-evaluation, if needed, and renders untrustworthy the proper identification of the involved segment of colon if definitive resection is deemed appropriate. The consulting physician is then left with the difficult task of stratifying risk-weighing the risk of residual or recurrent disease, the risk of lymph node metastasis and balancing them against the patient’s operative risk. Analysis of the existing body of data demonstrates that this is still a controversial topic that generally requires a multidisciplinary approach. This review will discuss the important prognostic features of malignant polyps that will most profoundly affect this risk profile. Additionally, we will discuss effective strategies for their overall management.
POLYP CLASSIFICATION AND ASSESSMENT OF RISK
Size and morphology
Polyps are initially characterized endoscopically by their size and morphology, which are two important features that may predict underlying malignancy and should ultimately guide how advanced polyps are managed. Morphologically, polyps can be broadly classified as either pedunculated or sessile. Pedunculated polyps are those attached to the colonic mucosa by a stalk of variable length, while sessile polyps grow in a more flattened pattern over the mucosa with less separation of the adenomatous epithelium from the underlying layers of the bowel wall[4]. The latter are often, understandably, more difficult to completely remove with conventional snare polypectomy, depending on their location within the colon and their size. Studies have shown that those polyps ≤ 5 mm have negligible risk of malignancy and are therefore more amenable to standard techniques for endoscopic removal. Using a prospective registry of colorectal polyps, Nusko et al[5] performed a multivariate analysis of 11188 adenomas detected at colonoscopy. Of the 5027 adenoma < 5 mm analyzed, none contained invasive carcinoma. Larger polyps between 1.5 and 3.5 cm have higher malignant potential ranging from 19%-43% and should be approached with more caution[5,6].
Ultimately, the endoscopic assessment of polyps can be a subjective process that can vary between endoscopists. Fortunately, as image resolution has improved, there has also been an appreciable improvement in the ability to further characterize the endoscopic appearance of these polypoid lesions. The uses of narrow band imaging and chromoendoscopy have been shown to be effective adjuncts in this process. Narrow band imaging uses light at specific wavelengths, which enhances visualization of the mucosal surface and the associated vessels. In conjunction with routine imaging, chromoendoscopy uses special dyes that stain the mucosa and provides contrast between normal and abnormal tissue[7,8]. Using these concepts, the Paris and Kudo pit classification systems further assess the degree of irregular contours, ulcerations, and pit patterns (using magnifying chromoendoscopy) to stratify risk of underlying malignancy[9]. While they can be helpful, these classifications are still poorly standardized globally, which can lead to marked interobserver variability[10].
Depth of invasion
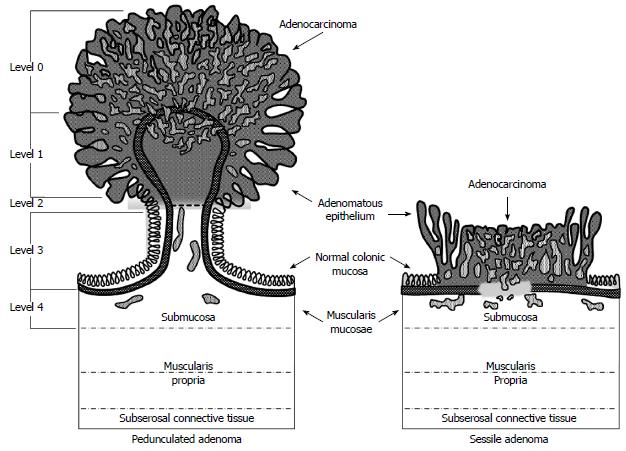
Histologically, polyps are classified by several factors but perhaps the most important feature is the depth of invasion. In 1985, Haggitt et al[11] put forward a classification system for pedunculated and sessile polyps based on the depth of invasion of adenocarcinoma. According to this classification system, pedunculated polyps can be classified as levels 0-4. Level 0 indicates cancer cells that are limited to the mucosa but do not penetrate through the muscularis mucosa (carcinoma in situ or intramucosal carcinoma). Levels 1 through 3 pertain to pedunculated polyps only. Level 1 indicates cancer cells invading through the muscularis mucosa into the submucosa but limited to the head of the polyp. When the cancer cells invade into the level of the neck (the junction of the head and the stalk) of the polyp, this denotes level 2. Level 3 indicates cancer cells invading any part of the stalk and level 4 signifies cancer cells invading into the submucosa of the bowel wall below the stalk of the polyp but above the muscularis propria. All sessile polyps with any degree of invasion were defined as level 4 (Figure 1). In this landmark study, they found that level 4 invasion was associated with statistically significant adverse prognostic factors[4,6,11]. These findings were confirmed in subsequent studies. Nivatvongs et al[12] reported a series of 151 patients undergoing colectomy for polyps with invasive carcinoma to determine the incidence of lymph node metastasis based on depth of invasion. They found that 10% of patients with sessile malignant polyps had evidence of lymph node metastasis. Overall, the incidence of lymph node metastasis in patients with pedunculated polyps was 6%; however, when the level of invasion reached level 4 the incidence increased to 27%[12].
