INTRODUCTION
Hepatocellular carcinoma (HCC) is the sixth most common type of cancer and with about 9.2% of all deaths it is the third most common cause of cancer death[1]. HCC is more common in males than in females and mostly occurs in developing countries. The increasing trend is mainly due to a cohort effect related to infection with hepatitis B and C viruses (HBV and HCV), the incidence of which peaked in the 1950s to 1980s. By contrast, in North America, Europe, and Japan, HCV infection is the main risk factor, together with alcohol use. Time trends in incidence of hepatocellular carcinoma of developed countries parallel the timing of HCV spread. In Japan and Europe, where HCV infection spread earlier than in the United States, the incidence of hepatocellular carcinoma has almost reached a plateau and in some areas it is declining; however, in the United States, incidence is still increasing and the infection could have a synergistic effect with other risk factors, such as non-alcoholic fatty liver disease.
In most cases, HCC is a multistage disease whose occurrence is linked to environmental, dietary and life-style factors[2]. Unlike other cancers, HCC usually arises on a previously damaged organ, mostly in the setting of chronic hepatopathy, cirrhosis[3,4], or in association with hereditary diseases such as hemochromatosis, Wilson’s disease and a-1-antitrypsin deficiency. However, in about 15%-20% of cases HCC may occur in the non-fibrotic liver or in livers with minimal portal fibrosis without any septal fibrosis[5].
Although histopathologic criteria for diagnosing classical, progressed hepatocellular carcinoma have not recently changed, diagnosis of small and early lesions has gained of importance due to the increased detection rate of these early lesions in routine screening programs. These lesions can be far more difficult to distinguish from one another than progressed HCC. Furthermore lesions thought to derive from progenitor cells have recently been reclassified in the WHO.
PRECURSOR LESIONS
HCC
Although a typical adenoma-carcinoma sequence does not represent a frequent pathway in hepatic carcinogenesis, hepatocellular adenoma (HCA) may rarely act as a precursor lesion of HCC[4]. It occurs predominantly in female patients using oral contraceptives, but has also been described female patients with maturity onset diabetes of the young type 3[6]. In males it is described in patients with glycogen storage disease or androgen treatment. This type is usually beta-catenin mutated and is reported to have a higher risk of malignant transformation[7]. Metabolic syndrome has recently also been described as an emerging risk factor for HCA[6]. Obesity and alcohol mostly lead to an inflammatory HCA, which express proteins such as Amyloid A and CRP. Generally, malignant transformation has been described in 4%-8% of patients. In some cases no etiology for the development of a hepatocellular adenoma can be determined. The differential diagnosis between HCA and well-differentiated HCC arising in non-cirrhotic liver can be challenging, especially when tumors histologically similar to HCA occur in unusual clinico-pathological settings, such as in a man or an older woman and/or display cytological or architectural atypia[8].
Dysplastic foci
Dysplastic foci are uniform lesions and their morphology, cytoplasmatic staining, nuclear size and cellular atypia discriminates them from the surrounding liver tissue. By definition, these clusters are < 1 mm in size and do not fulfill criteria for malignancy i.e. invasive growth[9]. A similar and hard to distinguish lesion is small cell dysplasia. These are round foci of small dysplastic cells with an increased nuclear/cytoplasmatic ratio. They are usually seen in cirrhotic livers and are also considered premalignant lesions due to their increased proliferation index and low rate of apoptosis[10].
Dysplastic nodules
In contrast to dysplastic foci, dysplastic nodules are defined as being larger than 1mm in size. These lesions are usually found in cirrhosis and are generally subdivided into low-grade and high-grade lesions. Both subtypes have been described as possible progenitor lesions to HCC but regression has also been described in literature. Low-grade dysplastic nodules only present minimal abnormality with a normal to slightly increased nuclear/cytoplasmatic ratio, minimal atypia, no mitoses and one to two cells wide cell plates. Portal tracts and the reticulin network are still present. The borders of the lesions can be rounded but usually do not show compression of the adjacent liver tissue. In addition, high-grade dysplastic nodules may display the following histological features: increased nuclear/cytoplasmatic ratio, nuclear hyperchromasia, irregular nuclear borders, peripheral location of the nucleus, occasional mitoses, cell plates > 2 cells, basophilic cytoplasm, pseudo gland formation and resistance to iron accumulation. Occasional unpaired arteries have also been described in high-grade dysplastic nodules[9,11,12].
