Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15925
Revised: May 4, 2014
Accepted: June 26, 2014
Published online: November 14, 2014
Processing time: 243 Days and 23.1 Hours
We report a case of intraductal papillary neoplasm of the bile duct (IPNB) that developed in a patient with primary sclerosing cholangitis. A 46-year-old woman was admitted to our hospital with obstructive jaundice. The liver function tests demonstrated increased serum liver enzyme levels. Computed tomography showed dilatation of the intrahepatic bile ducts. Abdominal ultrasonography revealed a highly echoic protruding lesion in the posterior bile duct near the right lobe of the liver. The lesion was suspected to be IPNB, but we were unable to confirm whether it was a carcinoma. A right hepatectomy was performed, and this showed that the dilated bile duct was filled with mucin and contained several yellowish papillary tumors. Histologically, the neoplastic biliary epithelium showed papillary growth in the dilated lumen. The tumor was diagnosed as IPNB, high-grade intraepithelial neoplasia secreting abundant mucin. No recurrence has been detected 3 years after surgery.
Core tip: Intraductal papillary neoplasm of the bile duct (IPNB) is a variant of bile duct carcinoma that is characterized by grossly visible lesions and better outcomes compared with cholangiocarcinoma (CCA). Primary sclerosing cholangitis (PSC) is considered to be a risk factor for CCA. We report a 46-year-old woman who presented with dilatation of the intrahepatic bile ducts and mucin in the common bile duct. This is the first case of PSC in which IPNB developed in the setting of high-grade intraepithelial neoplasia.