Figure 1 Haggitt classification of pedunculated and sessile polyps.
Reprinted permission from[29].
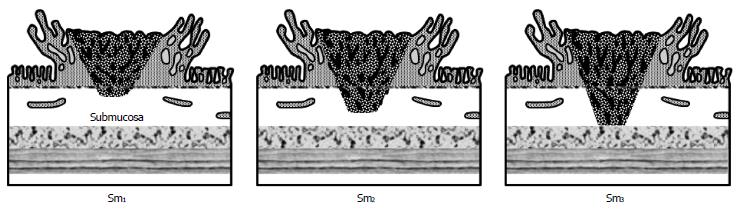
In the early 1990s Kudo et al[13] and Kikuchi et al[14] further classified the submucosal invasion of adenocarcinoma in sessile polyps into three levels (Figure 2): Sm1-invasion into the upper third of the submucosa; Sm2-invasion into the middle third of the submucosa; Sm3-invasion into the lower third of the submucosa.
Figure 2 Classification of submucosal (Sm) invasion of malignant polyps.
Reprinted permission from[29].
This apparently subtle distinction has proved quite significant, as further study has shown that the penetration of cancer cells into the lower third of the submucosa (Sm3) of sessile lesions is associated with a greater risk of lymphatic spread (up to 23%)[15]. However, use of this classification system can prove challenging for pathologists if the endoscopically resected specimen does not include a significant portion of the submucosa or some of the muscularis propria, which would define the deepest border of the submucosa. Others have modified this classification system in more practical terms by measuring the degree of submucosal invasion from the muscularis mucosa[16]. The results from a recent systematic review and meta-analysis of 23 studies of this topic demonstrated that a depth of submucosal invasion > 1 mm was significantly associated with lymph node metastasis[17].
Polypectomy resection margin
The requisite margin of a polypectomy resection is still a matter of much debate due to the risk of luminal recurrence. This ranges from 0% to 2% in malignant polyps with a margin of resection greater than 1 mm. However, when the resection margin is involved, or < 1 mm, the percentage of relapse ranges between 21% and 33%. Subsequently, many authors believe that a resection margin of ≥ 2 mm is ideal[2]. In 2012, Butte et al[18] reported a series of colectomies performed following polypectomy in 143 patients with clear or suspicious submucosal invasion. They found residual invasive disease in 11% of colectomy specimens. Analysis of margin status at polypectomy revealed that 16% of patients had residual invasive disease in the colon wall if the polypectomy margin was < 1 mm and 21% had residual invasive disease if the margin was indeterminate. None of the patients with a polypectomy margin ≥ 1 mm had residual invasive disease. The overall rate of lymph node metastasis in this study was 7%[18].
Other pathologic predictors
In addition to the depth of invasion and margin status, invasive adenocarcinomas can also be classified by distinct histologic findings, namely tumor budding architecture, degree of differentiation, or the presence of lymphovascular invasion. These are among the more commonly studied pathologic features, which can be of important prognostic significance that may ultimately influence management.
As compared with grade 1 (well-differentiated) adenocarcinomas, grade 3 (poorly-differentiated) cancers have been shown to be associated with adverse outcomes. Similarly, the presence of lymphovascular invasion has been significantly associated with increased lymph node metastasis[2,19,20].
Tumor budding refers to small clusters of undifferentiated cancer cells ahead of the invasive front of the lesion. While this is not a routinely examined pathologic parameter, there is increasing evidence that the quantitative assessment of tumor budding reflects clinical aggressiveness of colon cancers. This has also been shown by some to be a poor prognostic feature[20-22].
MANAGEMENT
Although the diagnosis of invasive adenocarcinoma in polyps is ultimately based on histological examination, the overall clinical management of malignant polyps should begin with their initial assessment at the time of index endoscopy-based on the size and morphology. Those suspicious for submucosal invasion or not deemed amenable for endoscopic removal should be referred for definitive surgical resection. It is important that the polyp site be marked to facilitate identification at the time of surgery.