GROSS PATHOLOGY
Up until only two decades ago, it was unknown how HCC arose and how it evolved from early to advanced cancer in humans. With the remarkable advances of various diagnostic imaging techniques and the establishment of a follow-up system for the high-risk population, the numbers of surgically resected cases of early stage small HCCs and the amount of biopsy material form minute HCCs has increased. Extensive morphologic studies of this material have revealed that many HCCs arise in equivocal nodular lesions, such as dysplastic nodules in the cirrhotic liver and are highly differentiated in the early stages. At the same time, it has been established that well-differentiated HCC in the early stages evolves to advanced and dedifferentiated tumor in a multistep fashion[13,14]. This is particularly true for patients with chronic HBV and HCV infections.
An additional characteristic feature of HCC is its frequent occurrence in the form of multiple nodules[15]. In HCC, the simultaneous occurrence of multiple HCCs may reflect either the dissemination of malignant cells from a single primary tumor to form satellite tumor nodules (intrahepatic metastasis), or the synchronous development of several independent tumors. The two possible mechanisms of development of multiple HCCs reflect important differences in pathogenesis that appear to have an impact on treatment and prognosis[16]. Differences in prognosis between these two categories probably result from the fact that multiple HCCs developing from intrahepatic metastasis are more aggressive and more poorly differentiated than multiple HCCs that are composed of several independent tumours that emerge more or less simultaneously. Molecular analysis of the HBV integration patterns and genetic changes has indicated independent multicentric development of these nodules[15,17-20].
During histological assessment, small and early HCCs should be distinguished from advanced disease. While the diagnosis is mostly clear in advanced stage cases, small, early and therefore mostly well-differentiated lesions can be problematic. Due to established screening programs in cirrhotic patients, biopsies of these lesions problematic lesions have increased in number.
The international consensus group for hepatocellular carcinoma[21] and the WHO[22] propose the following classification: (1) early HCC: a: well differentiated; b: small size (< 2 cm); and c: poorly defined margins, vaguely nodular type; and (2) progressed HCC: a: > 2 cm; b: small size (< 2 cm), but moderately differentiated, distinctly nodular type.
HCCs of the vaguely nodular type occur more often in cirrhosis, are usually smaller in size and less often show portal vein invasion than the distinctly nodular type. Furthermore they are hypovascular and almost never show intrahepatic metastasis. In resected specimens, they are sometimes difficult to localize, because their margins to the surrounding liver tissue are not well defined and portal tracts are retained within the tumor[11]. The distinctly nodular subtype has a discernible capsule and usually occurs in a cirrhotic liver.
Progressed hepatocellular carcinoma can grossly be classified into the following macroscopic groups: nodular, massive and diffuse. The nodular type can either consist of a single or multiple nodules. Single nodules are usually encapsulated and may show extracapsular growth in the vicinity of the primary nodule. The multinodular type is an aggregation of a varying amount of small nodules. The massive type is defined as a large tumor with irregular demarcation. This morphologic appearance can also been seen in advanced stage nodular HCC. The diffuse type is described to have many small nodules in a liver lobe or the whole organ[11].
Rarely a pedunculated or protruded growth can be observed. If the HCC grows extrahepatically with a peduncle, this should be termed “pedunculated”. If a peduncle is absent the term “protruding” is adequate[11].