- Citation: Hachiya H, Kita J, Shiraki T, Iso Y, Shimoda M, Kubota K. Intraductal papillary neoplasm of the bile duct developing in a patient with primary sclerosing cholangitis: A case report. World J Gastroenterol 2014; 20(42): 15925-15930
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15925.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15925
According to the 2010 World Health Organization (WHO) classification of tumors, there are two types of precancerous/early cancer of the bile duct. The first type is characterized by flat or micropapillary growth of atypical biliary epithelium and is known as biliary intraepithelial neoplasm. The other type is intraductal papillary neoplasm of the bile duct (IPNB) with malignant potential, which is characterized histologically by prominent papillary growth of atypical biliary epithelium.
IPNB is characteristic of producing abundant mucin, and clinically, it can cause biliary dilation, obstructive jaundice, and cholangitis. Furthermore, patients with Primary sclerosing cholangitis (PSC) are known to be at increased risk of developing cholangiocarcinoma (CCA). Recent studies have demonstrated that CCA may arise from premalignant lesions such as IPNB[1]. Here, we describe a case of IPNB developing in a patient with PSC.
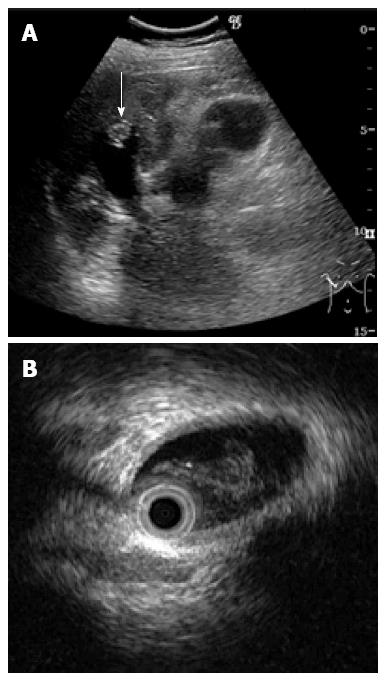