Larger, sessile polyps should be referred to advanced endoscopists for consideration for EMR or ESD with the ultimate goal of complete, intact resection for histological evaluation. Endoscopic mucosal resection was developed for removal of sessile polyps confined to the mucosa and submucosa and is typically used for complete excision of lesions up to 2 cm. There are several techniques for EMR that have been described including cap-, and ligation-assisted EMR; however, injection-assisted EMR is most commonly employed. This typically involves an initial submucosal injection of saline, or other suitable injectates, which elevates the identified lesion and facilitates its removal from the deeper layers with an electrocautery snare[23,24]. The inability to lift a polyp with submucosal injection heralds the potential for deeper invasion by malignancy, and indicates suitability for endoscopic management. Endoscopic submucosal dissection is generally employed for larger GI lesions but has not been widely adopted for advanced colorectal polyps. Similar to EMR, ESD initially involves the saline lift of the polyp; however, this is followed by a mucosal incision and submucosal dissection with specialized endoscopic electrosurgical knives[23,25]. These techniques are more technically challenging and are associated with slightly higher risk of serious complications (bleeding and perforation). Again it is paramount that the polypectomy site be marked endoscopically so that the area can be reassessed for surveillance or can be identified if surgery is needed.
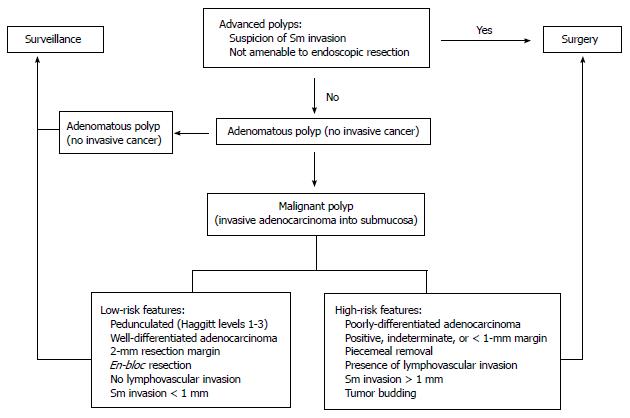
Based on the aforementioned prognostic features, the management of malignant polyps that have been previously excised depends on the risk of residual disease in the colon wall, the risk of lymph node metastasis, and the patient’s general medical condition (Figure 3). High-risk polyps are characterized by poor differentiation, the presence of lymphovascular invasion, deep submucosal invasion (> 1 mm), margin < 1 mm, and piecemeal resection (inability to completely assess the resected margin). These patients should be referred for definitive oncologic segmental resection, if medically fit for surgery[26]. Colectomy can be carried out in the traditional open technique or with a laparoscopic approach. Laparoscopy provides the benefits of less postoperative pain, quicker recovery of bowel function postoperatively, shorter hospital stays, improved cosmesis, and earlier return to normal activities without compromising oncologic results[26,27]. Low-risk polyps are characterized by the lack of these poor prognostic features and, if completely excised, can be managed adequately with conventional polypectomy and appropriate surveillance.
Figure 3 Algorithm for the management of malignant colon polyps.
Sm: Submucosal; EMR: Endoscopic submucosal resection.
Using this risk stratification, Choi et al[22] reported a series of 87 patients that were followed prospectively after endoscopic resection of a malignant polyp. Among the 30 high-risk patients that opted for surgical resection, 20% were found to have lymph node metastasis. Twenty patients with high-risk features opted for surveillance or had prohibitive factors for radical surgery. Of these patients, 3 (15%) had a recurrence. There were 30 patients without risk factors, and none developed lymph node metastasis or recurrent cancer after opting for surgery or surveillance[22].
SURVEILLANCE
The United States Multi-Society Task Force on colorectal cancer and other international organizations have established clear guidelines on colonoscopy surveillance after polypectomy based on the size and number of adenomatous polyps excised[8,28]. However, currently there is no established standard for surveillance after endoscopic removal of malignant polyps in patients that do not undergo surgery. Most authors suggest initial follow up endoscopy in 3-6 mo but the duration of subsequent surveillance varies[8,10]. There does not appear to be a role for routine CT imaging due to its poor sensitivity.
CONCLUSION
The management of malignant polyps can be challenging and often requires a multidisciplinary approach. Emphasis should be placed on the proper initial endoscopic assessment of these polyps and appropriate, complete resection using conventional snare polypectomy or more advanced techniques, such as endoscopic mucosal resection. After successful polypectomy, regardless of technique, appropriate decision analysis must be applied to those polyps deemed “malignant”. Patients with polyps that are concerning for malignancy during endoscopy or resected polyps with high-risk features (positive or indeterminate resection margins, margin < 1 mm, lymphovascular invasion, poor differentiation, Sm3 invasion, or tumor budding) should be referred for consultation for segmental colectomy, if medically fit, as the incidence of lymph node metastasis is high. Similarly, appropriate surveillance after polypectomy is critical to mitigate the risk of recurrent or metachronous disease. By understanding the risk factors associated with lymph node metastases based on the anatomic and histologic features of polyps, we as clinicians, can help risk stratify our patients and make rational, safe and informed choices for surgery.
P- Reviewer: Wang WH, Zhong JH S- Editor: Qi Y L- Editor: A E- Editor: Zhang DN