HISTOPATHOLOGY
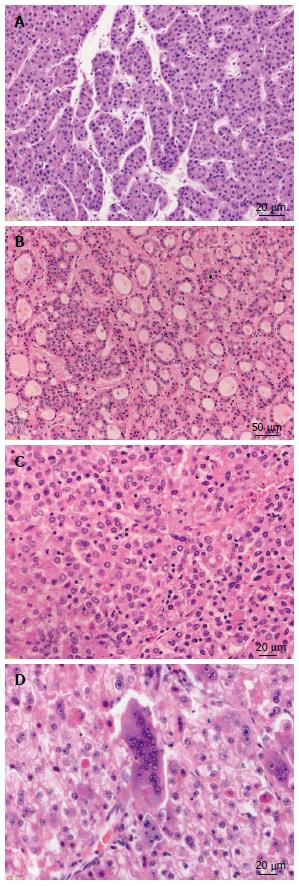
The classical histomorphologic features of HCC are the following: well vascularized tumors with wide trabeculae (> 3 cells), prominent acinar pattern, small cell changes, cytologic atypia, mitotic activity, vascular invasion, absence of Kupffer cells and the loss of the reticulin network[23]. The most common histologic growth patterns are: trabecular-resembling normal liver tissue, pseudoglandular or acinar with possible bile or fibrin content and the compact or solid pattern (Figure 1). Bile production can frequently be observed. Within the tumor cells Mallory-bodies and pale bodies can be also present[22].
Figure 1 Growth patterns of progressed hepatocellular carcinoma.
A: Hepatocellular carcinoma (HCC) with trabecular growth pattern [hematoxylin and eosin (HE), × 300]; B: HCC with pseudoglandular growth pattern (HE, × 100); C: HCC with solid growth pattern (HE, × 200); D: HCC with giant cell formation (HE, × 200).
Histomorphologic appearance of hepatocellular carcinoma varies greatly from patient to patient and even in a single patient, different stages of intratumoral differentiation and growths patterns can be observed. Some authors postulate a step-wise dedifferentiation of an initially well differentiated small lesion into a larger, less differentiated tumor which leads to intratumoral heterogeneity. The well differentiated lesion is usually replaced by tissue of the dedifferentiated component in advanced disease and therefore leads to a nodule in nodule appearance. Especially in very advanced stage, but sometimes also in small tumors, the initial well-differentiated tumor component may not be discernible[13,14,24,25].
In contrast, progressed HCC shows an expansive and infiltrative histologic growth pattern with complete neovascularization with unpaired arteries and possible vascular infiltration. There are no portal tracts seen within the tumor and all the classical histologic patterns (i.e., trabecular/sinusoidal, pseudoglandular, solid and undifferentiated) are usually present (Figure 1). The tumors are mostly encapsulated and septae are detected. Encapsulation is reported to be more frequent in tumor arising in a cirrhotic liver than in non-cirrhotic livers[11]. Most of these tumors show satellite nodules within 2 cm of the primary tumor nodule as well as metastasis in the liver.
In HCC, angioarchitecture plays a very important role during tumor growth and is also essential part of modern imaging modalities. Progressed HCC has classical unpaired arteries positive for SMA and CD34. These arteries are not associated with a portal tract and therefore have no contact to bile ducts. They also show less elastic fibers compared to normal intrahepatic arteries. Early HCC of the vaguely nodular type has a reduced density of unpaired arteries compared to progressed HCC and therefore appears hypovascular in imaging. Due to the presence of portal tracts within tumor, although less in number than in normal liver tissue, these lesions also receive blood from the portal vein[26,27]. Early HCC of the distinctly nodular type as well as progressed-HCC appear hypervascular because of earlier neovascularization with unpaired arteries.
