Published online Nov 21, 2014. doi: 10.3748/wjg.v20.i43.16153
Revised: May 1, 2014
Accepted: June 13, 2014
Published online: November 21, 2014
Processing time: 266 Days and 2.1 Hours
The introduction of colorectal endoscopic submucosal dissection (ESD) has expanded the application of endoscopic treatment, which can be used for lesions with a low metastatic potential regardless of their size. ESD has the advantage of achieving en bloc resection with a lower local recurrence rate compared with that of piecemeal endoscopic mucosal resection. Moreover, in the past, surgery was indicated in patients with large lesions spreading to almost the entire circumference of the rectum, regardless of the depth of invasion, as endoscopic resection of these lesions was technically difficult. Therefore, a prime benefit of ESD is significant improvement in the quality of life for patients who have large rectal lesions. On the other hand, ESD is not as widely applied in the treatment of colorectal neoplasms as it is in gastric cancers owing to the associated technical difficulty, longer procedural duration, and increased risk of perforation. To diversify the available endoscopic treatment strategies for superficial colorectal neoplasms, endoscopists performing ESD need to recognize its indications, the technical issues involved in its application, and the associated complications. This review outlines the methods and type of devices used for colorectal ESD, and the training required by endoscopists to perform this procedure.
Core tip: For treatment of superficial colorectal tumors, endoscopists should be aware of the treatment strategies including the indications, technical issues, and adverse events of each technique. The most appropriate treatment should be selected according to this information and in accordance with the accurate endoscopic diagnosis. In terms of endoscopic submucosal dissection (ESD), the most serious complication is iatrogenic perforation at a rate of < 3%. However, most of our patients with this complication did not need emergency surgery. Hence, ESD is considered a safe and appropriate procedure when performed by experienced endoscopists who have acquired the necessary technical skills through adequate training.
- Citation: Sakamoto T, Mori G, Yamada M, Kinjo Y, So E, Abe S, Otake Y, Nakajima T, Matsuda T, Saito Y. Endoscopic submucosal dissection for colorectal neoplasms: A review. World J Gastroenterol 2014; 20(43): 16153-16158
- URL: https://www.wjgnet.com/1007-9327/full/v20/i43/16153.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i43.16153
Endoscopic treatment for superficial colorectal neoplasms has shown rapid progress in the past decade with the introduction of endoscopic submucosal dissection (ESD). ESD is a state-of-the art technique that enables en bloc resection regardless of the lesion size[1-4]. Before the advent of this technique, snare polypectomy or endoscopic mucosal resection (EMR) using an electric snare was the most popular procedure for local endoscopic excision. EMR is a simple and safe technique and can be applied for lesions < 20 mm in size that require en bloc resection. If the lesion size is > 20 mm, en bloc resection is usually difficult and piecemeal resection[5-7], which has a high local recurrence incidence and clinically is associated with an inaccurate pathological evaluation, is performed. Patients who undergo piecemeal EMR require intensive colonoscopic surveillance with repeated examinations recommended every 12 mo. Hence, endoscopists should be knowledgeable of the recent advances in treatment techniques for superficial colorectal neoplasms to provide patients with the best care available. This review outlines the methods and type of devices used for colorectal ESD, and the training required by endoscopists to perform this procedure.
Endoscopic treatment is recommended only for lesions diagnosed as non-invasive tumors with a low metastatic potential. The risk factors for lymph node metastasis are as follows: poorly differentiated, signet-ring cell, and mucinous adenocarcinoma histological types; depth invasion; lymphovascular invasion; and tumor budding[8]. Depth invasion can be predicted using magnifying endoscopy or endoscopic ultrasound prior to treatment. We believe that pit pattern analysis using magnifying endoscopy has the highest diagnostic accuracy and is helpful in the treatment selection process between endoscopic treatment and surgery. Specifically, the invasive type V pit pattern with a clear demarcated area is crucial in the prediction of submucosal deep (≥ 1000 μm) invasive cancer; patients with tumors that do not have an invasive pattern are candidates for endoscopic treatment[9].