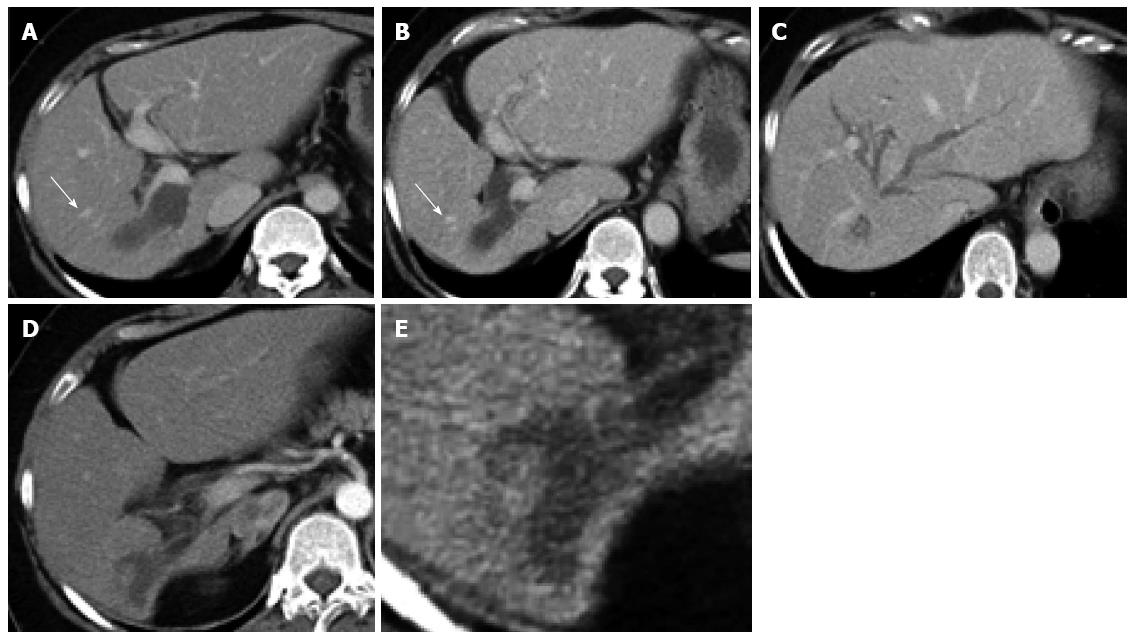
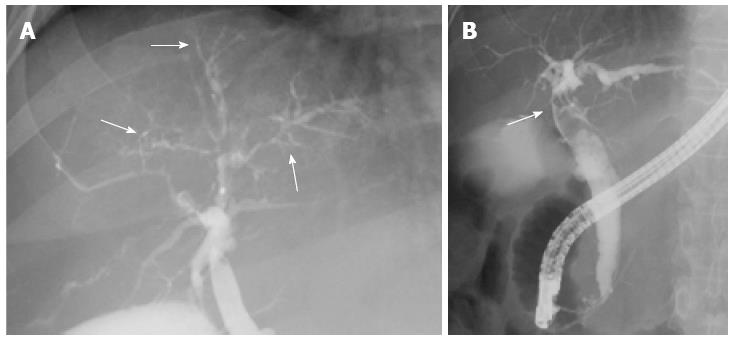
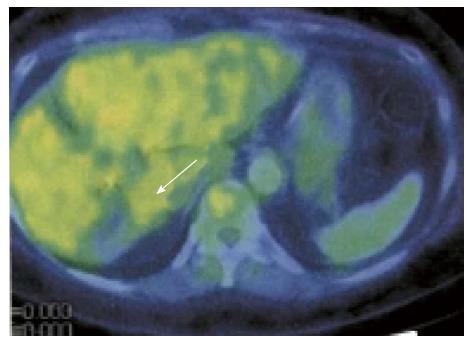
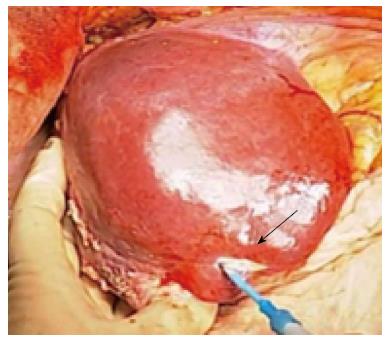
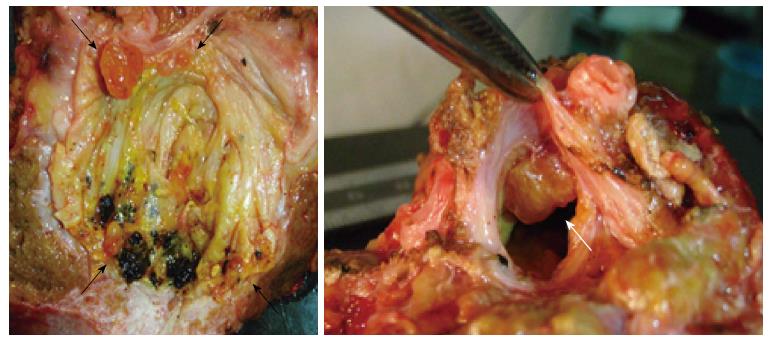
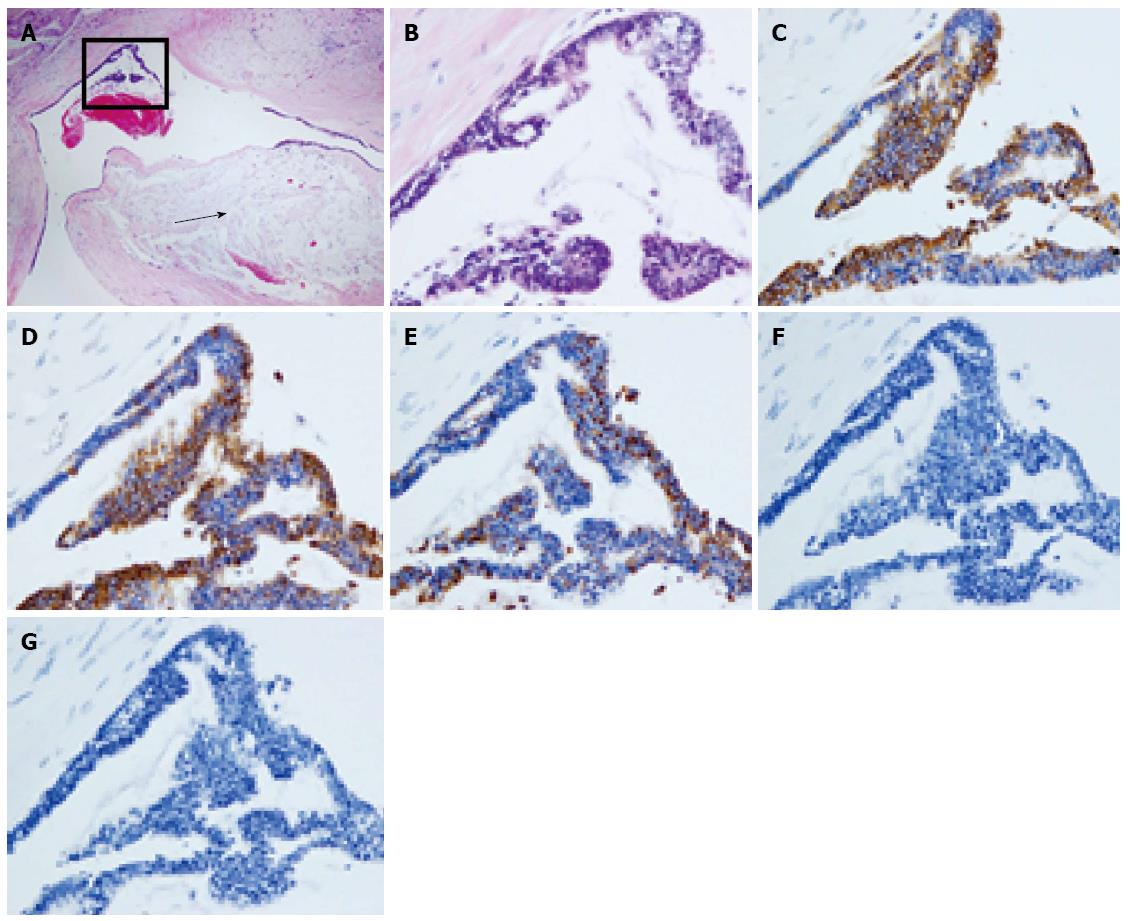
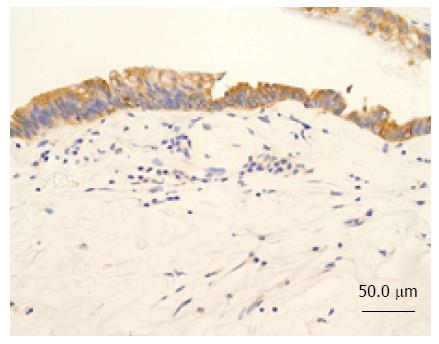
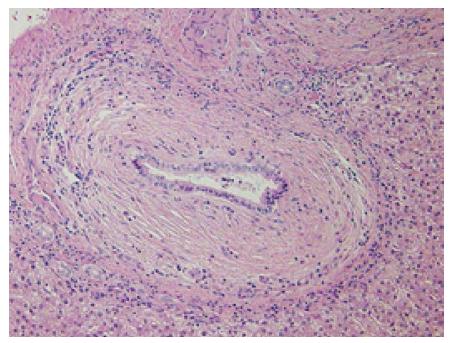
A 46-year-old woman with an 8-year history of Behçet’s disease was hospitalized with obstructive jaundice. On admission, the liver function tests demonstrated increased serum liver enzyme levels: aspartate aminotransferase, 104 U/I; alanine aminotransferase, 101 U/I; alkaline phosphatase, 929 U/I; γ-glutamyl transpeptidase, 667 U/I; and total bilirubin, 2.2 mg/dL. The levels of tumor markers, such as α-fetoprotein, CA 19-9, and carcinoembryonic antigen, were normal. Antibodies against hepatitis C virus and hepatitis B surface antigen were negative. The indocyanine green clearance rate at 15 min was 5.4%. Colonoscopy revealed inflammatory bowel disease unclassified (IBDu). Abdominal ultrasonography revealed a highly echoic protruding lesion in a dilated posterior bile duct of the right lobe (Figure 1A). Intraductal ultrasonography demonstrated low-echoic mucin in the common bile duct (Figure 1B). CT showed dilatation of the intrahepatic bile ducts of the bilateral lobes and a papillary lesion in the posterior segment, which was poorly enhanced with contrast. The right lobe atrophied from 410 g to 230 g (expected liver weight) over 18 mo, and the left lobe became enlarged (Figure 