HISTOLOGIC VARIANTS
Fibrolamellar HCC
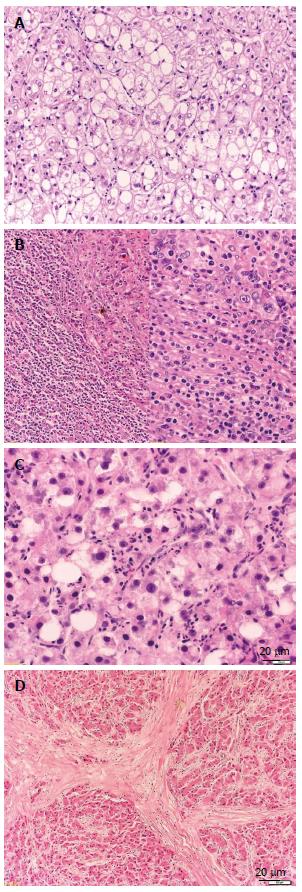
Fibrolamellar HCC is a rare subtype first described by Edmondson accounting for less than 1% of all tumors[31]. This subtype is seen in young patients without liver cirrhosis and with no other known predisposing factors and has a better prognosis than classical HCC[32,33]. Grossly it shows many fibrous septae and may have a central scared zone with possible calcification, therefore mimicking focal nodular hyperplasia (Figure 2D). Histologically the tumor cells grow in sheets and trabeculae that are separated by collagen fibers which are often hyalinized and show a characteristic lamellar pattern[11]. Cytoplasmatic inclusions such as ground glass pale bodies and cytoplasmatic globules which are PAS-positive and immunoreactive to anti fibrinogen are often seen. Van Eyken and colleagues report an abundant CK7 and a focal CK19 expression in this subtype[34].
Figure 2 Histologic variants of hepatocellular carcinoma.
A: Hepatocellular carcinoma (HCC), clear cell variant [hematoxylin and eosin (HE), × 100]; B: HCC with lymphoid stroma (HE, × 100/× 200). C: HCC, steatohepatic variant (HE, × 200); D: HCC, fibrolamellar variant (HE, × 50).
Sarcomatous HCC
Sarcomatous HCC is another subtype which can occur by itself, but also within classical HCC. The tumor cells are spindle-shaped and show bizarre anaplastic figures. Giant cells are often present, but they can also been seen in other types of HCC. In cases where there is no adjacent classical HCC, these tumor can be difficult to distinguish from leiomyosarcoma and fibrosarcoma[35,36].
Scirrhous HCC
Scirrhous HCC shows diffuse fibrotic change which can occur after various antitumoral treatments and seldom in untreated tumors. These fibrotic changes often lead to misdiagnosis as cholangiocellular carcinoma or metastasis in preoperative imaging. This type of tumor histologically shows fibrosis along the sinusoid-like blood spaces, with atrophy of the trabeculae. In immunohistochemistry HePar-1 and CK7 (65%) can be positive[37]. Kojiro[11] reports a marked CD8+ predominant lymphocyte infiltrate in 84% of the cases. In his series he also describes a unique directly subcapsular location of most of these tumors which leads to a possible pedunculated macroscopy.
Clear-cell variant of HCC
The clear-cell variant of HCC is usually arranged in a trabecular pattern and is characterized by clear cytoplasm that contains glycogen and a variable amount of fat vesicles[38]. Mostly only parts of the tumor show these clear-cell changes (Figure 2A). A male predominance of variable degree is reported of this particular subtype of HCC[11,39].
Steatohepatic HCC
Steatohepatic HCC is characterized by a steatotic appearance of > 5% of the tumor, presence of Mallory-bodies, fibrosis, inflammation and ballooning of the hepatocytes as in steatohepatitis (Figure 2C). The inflammatory infiltrate usually consists of neutrophils, plasma cells and lymphocytes. Fibrosis usually appears in a pericellular and trabecular form. These patients often suffer from non-alcoholic steatohepatitis but this phenotype of carcinoma is also seen in patients without steatohepatitic changes in the non-neoplastic liver tissue[40].
HCC with lymphoid stroma
HCC with lymphoid stroma is an entity only described in a few case reports. This is a tumor consisting of a massive inflammatory infiltrate, often with very few identifiable tumor cells in HE stain (Figure 2B). Most of the cells are lymphocytes, some macrophages, giant cells, plasma cells and neutrophils can also be detected. Immunohistochemical subtyping of the lymphoid cells show more CD3+ T-Cells than CD20+ B-Cells. The T-Cells are mostly of the CD4+ subtype[41,42]. It is still unclear if this is a regression phenomenon or an entity by its own.