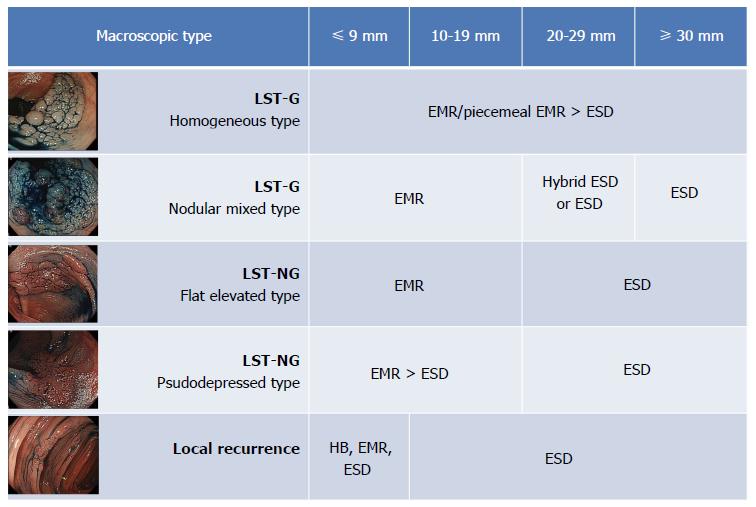
To make the appropriate treatment choice, we must identify lesions that require en bloc resection. The morphological type is a very important factor and must be taken into consideration when deciding the treatment strategy (Figure 1). Laterally spreading tumors (LSTs) are commonly used to describe flat or depressed type lesions. LSTs are divided into 2 main types: granular (LST-G) and non-granular (LST-NG) types. We know that LST-NG tumors ≥ 20 mm and LST-G tumors ≥ 30 mm have a significantly higher possibility of submucosal invasion and require an en bloc resection for an accurate pathological evaluation. Hence, ESD is indicated in these types of lesions. ESD is also indicated in lesions that are technically difficult to treat with conventional EMR, which includes those that are non-lifting after submucosal injection and those that demonstrate local recurrence. Piecemeal EMR is recommended in adenomatous lesions, diagnosed during pre-endoscopic examination, and are representative of the LST-G homogeneous type.
We used the water jet endoscope (PCF-Q260JI and GIF-Q260J; Olympus Medical System Co., Tokyo, Japan). When handling of the scope as the operator intends during the ESD procedure is difficult due to the lesion location or paradoxical movement, double-balloon colonoscope (EC-450BI, Fujifilm Corp., Tokyo, Japan) can be used to provide precise control of the endoscope[10].
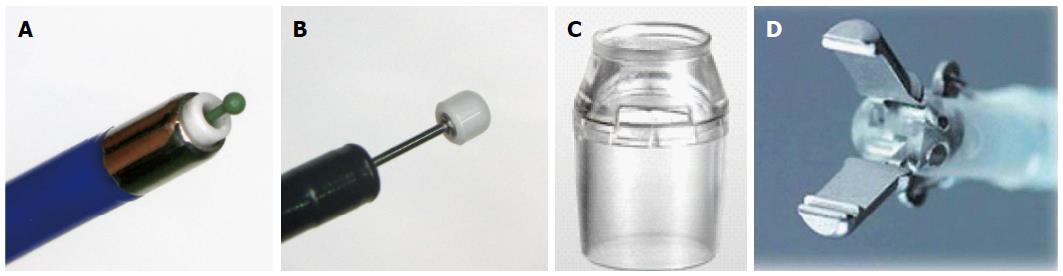
A ball-tip bipolar needle knife with water jet function (Jet B knife; XEMEX Co., Tokyo, Japan) is used for mucosal incision and submucosal dissection in the initial part of the procedure (Figure 2A). A notable characteristic of this device is use of the bipolar current system, which minimizes damage to deeper tissues and reduces the risk of perforation[11,12]. Following that, an insulation-tipped electrosurgical knife (IT knife nano, KD-612Q; Olympus Optical Co., Tokyo, Japan), in which the insulation-tip is smaller and the short blade is designed as a small disk to reduce the burning effect on the muscular layer, is commonly used to shorten the procedure time (Figure 2B).
As for the distal attachment, we used the short-type ST hood (DH-28GR and 29CR; Fujifilm Medical Co., Tokyo, Japan), which makes it easy to broaden the operator’s horizons and cut the submucosal layer due to its characteristic tapering shape (Figure 2C).