2). Endoscopic retrograde cholangiography (ERC) showed a filling defect in the common bile duct and narrowing of the intrahepatic bile duct, creating asymmetry with a withered branch-like appearance, compatible with PSC (Figure 3). Positron emission tomography (PET) demonstrated no abnormal accumulation of FDG (Figure 4). Based on a diagnosis of IPNB, the patient underwent right hepatectomy. The operation time was 501 min, and operative blood loss was 778 mL. The right lobe was atrophic and the resected liver volume was 122 g (Figure 5). Macroscopically, the lumen of the intrahepatic bile duct was filled with mucin, and there were several yellowish papillary tumors (Figure 6). Histologically, the epithelia of the dilated bile duct exhibited papillary tumors, which secreted abundant mucin. In the subepithelial stroma, no ovarian-like mesenchymal stroma was evident. The tumor was diagnosed as IPNB on a background of high-grade intraepithelial neoplasia with negative surgical margins, and the epithelial subtype of IPNB was the intestinal type. Immunohistochemical staining revealed that the tumor epithelia was positive for cytokeratin (CK) 7, CK20, and mucin (MUC) 2, and negative for MUC5AC and MUC6 (Figure 7). In the parenchyma of the protuberant mass, no cells were positive for CK7 or CK20 (Figure 8). Periductal concentric fibrosis of the bile ducts (onion skin-like appearance) suggested the presence of PSC (Figure 9). At 3 years after the operation, the patient had no signs of recurrence.