IMMUNOHISTOCHEMISTRY AND DIFFERENTIAL DIAGNOSIS
Especially in early stage and therefore mostly well differentiated hepatocellular carcinoma immunohistochemistry can be helpful for distinguishing adenoma, focal nodular hyperplasia and dysplastic nodules from HCC[23].
Adenoma vs well-differentiated HCC
In conventional histology hepatocellular adenoma resembles normal liver tissue but may show pseudoglandular architecture, especially when arising in a patient with anabolic use. The tumor cells often have an increased cell size due to increased intracellular glycogen or fat accumulation but show regular nuclei without atypia. The cell sheets are 2 layers thick. HCC arising from transformed adenoma is usually well- to moderately-differentiated and only few cases showed vascular invasion or satellite nodules[43].
Beta-catenin mutations are reported in 20% of all HCCs, with a higher rate in HCC developing from livers with chronic hepatitis C[44]. Hepatocellular adenoma can also be beta-catenin activated in 10%-15% of the cases. The beta-catenin mutation leads to a series of upregulated genes which are important for cell proliferation. One of these genes is glutamine-synthetase, which is therefore diffusely positive in beta-catenin mutated tumors. Normal liver tissue shows glutamine-synthetase expression in the pericentral hepatocytes but not in periportal or midzonal localized hepatocytes. In hepatic adenoma, glutamine-synthetase can either be completely negative, localized in the pericentral area as in normal liver, or show patch-like expression with no particular pattern. In beta-catenin mutated hepatic adenoma diffuse and strong expression is seen[45].
Glypican-3 is a proteoglycan which is a histochemical and serological marker for HCC. In normal liver it is not expressed but it occurs in fetal liver and the placenta. Glypican-3 has been reported in 70%-90% of all HCCs[46-49].
High-grade dysplastic nodule vs well-differentiated HCC
Although these criteria are still controversial, in cases where cell plates are thick (> 3 cells), there is a focal pseudoglandular growth pattern and a loss of the reticulin network, HCC can possibly be diagnosed with conventional histology. Unfortunately not all HCCs show these patterns and high-grade dysplastic nodules can mimic similar histological features[12].
High-grade dysplastic nodules can either be of the distinctly or vaguely nodular type in a cirrhotic liver. These lesions show atypia which is insufficient for the diagnosis of HCC, usually have an increased cell density compared to the surrounding liver tissue and show an irregular trabecular pattern. The most common form of cytological atypia is small cell change/dysplasia characterized by small cells with decreased cytoplasma and moderately enlarged nuclei[50]. Unpaired arteries have also been described in these lesions. The best criterion to differentiate these lesions from early HCC is stromal invasion, but this can be very difficult to assess, especially in biopsy specimens. In a cirrhotic liver, this feature can be difficult to distinguish from small groups of hepatocytes entrapped within fibrous septae. In these cases, a CK7/19 stain can be helpful to highlight the ductular reaction around these foci, since these non-neoplastic intraseptal hepatocytes and the adjacent ductular reaction in dysplastic nodules are thought to derive from a common progenitor cell. This ductular reaction should be present around 50% of the circumference of a nodule and is mostly absent in HCC[21,51-53].
Furthermore high-grade dysplastic nodules may show peripheral and focal staining for CD34, whereas diffuse sinusoidal staining is typically seen in nodular HCC. Some authors suggest using CD34 combined with Glypican-3 to achieve a higher sensitivity. Numbers on Glypican-3 expression in dysplastic nodules vary widely (3%-76%). CD34 in combination with SMA also marks unpaired muscularised arteries. Unfortunately neovascularization is rarely complete in early HCC[46,54,55].
Gluthamine-synthetase (GS) expression is seen more frequently in HCC than in high-grade dysplastic nodules (69.8% vs 13.6%). In these positive dysplastic nodules GS was expressed in less than 50% of the cells whereas in HCC it was reported positive in over 50% of the cells[56].