We used the ERBE VIO 300 D (Erbe, Tubingen, Germany). The output setting for ESD procedures is described in Table 1.
| Device | Cut mode | Coagulation mode | |
| Mucosal incision | Jet B knife | Dry cut, [E]3 100 W | |
| Submucosal dissection | Jet B knife | Dry cut, [E]3 100 W | Forced coag, [E]2 50 W |
| IT knife nano | Dry cut, [E]3 100 W | Swift coag, [E]2 50 W | |
| Hemostasis | Hemostat-Y | Bipolar, [E]5 25 W |
Maintenance of sufficient submucosal elevation with injection is crucial for the success of the ESD procedure. A long-lasting maintenance of submucosal elevation with submucosal injection is therefore preferable. Considering this, we commonly use 2 solutions; Glyceol (10% glycerin and 5% fructose; Chugai Pharmaceutical Co., Ltd., Tokyo, Japan) mixed with a small amount of Indigo Carmine and epinephrine, and 0.4% sodium hyaluronate solution (MucoUp; Seikagaku Corp, Tokyo, Japan)[13]. A small amount of Glyceol is injected into the submucosal layer to confirm the appropriate submucosal layer elevation first, and MucoUp is injected into the properly elevated submucosal layer next. Finally, a small amount of Glyceol is injected again to flush any residual MucoUp[14].
Use of carbon dioxide (CO2) gas for insufflation of the colonic lumen has been proven effective[15,16]. Insufflation of CO2 can reduce the risk of pneumoperitoneum in cases of perforation and abdominal problems before and/or after treatment.
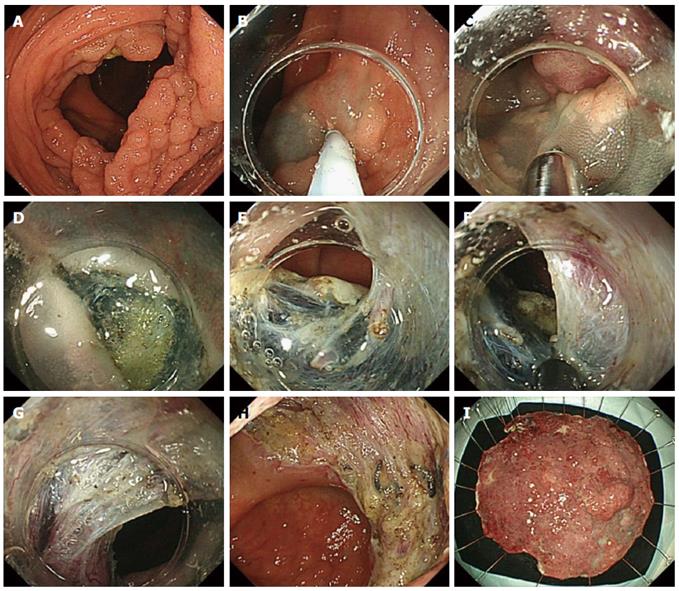
The endoscopic submucosal dissection procedure see the Figure 3. We initiate the process in retroflex view because the endoscope can be stabilized and handled better than in the forward view. Then, after creating sufficient submucosal elevation by injection, the initial mucosal incision is made with the Jet B knife from the distal side of the lesion.
It is usually difficult to insert the tip of the endoscope into the submucosal layer immediately after the initial mucosal incision. This is followed by trimming. The available cutting space is not enough to perform submucosal dissection during trimming; the submucosal layer near the mucosal layer is then carefully cut.
After securing a good visual field of the submucosal layer, submucosal dissection is continued with the same Jet B-knife. ESD is advantageous in that it allows good recognition of the structures in the submucosal layer and makes it possible to prevent bleeding by pre-coagulation of vessels. If the vessel is thin, pre-coagulation is performed with cutting devices. On the other hand, if it is a thick or pulsatile artery, coagulation forceps should be applied. In our institution, Hemostat-Y forceps (H-S2518; Pentax Co., Tokyo, Japan) were used in bipolar mode (25 W) to control visible bleeding and minimize the risk of any burning effect on the muscle layer (Figure 2D). Moreover, it is also possible to adjust the cutting line during submucosal dissection. In case of adenomatous lesions, the cutting line could be set near the mucosal layer to reduce perforation. On the other hand, in lesions that are possibly submucosal invasive cancer, it is necessary to achieve R0 resection, and the cutting line should be set deeper (near the muscularis propria) despite the higher risk of perforation.
After obtaining an adequate visual field with the process mentioned above, an IT knife nano is used for continuous dissection. It knife nano, “blade” type knife having longer useful section, could shorten the procedure time compared with the “needle” type knife. Throughout the procedure, repeated submucosal injections should be added to maintain good submucosal elevation.
After the colorectal ESD was completed, a routine colonoscopic review to detect any possible perforation or exposed vessels was conducted, and minimum coagulation was performed using the hemostat-Y forceps on non-bleeding visible vessels to prevent postoperative bleeding.
The Japan Society for Cancer of the Colon and Rectum conducted a multicenter, observational study for all patients treated by conventional endoscopic resection and ESD for colorectal neoplasms exceeding 20 mm in size from October 2007 to December 2010[17]. A total of 816 lesions were treated by ESD and the short-term outcomes were as follows. The mean lesion size was about 40 mm in diameter. En bloc resection was achieved in more than 90% of the cases, regardless of lesion size, with a perforation rate of 2.0% and delayed bleeding rate of 2.2%. None of the perforation cases needed emergency surgery as most iatrogenic perforations are very small, and can be successfully closed with endoscopic clip placement alone followed by intravenous antibacterial therapy (nothing per os).
The ESD procedure requires a high level of endoscopic skill and experience because of the high risk of complications associated with the anatomical characteristics of the colon. Therefore, a greater understanding of the learning curve for ESD is required to standardize the training and achieve wider acceptance of this technique. At our institution, endoscopists who intend to perform colorectal ESD must meet the following prerequisites: a high skill level in the non-loop insertion colonoscopy technique (more than 10 cases of total colonoscopies completed within 5 min without any patient complaints of abdominal discomfort), skill in conventional EMR or piecemeal EMR techniques, experience with > 20 gastric ESD cases, and assisting during > 20 colorectal ESDs conducted by experienced endoscopists[18]. However, in western countries, gastric cancer is not as common as colorectal cancer, and it may be difficult to introduce trainees to the resection of this lesion as the first step of ESD training. If required, trainees should begin with clinical training for colorectal ESD for lower rectal lesions, which have a lower risk of perforation and have a setting similar to that of gastric lesions.
We reported the short-term outcomes of colorectal ESD performed by less-experienced endoscopists[18,19]. In terms of the learning curve, they can perform it safely and independently after preparatory training and experience with ≥ 30 cases. On the other hand, most of LST-G tumors ≤ 40 mm were treated safely within 120 min procedure time, without any adverse events. Therefore, we suggest that an LST-G tumor < 40 mm in size is likely to be suitable for introducing trainees to ESD.
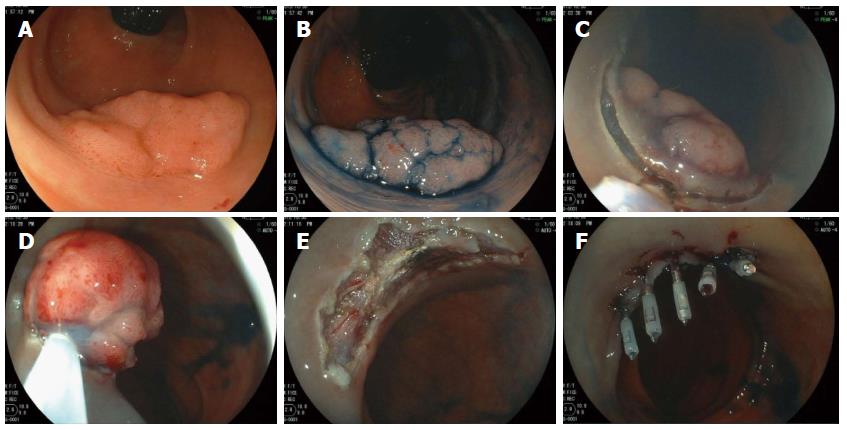
Hybrid ESD, which was first reported as “endoscopic resection with local injection of hypertonic saline-epinephrine” by Hirao et al[20] in 1986, is considered an alternative for ESD. It may enable en bloc resection or at least fewer piecemeal resections for large colorectal neoplasms in a manner that is both safe and relatively quick. The technique is simple; the first step is circumferential mucosal incision, and the next step involves placing the snare around it via the circumferential incision and tightening it (Figure 4)[21,22]. However, this technique has limitations. From our limited experience, it is often difficult to achieve en bloc resection in lesions ≥ 35 mm or LST-NG pseudo-depressed type tumors, and therefore, we believe that hybrid ESD is most suitable in lesions 20-30 mm in size.
Herein, we have described the outline for endoscopic treatment of colorectal neoplasms. ESD is a reliable method for achieving en bloc resection of relatively large colorectal superficial neoplasms, with superior curability and allows for an accurate pathological evaluation compared to piecemeal EMR. Moreover, colorectal ESD has succeeded in reducing extensive surgery of mucosal carcinomas and improving the overall quality of life for most patients with lower rectal lesions. On the other hand, associated technical difficulties and complications such as iatrogenic perforation have affected worldwide acceptance and generalization of this technique. Considering the technical difficulties associated with ESD, we should select the treatment method according to tumor characteristics identified by pre-treatment examination including magnifying endoscopy or endoscopic ultrasound. Moreover, the technical difficulty encountered depends on the macroscopic type of the lesions; treatment of the LST-NG type lesion or local recurrence is generally more difficult technically than that of the LST-G type[23,24].
P- Reviewer: Naito Y, Trecca A S- Editor: Ma YJ L- Editor: A E- Editor: Wang CH
| 1. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Fu KI, Sano Y, Saito D. Endoscopic treatment of large superficial colorectal tumors: a case series of 200 endoscopic submucosal dissections (with video). Gastrointest Endosc. 2007;66:966-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 282] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 2. | Fujishiro M, Yahagi N, Kakushima N, Kodashima S, Muraki Y, Ono S, Yamamichi N, Tateishi A, Oka M, Ogura K. Outcomes of endoscopic submucosal dissection for colorectal epithelial neoplasms in 200 consecutive cases. Clin Gastroenterol Hepatol. 2007;5:678-683; quiz 645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 272] [Cited by in RCA: 275] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 3. | Tanaka S, Oka S, Kaneko I, Hirata M, Mouri R, Kanao H, Yoshida S, Chayama K. Endoscopic submucosal dissection for colorectal neoplasia: possibility of standardization. Gastrointest Endosc. 2007;66:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 349] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 4. | Saito Y, Uraoka T, Yamaguchi Y, Hotta K, Sakamoto N, Ikematsu H, Fukuzawa M, Kobayashi N, Nasu J, Michida T. A prospective, multicenter study of 1111 colorectal endoscopic submucosal dissections (with video). Gastrointest Endosc. 2010;72:1217-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 591] [Cited by in RCA: 591] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 5. | Soetikno RM, Inoue H, Chang KJ. Endoscopic mucosal resection. Current concepts. Gastrointest Endosc Clin N Am. 2000;10:595-617, vi. [PubMed] |
| 6. | Tanaka S, Haruma K, Oka S, Takahashi R, Kunihiro M, Kitadai Y, Yoshihara M, Shimamoto F, Chayama K. Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc. 2001;54:62-66. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 232] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 7. | Tamura S, Nakajo K, Yokoyama Y, Ohkawauchi K, Yamada T, Higashidani Y, Miyamoto T, Ueta H, Onishi S. Evaluation of endoscopic mucosal resection for laterally spreading rectal tumors. Endoscopy. 2004;36:306-312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Watanabe T, Itabashi M, Shimada Y, Tanaka S, Ito Y, Ajioka Y, Hamaguchi T, Hyodo I, Igarashi M, Ishida H, Ishiguro M, Kanemitsu Y, Kokudo N, Muro K, Ochiai A, Oguchi M, Ohkura Y, Saito Y, Sakai Y, Ueno H, Yoshino T, Fujimori T, Koinuma N, Morita T, Nishimura G, Sakata Y, Takahashi K, Takiuchi H, Tsuruta O, Yamaguchi T, Yoshida M, Yamaguchi N, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2010 for the treatment of colorectal cancer. Int J Clin Oncol. 2012;17:1-29. [PubMed] |
| 9. | Matsuda T, Fujii T, Saito Y, Nakajima T, Uraoka T, Kobayashi N, Ikehara H, Ikematsu H, Fu KI, Emura F. Efficacy of the invasive/non-invasive pattern by magnifying chromoendoscopy to estimate the depth of invasion of early colorectal neoplasms. Am J Gastroenterol. 2008;103:2700-2706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 259] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 10. | Ohya T, Ohata K, Sumiyama K, Tsuji Y, Koba I, Matsuhashi N, Tajiri H. Balloon overtube-guided colorectal endoscopic submucosal dissection. World J Gastroenterol. 2009;15:6086-6090. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 29] [Cited by in RCA: 35] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Sano Y, Fu KI, Saito Y, Doi T, Hanafusa M, Fujii S, Fujimori T, Ohtsu A. A newly developed bipolar-current needle-knife for endoscopic submucosal dissection of large colorectal tumors. Endoscopy. 2006;38 Suppl 2:E95. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Nonaka S, Saito Y, Fukunaga S, Sakamoto T, Nakajima T, Matsuda T. Impact of endoscopic submucosal dissection knife on risk of perforation with an animal model-monopolar needle knife and with a bipolar needle knife. Dig Endosc. 2012;24:381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Uraoka T, Fujii T, Saito Y, Sumiyoshi T, Emura F, Bhandari P, Matsuda T, Fu KI, Saito D. Effectiveness of glycerol as a submucosal injection for EMR. Gastrointest Endosc. 2005;61:736-740. [PubMed] |
| 14. | Yamamoto H, Kawata H, Sunada K, Sasaki A, Nakazawa K, Miyata T, Sekine Y, Yano T, Satoh K, Ido K. Successful en-bloc resection of large superficial tumors in the stomach and colon using sodium hyaluronate and small-caliber-tip transparent hood. Endoscopy. 2003;35:690-694. [PubMed] |
| 15. | Saito Y, Uraoka T, Matsuda T, Emura F, Ikehara H, Mashimo Y, Kikuchi T, Kozu T, Saito D. A pilot study to assess the safety and efficacy of carbon dioxide insufflation during colorectal endoscopic submucosal dissection with the patient under conscious sedation. Gastrointest Endosc. 2007;65:537-542. [PubMed] |
| 16. | Kikuchi T, Fu KI, Saito Y, Uraoka T, Fukuzawa M, Fukunaga S, Sakamoto T, Nakajima T, Matsuda T. Transcutaneous monitoring of partial pressure of carbon dioxide during endoscopic submucosal dissection of early colorectal neoplasia with carbon dioxide insufflation: a prospective study. Surg Endosc. 2010;24:2231-2235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Nakajima T, Saito Y, Tanaka S, Iishi H, Kudo SE, Ikematsu H, Igarashi M, Saitoh Y, Inoue Y, Kobayashi K. Current status of endoscopic resection strategy for large, early colorectal neoplasia in Japan. Surg Endosc. 2013;27:3262-3270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 182] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 18. | Sakamoto T, Saito Y, Fukunaga S, Nakajima T, Matsuda T. Learning curve associated with colorectal endoscopic submucosal dissection for endoscopists experienced in gastric endoscopic submucosal dissection. Dis Colon Rectum. 2011;54:1307-1312. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 19. | Sakamoto T, Sato C, Makazu M, Sekiguchi M, Mori G, Yamada M, Kinjo Y, Turuki E, Abe S, Otake Y. Short-term outcomes of colorectal endoscopic submucosal dissection performed by trainees. Digestion. 2014;89:37-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Hirao M, Masuda K, Nakamura M. [Endoscopic resection with local injection of HSE (ERHSE) in early gastric carcinomas]. Gan No Rinsho. 1986;32:1180-1184. [PubMed] |
| 21. | Terasaki M, Tanaka S, Oka S, Nakadoi K, Takata S, Kanao H, Yoshida S, Chayama K. Clinical outcomes of endoscopic submucosal dissection and endoscopic mucosal resection for laterally spreading tumors larger than 20 mm. J Gastroenterol Hepatol. 2012;27:734-740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 22. | Sakamoto T, Matsuda T, Nakajima T, Saito Y. Efficacy of endoscopic mucosal resection with circumferential incision for patients with large colorectal tumors. Clin Gastroenterol Hepatol. 2012;10:22-26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Toyonaga T, Man-i M, Fujita T, East JE, Nishino E, Ono W, Morita Y, Sanuki T, Yoshida M, Kutsumi H. Retrospective study of technical aspects and complications of endoscopic submucosal dissection for laterally spreading tumors of the colorectum. Endoscopy. 2010;42:714-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 109] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 24. | Sakamoto T, Saito Y, Matsuda T, Fukunaga S, Nakajima T, Fujii T. Treatment strategy for recurrent or residual colorectal tumors after endoscopic resection. Surg Endosc. 2011;25:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 61] [Article Influence: 4.1] [Reference Citation Analysis (0)] |