IPNB was first proposed as a distinct entity in the 2010 revision of the WHO classification of tumors of the liver and intrahepatic bile duct[2,3]. Nakanuma et al[4] have reported that IPNB is characterized by proliferation of neoplastic epithelial cells with fibrovascular stalks in the bile duct lumen, mucin hypersecretion, and considerable dilatation or multilocular changes of the affected bile ducts. IPNB frequently arises during a reactive change in chronic biliary tract disease, such as PSC, intrahepatic calculosis and hepatolithiasis[5]. It is difficult to make an accurate preoperative diagnosis because of IPNB’s low incidence and the lack of specificity in its clinical manifestation. The pathognomonic features of IPNB include cholangiectasis and obstructive jaundice due to the production of a large quantity of mucin. This causes various symptoms such as abdominal pain, pyrexia, anorexia and vomiting. Laboratory analysis commonly reveals elevated levels of hepatobiliary enzymes. Many cases are discovered during routine medical examination or follow-up for other disorders, as most patients are negative for hepatitis virus markers. US, CT, MRI, ERC, cholangioscopy and IDUS are all useful for diagnosing tumor extension, involvement of the bile duct and the presence of mucin.
PSC is a chronic cholestatic syndrome of unknown etiology characterized by fibrosing inflammatory destruction of the intrahepatic and/or extrahepatic bile ducts[6]. It has been suggested that PSC may have some involvement in stepwise progression to CCA via the metaplasia-dysplasia-carcinoma sequence[7], and biliary tract carcinogenesis may thus be a multistep process resulting from chronic biliary inflammation. According to one report, among 200 patients followed up for an average of 12.4 years, 107 (60%) had IBD, 8 (4.5%) had IBDu and 13 (6.5%) had CCA[8]. Both PSC and Behçet’s disease are associated with bowel lesions. To our knowledge, there has been no case report in which PSC and Behçet’s disease coexisted. Although PSC is considered to be a risk factor for CCA, the frequency of CCA in patients with PSC has been reported to vary from 7% to 30%[1]. In a retrospective study of 100 consecutive orthotopic liver transplantations performed for PSC, Lewis et al found 30 patients with CCA, and among the remaining 70 patients, 11 had papillary lesions[1]. No association between the duration of PSC and the incidence of CCA has been demonstrated[6].
In our present patient, IPNB developed on a background of PSC. Fortunately, IPNB was found at the precancerous stage, due to clinical signs and symptoms attributable to abundant mucin production. To our knowledge, this is the third reported case of PSC with concurrent IPNB. The first such case occurred in a 60-year-old woman, and IPNB was diagnosed on the basis of bile duct dilatation and elevation of hepatobiliary enzyme levels one year after the patient had been diagnosed with PSC[9]. The patient underwent extended resection of the left lobe of the liver and was found to have an intraductal papillary neoplasm with an associated invasive carcinoma. The patient had no signs of recurrence 27 mo after surgery. The second case of IPNB occurred in a 50-year-old woman who had been diagnosed with PSC 26 years previously[10]. The patient underwent orthotopic liver transplantation because of multiple episodes of acute cholangitis. Pathological examination demonstrated intraductal papillary neoplasia with an associated invasive carcinoma. Therefore, our present case of IPNB is the first to have been diagnosed at the premalignant stage.
Surgical resection is the first choice of treatment for IPNB. Curative surgical resection, including any invasive carcinoma, has been reported to yield good outcomes, with 1-, 3-, and 5-year survival rates of 96%, 84% and 81%, respectively[11]. If intraoperative pathological examination demonstrates a positive surgical margin including dysplasia, additional resection should be performed to obtain cancer-negative surgical margins because positive resection margins are strongly associated with shorter overall and recurrence-free survival[12]. In fact, after curative resection, our patient is currently doing well without any signs of recurrence 3 years after surgery.
In conclusion, we have described the first case of PSC in which IPNB developed on a background of high-grade intraepithelial neoplasia. Patients with PSC should be recognized as being at risk of developing IPNB and subsequent CCA.
A 46-year-old woman with a history of resected intraductal papillary neoplasm of the bile duct (IPNB) with primary sclerosing cholangitis (PSC).
Obstructive jaundice, cholangitis.
Mass-forming cholangiocarcinoma, mucinous cystic neoplasm, pyogenic cholangitis with bile duct stones.
The liver function tests demonstrated increased serum liver enzyme levels: aspartate aminotransferase, 104 U/L; alanine aminotransferase, 101 U/L; alkaline phosphatase, 929 U/L; γ-glutamyl transpeptidase, 667 U/L; and total bilirubin, 2.2 mg/dL.