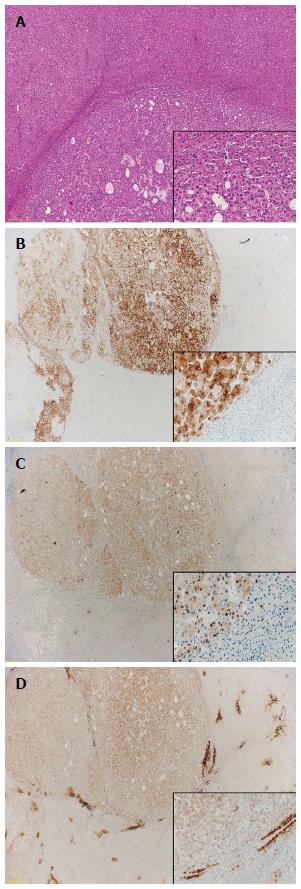
In addition to Glypican-3 and GS, Di Tommaso and colleagues also reported heat shock protein 70 (HSP70) to be positive in 73.5% of HCCs while only a single case of dysplastic nodule was positive. They therefore suggest using the combination of HSP70, GS and Glypican-3 for discriminating HCC from high-grade dysplastic nodule. When using these three markers they could achieve a sensitivity of 72% and a specificity of 100% for the detection of malignancy with a cut-off of any two positive markers in resection specimens (Figure 3). In biopsy specimens the markers are less sensitive due to unequal distribution of positive cells. It is therefore recommended to use clathrin heavy chain as an additional marker in small biopsy specimens to increase sensitivity[57,58].
Figure 3 Immunohistochemistry.
A: Well-differentiated hepatocellular carcinoma (HCC) [hematoxylin and eosin (HE), × 20]; B: Glypican-3 (HE, × 10/× 200); C: Heat-shock-protein 70 (HE, × 10/× 200); D: Gluthamine-synthetase (HE, × 10/× 200).
In conclusion the loss of the reticulin fiberwork, stromal invasion and a positivity of at least 2 out of 3 markers (HSP70, GS, Glypican 3) are considered the strongest parameters in the diagnosis of early HCC in resection specimens[55].
Molecular analysis can be useful for proving clonality of a lesion, but since well differentiated HCC and high-grade dysplastic nodules can both have monoclonal growth patterns this analysis is not particularly helpful in this differential diagnosis[11].
HCC vs metastasis
The differential diagnosis of HCC varies greatly depending on the underlying liver disease. In hepatitic/cirrhotic livers, HCC, its precursor lesions and cholangiocellular carcinoma occur more frequently than malignant lesions of non-hepatic origin. In patients without underlying liver disease, HCC accounts only for about 2% of malignant liver neoplasms. The most common primary sites that metastasize into the liver are lung, colon, pancreas and breast[59]. In some cases metastasis can mimic hepatocellular carcinoma. The primary tumors resembling HCC include clear-cell renal cell carcinoma, clear-cell adenocarcinoma of the female genital organs, adrenal carcinoma and hepatoid adenocarcinoma of the stomach. Sometimes metastatic neuroendocrine tumors of the gastrointestinal tract, especially with trabecular growth pattern can also be difficult to distinguish from HCC.
In cases where the hepatic origin of a tumor is unclear, polyclonal CEA (pCEA) or Hepatocyte Paraffin 1 antigen (Hep Par-1) can be useful to prove the hepatic origin while the latter is considered the most sensitive and specific marker. These markers are also positive in normal liver tissue and Hep Par-1 positivity has also been described in adenocarcinoma of the lung, pancreas, stomach, esophagus, gall bladder, small intestine, adrenal gland, melanoma, paraganglioma and the urinary bladder. Hep Par-1 shows a cytoplasmatic granular positivity in almost all well-differentiated HCCs, but this number diminishes to < 50% in poorly differentiated HCC. pCEA shows diffuse cytoplasmatic expression in most adenocarcinomas, but in HCC there is a distinct “chicken-wire fence” pattern around the canaliculi. As with Hep Par-1, the sensitivity and specificity of pCEA decline with dedifferentiation of the tumor[11,59,60]. In the adult, alpha-fetoprotein (AFP) is positive in HCC and germ cell tumors while normal liver tissue does not stain for AFP. In contrast to the previous two markers, the percentage of AFP-positive tumor cells increases with dedifferentiation. In HCC CD10 shows a similar staining pattern as pCEA, but is negative in adenocarcinomas. MOC-31 is a marker which is expressed in glandular epithelia of various sites, but is negative in HCC, melanoma and lymphoma. HCC is usually positive for CK8 and CK18 and negative for CK7, CK19 and CK20 in about 70% of the cases[59].
Combined hepatocellular-cholangiocarcinoma
Less than 1% of all liver carcinomas are combined hepatocellular-cholangiocarcinoma. In contrast to classical HCC which is thought to develop from mature hepatocytes in a multistep carcinogenesis, recent studies suggest a common progenitor cell capable of differentiating into hepatocytes and cholangiocytes that is activated when liver damage occurs and therefore leads to various types of combined carcinomas[61,62]. These combined carcinomas usually show a staining for CK7/19 although this has also been described in classical HCC. The expression of these markers can either be interpreted as transdifferentiation into cholangiocytes or dedifferentiation in a common progenitor cell[63]. The WHO subclassifies these carcinomas into the classical subtype and the subtype with stem cell features. The latter is the case when the stem cell features predominate and is then subdivided into the typical, intermediate and cholangiolocellular subtype[22].
In the classical subtype the hepatocellular and biliary component have the typical morphology of HCC and biliary adenocarcinoma respectively and may have any differentiation. The hepatocellular component can be highlighted with a Hep Par-1, CD10 or pCEA staining, the biliary component with a D-PAS or mucicarmine stain to show mucin production and immunohistochemically with CK7/19 and epithelial membrane antigen (EMA). A low expression of Hep Par-1, CD56, c-KIT and epithelial cell adhesion molecule (EpCAM) has also been reported. CD133 and vimentin are usually negative. The biliary component often shows abundant desmoplastic stroma. The hepatocellular component may also stain positive for CK7/19. In the zone where these components intersect, cells often have phenotypical and/or immunohistochemical properties of stem/-progenitor cells[64-67].
Since it remains unclear if the subtypes with stem-cell features are all distinctive entities, they are, for the time, according to the WHO, subsumed under the term “combined hepatocellular-cholangiocarcinoma with stem cell features”. In contrast to the classical type, the following subtypes are not mucin-producing. While Hep Par-1 expression is less frequent in these subtypes with stem cell features than in the classical subtype, expression of CD133, EpCAM and vimentin is significantly higher[67]. These subtypes are often intermixed but their major components are defined as follows:
The typical subtype shows mature hepatocytes with peripheral aggregates of small cells with a high nuclear/cytoplasmatic ratio and hyperchromatic nuclei. These peripheral cells are positive for CK7/19, EMA, CD56, c-KIT and EpCAM and don’t show any mucin production. Adjacent to these cells abundant stroma can be observed.
The intermediate cell subtype is composed of cells with intermediate features of both hepatocytes and cholangiocytes. The cells are gathered in stands, solid nests or trabeculae. The cells are small and have scant cytoplasm with an increased nuclear/cytoplasmatic ratio and are usually embedded within less stroma than the typical subtype. Expression of CK7/19 and EMA is frequent, while positivity for HePar-1, CD56, CD133, EpCAM is observed less often.
The cholangiolocellular subtype, previously designated “cholangiolocellular carcinoma”, shows small monotonous glands with antler-like intersection patterns. The tumor cells are cuboidal, embedded in thick stroma, but smaller in size than non-neoplastic hepatocytes with a high nuclear/cytoplasmatic ratio and with pronounced nuclei. Biliary markers (CD7/19, EMA) are expressed, while Hep Par-1 is negative. This subtype is reported to have a significantly higher CK19 expression compared to the classical subtype. Furthermore an increased expression of CD133, EpCAM and vimentin over the classical and intermediate cell subtypes has also been observed[67].
In addition to these WHO listed subtypes, Terada[68] suggest another subtype with features of ductal plate malformations.