Computed tomography/Endoscopic retrograde cholangiography showed dilatation of the intrahepatic bile ducts and mucin in the common bile duct.
The tumor was diagnosed as IPNB on a background of high-grade intraepithelial neoplasia.
The patient underwent right hepatectomy.
There are two cases of PSC with concurrent IPNB.
IPNB was found at the precancerous stage due to clinical signs and symptoms attributable to abundant mucin production.
When cystic lesions are observed in PSC patients, IPNB is unlikely and the patient should undergo regular scans.
This article is the first case of PSC in which IPNB developed on a background of high-grade intraepithelial neoplasia.
P- Reviewer: Brogna A, Pan GD, Sandblom G S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Lewis JT, Talwalkar JA, Rosen CB, Smyrk TC, Abraham SC. Precancerous bile duct pathology in end-stage primary sclerosing cholangitis, with and without cholangiocarcinoma. Am J Surg Pathol. 2010;34:27-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 2. | WHO classification of tumours of the Digestive System. 4th Edition. 2010;. |
| 3. | Nakanuma Y. Biliary tract clinicopathology with an emphasis on biliary intraepithelial neoplasia. JJBA. 2011;25:31-42. |
| 4. | Nakanuma Y, Zen Y, Harada K, Ikeda H, Sato Y, Uehara T, Sasaki M. Tumorigenesis and phenotypic characteristics of mucin-producing bile duct tumors: an immunohistochemical approach. J Hepatobiliary Pancreat Sci. 2010;17:211-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Zen Y, Sasaki M, Fujii T, Chen TC, Chen MF, Yeh TS, Jan YY, Huang SF, Nimura Y, Nakanuma Y. Different expression patterns of mucin core proteins and cytokeratins during intrahepatic cholangiocarcinogenesis from biliary intraepithelial neoplasia and intraductal papillary neoplasm of the bile duct--an immunohistochemical study of 110 cases of hepatolithiasis. J Hepatol. 2006;44:350-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 180] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 6. | Burak K, Angulo P, Pasha TM, Egan K, Petz J, Lindor KD. Incidence and risk factors for cholangiocarcinoma in primary sclerosing cholangitis. Am J Gastroenterol. 2004;99:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 415] [Cited by in RCA: 365] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 7. | Zen Y, Quaglia A, Heaton N, Rela M, Portmann B. Two distinct pathways of carcinogenesis in primary sclerosing cholangitis. Histopathology. 2011;59:1100-1110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Fevery J, Henckaerts L, Van Oirbeek R, Vermeire S, Rutgeerts P, Nevens F, Van Steenbergen W. Malignancies and mortality in 200 patients with primary sclerosering cholangitis: a long-term single-centre study. Liver Int. 2012;32:214-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 9. | Yokomuro S, Arima Y, Mizuguchi Y, Shimizu T, Kawahigashi Y, Kannda T, Arai M, Uchida E, Akimaru K, Tajiri T. Mucin-producing bile duct carcinoma arising from primary sclerosing cholangitis: a case report. J Nippon Med Sch. 2007;74:61-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Bu-Ghanim M, Suriawinata A, Killackey M, Schwartz ME, Jaffe D, Fiel MI. Invasive colloid carcinoma arising from intraductal papillary neoplasm in a 50-year-old woman with primary sclerosing cholangitis. Semin Liver Dis. 2004;24:209-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 9] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Kubota K, Nakanuma Y, Kondo F, Hachiya H, Miyazaki M, Nagino M, Yamamoto M, Isayama H, Tabata M, Kinoshita H. Clinicopathological features and prognosis of mucin-producing bile duct tumor and mucinous cystic tumor of the liver: a multi-institutional study by the Japan Biliary Association. J Hepatobiliary Pancreat Sci. 2014;21:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 87] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 12. | Jung G, Park KM, Lee SS, Yu E, Hong SM, Kim J. Long-term clinical outcome of the surgically resected intraductal papillary neoplasm of the bile duct. J Hepatol. 2012;57:787-793. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |