Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15820
Revised: February 27, 2014
Accepted: July 15, 2014
Published online: November 14, 2014
Processing time: 328 Days and 0.2 Hours
AIM: To evaluate the long-term results of conventional chemoradiotherapy and laparoscopic mesorectal excision in rectal adenocarcinoma patients without adjuvant therapy.
METHODS: Patients with biopsy-proven adenocarcinoma of the rectum staged cT3-T4 by endoscopic ultrasound or magnetic resonance imaging received neoadjuvant continuous infusion of 5-fluorouracil for five weeks and concomitant radiotherapy. Laparoscopic surgery was planned after 5-8 wk. Patients diagnosed with ypT0N0 stage cancer were not treated with adjuvant therapy according to the protocol. Patients with ypT1-2N0 or ypT3-4 or N+ were offered 5-fluorouracil-based adjuvant treatment on an individual basis. An external cohort was used as a reference for the findings.
RESULTS: One hundred and seventy six patients were treated with induction chemoradiotherapy and 170 underwent total mesorectal excision. Cancer staging of ypT0N0 was achieved in 26/170 (15.3%) patients. After a median follow-up of 58.3 mo, patients with ypT0N0 had five-year disease-free and overall survival rates of 96% (95%CI: 77-99) and 100%, respectively. We provide evidence about the natural history of patients with localized rectal cancer achieving a complete response after preoperative chemoradiation. The inherent good prognosis of these patients will have implications for clinical trial design and care of patients.
CONCLUSION: Withholding adjuvant chemotherapy after complete response following standard neoadjuvant chemoradiotherapy and laparoscopic mesorectal excision might be safe within an experienced multidisciplinary team.
Core tip: This study shows that patients with localized rectal cancer can achieve a complete response after preoperative chemoradiation. The inherent good prognosis of these patients will have implications for clinical trial design and care of patients. Withholding adjuvant chemotherapy after complete response following standard neoadjuvant chemoradiotherapy and laparoscopic mesorectal excision might be safe within an experienced multidisciplinary team.
- Citation: García-Albéniz X, Gallego R, Hofheinz RD, Fernández-Esparrach G, Ayuso-Colella JR, Bombí JA, Conill C, Cuatrecasas M, Delgado S, Ginés A, Miquel R, Pagés M, Pineda E, Pereira V, Sosa A, Reig O, Victoria I, Feliz L, María de Lacy A, Castells A, Burkholder I, Hochhaus A, Maurel J. Adjuvant therapy sparing in rectal cancer achieving complete response after chemoradiation. World J Gastroenterol 2014; 20(42): 15820-15829
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15820.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15820
Randomized clinical trials demonstrate that preoperative chemoradiotherapy (CRT) and radiotherapy have better local control, lower toxicity and higher compliance than postoperative adjuvant CRT for all patients, including those selected as high-risk[1,2]. Only one study has shown an improvement in disease-free survival (DFS) with the preoperative CRT strategy[3]. However, these results should be interpreted with caution as the analysis was carried out in only 267 of the initially planned 900 patients. In addition, presurgical CRT was better than presurgical radiotherapy alone in terms of local recurrences in both resectable[4-6] and non-resectable patients[7]. No benefit in DFS or overall survival (OS) was seen when comparing CRT with radiotherapy in resectable tumors. However, a significant benefit was obtained in time-to-treatment failure and cancer-specific survival, favoring CRT in non-resectable patients[7].
Although the strategy of presurgical CRT is well established, the benefit of adjuvant therapy after neoadjuvant CRT and total mesorectal excision (TME) is not supported by randomized clinical trials. Nevertheless, the National Comprehensive Cancer Network guidelines recommended postoperative chemotherapy for all patients undergoing preoperative CRT, regardless of surgical pathology results[8], and the European Society for Medical Oncology has recommended a similar strategy for colon cancer (high-risk stage II and stage III)[9]. Interestingly, Bujko et al[10] questioned the value of adjuvant therapy, specifically in the subset of patients that were in remission. Although patients with complete pathologic remission (no residual tumor and mesorectal lymph nodes are negative for metastases) fare well in multiple series[11,12], there is uncertainty as to whether this is due to the induction (CRT), the adjuvant or to both therapies.
The aim of this single-institution prospective study was to evaluate DFS and OS when adjuvant chemotherapy was omitted in patients with complete pathologic remission after conventional CRT and laparoscopic TME in a tertiary-care setting. An external cohort of patients drawn from a randomized clinical trial[13] was used to compare the findings.
From November 2000 to November 2008, all patients with a biopsy-proven adenocarcinoma of the rectum admitted to the Colorectal Cancer Unit at our hospital were evaluated for inclusion in the prospective cohort. Exclusion criteria for CRT treatment in the study were: (1) early stage (cT1-2N0); (2) cT3Nx located above the line crossing the promontorium and the acetabulum in a lateral projection of the barium enema; (3) elderly patients with frailty criteria or over 85 years of age; (4) patients unfit for CRT or with previous pelvic radiotherapy; (5) patient refusal to participate; and (6) metastatic disease. The study protocol was approved by the Ethics Committee of the Hospital Clínic of Barcelona, Spain.
Staging was performed in all cases by endoscopic ultrasound, abdominal spiral computed tomography, barium enema, chest X-ray and, after February 2006, pelvic magnetic resonance imaging. Patients received neoadjuvant chemotherapy comprised of a continuous infusion of 225 mg/m2 per day 5-fluorouracil for five weeks, with concurrent radiotherapy (45 Gy).
Surgery was performed by two surgeons (AML and SD have both performed approximately 50 laparoscopic rectal surgeries yearly since 1997) and included abdominoperineal resection and low anterior resection by laparoscopy. All resections were performed according to TME principles 5-8 wk after completion of CRT.
Patients were considered as having achieved a “complete response” if there was no residual tumor and mesorectal lymph nodes were negative for metastases (ypT0N0). Hematoxylin and eosin stained specimens were reviewed following standard protocols by three examiners (JAB, MC, RM) blinded to patient outcome. Patients with ypT1-2N0 stage cancer were considered as having achieved an “intermediate response”. Patients with ypT3-4 specimens or with the presence of pathologic lymph node involvement (ypN1) were considered to be “poor responders”.
Patients with residual disease (intermediate and non-responders) were offered adjuvant chemotherapy with 48 h continuous infusion of 5-fluorouracil (3 g/m2) with folinic acid (200 mg/m2) every two weeks for six cycles. Patients showing complete pathologic response were not offered adjuvant chemotherapy after surgery (“wait and see” approach).
Follow-ups consisted of visits to the surgery and/or oncology outpatient clinics every three months during the first two years, every six months for the following three years, and yearly thereafter. General laboratory work-up with carcinoembryonic antigen (CEA) levels was obtained and physical examination was performed during all visits. Abdominal and pelvic computed tomography scans were scheduled every six months for two years and annually after the second year of follow-up. Chest radiograph was performed annually and colonoscopy was carried out every three years in all patients. When recurrence was suspected, histologic confirmation was attempted whenever possible.
Given that the results of our single-institution non-randomized cohort may reflect a selection of patients rather than the effect of a therapeutic approach, a second cohort of patients following a similar treatment program was taken from a randomized clinical trial (NCT01500993) as a reference to evaluate the results. The NCT01500993 study is a non-inferiority clinical trial comparing capecitabine with fluorouracil in chemoradiotherapy for locally advanced rectal cancer[13]. From this trial, we selected patients undergoing the same therapeutic strategy as the main cohort: neoadjuvant chemoradiotherapy followed by surgery and adjuvant chemotherapy (both CRT and adjuvant chemotherapy could be capecitabine- or fluorouracil-based), and for whom information was available regarding the degree of pathologic response achieved after chemoradiotherapy. Details on dosing and schedules can be found in the main publication[13]. With the exception of two covariates (days in hospital and type of surgery in terms of open vs laparoscopy), the same information was available from this cohort.
Statistical analyses were performed using SAS V9.3 software (SAS Institute, Cary, NC, United States). Medians were compared using a Wilcoxon score test and proportions were compared with a χ2 test. Logistic regression was used to evaluate presurgical determinants for achieving a complete response. DFS was defined as the time from diagnosis until local and/or distant recurrence or death for any cause, whichever occurred first. OS was defined as time from diagnosis to death from any cause. Administrative censoring was established on December 1, 2011. Kaplan-Meier curves were used to plot survival and compute five-year DFS and OS. Cox proportional hazards regression with Efron method for ties was used to perform the survival analysis. Multivariate analysis was built using those variables with a P < 0.10 in the univariate analysis. Radial margin involvement was excluded from the multivariate analysis given its collinearity with the exposure of interest by definition. Continuous variables were entered as such in the models. The proportional hazards assumption was verified by plotting the cumulative marginal residuals and assessing for significance.
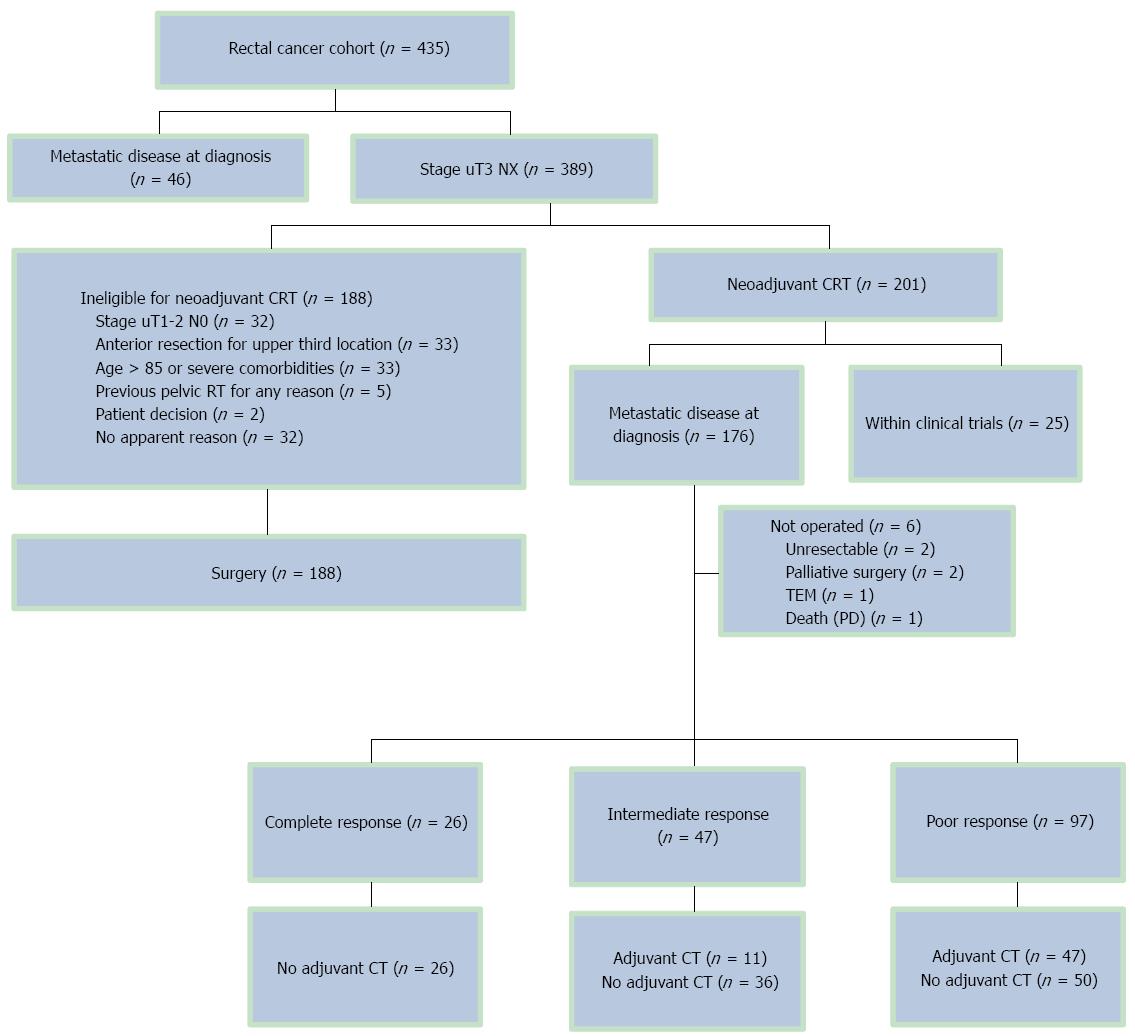
From November 2000 to November 2008, 435 patients were evaluated for inclusion. A total of 201 patients underwent preoperative CRT. Reasons for exclusion are shown in Figure 1. Twenty-five patients were included in two clinical trials[14,15] and were not analyzed in the current cohort, and six patients did not have radical surgery after CRT. Therefore, 170 patients constituted the basis of this analysis. The median age of all patients was 67 years (range: 40-85 years) and 68% (116/170) were male (Table 1). A fully laparoscopic approach was intended in 161 (95%) patients, of whom 17 were switched to open surgery (17/161; 4%). A low-anterior resection was performed in 119 (70%) patients and abdominoperineal resection was performed in 35 (21%) patients. In the surgical specimens of 81 (48%) patients, 12 or more lymph nodes could be identified. The overall median follow-up was 58.3 mo (range: 3.8-129.8 mo). Radial margin assessment was carried out in 147 patients (89%). Patients with ypT0N0 were considered by definition as R0 independently of radial margin assessment. R0 resection was performed in 132/147 (90%) assessed patients. Complete pathologic response was obtained in 15% (26/170) of patients, and an intermediate response was obtained in 28% (47/170). A median number of 11 lymph nodes were retrieved, and an absence of lymph node involvement (ypN0) was found in 130/170 specimens (76%).
| Variable | Complete1 | Intermediate2 | Poor3 | P value |
| (n = 26) | (n = 47) | (n = 97) | ||
| Age, yr | 65.5 (39.9-84.9) | 65.7 (42.9-84.0) | 65.5 (41.3-83.5) | 0.8407 |
| Female | 11 (42) | 14 (30) | 29 (30) | 0.4551 |
| CEA4 | 0.0122 | |||
| < 3.6 μg/L | 21 (81) | 26 (55) | 47 (49) | |
| 3.6-20.9 μg/L | 5 (19) | 20 (43) | 41 (42) | |
| > 20.9 μg/L | 0 | 0 | 9 (9) | |
| Presurgical hemoglobin, g/dL | 13.1 (9.2-16.0) | 14.0 (7.9-18.2) | 13.4 (7.6-18.4) | 0.0580 |
| Clinical stage | 0.1325 | |||
| cT2N0 | 0 | 25 (4) | 0 (0) | |
| cT2N1 | 0 | 3 (6) | 3 (3) | |
| cT3N0 | 11 (42) | 21 (45) | 38 (39) | |
| cT3N1 | 14 (54) | 14 (30) | 38 (39) | |
| cT4N0 | 1 (4) | 0 (0) | 2 (2) | |
| cT4N1 | 0 | 0 | 3 (3) | |
| Severe stenosis precluding staging | 0 | 7 (15) | 13 (13) | |
| Distance from tumor to anal margin, cm | 7.0 (2.0-12.0) | 5.0 (1.0-15.0) | 8.0 (1.0-15.0) | 0.0697 |
| Previous abdominal surgery | 14 (54) | 16 (34) | 39 (40) | 0.2546 |
| Type of surgery | 0.5368 | |||
| APR or miles intervention | 4 (15) | 8 (17) | 23 (24) | |
| Low-anterior resection | 21 (81) | 33 (70) | 65 (67) | |
| Other | 1 (4) | 6 (13) | 9 (9) | |
| Laparoscopic vs open surgery | 0.1748 | |||
| Fully laparoscopic | 25 (96) | 42 (89) | 77 (79) | |
| Intraoperative conversion to open | 1 (4) | 4 (9) | 12 (12) | |
| Open from the beginning | 0 | 1 (2) | 8 (8) | |
| Hospital stay, d | 5.0 (3.0-16.0) | 6.5 (4-55) | 7 (2-145) | 0.0164 |
| Number of lymph nodes resected | 11 (1-27) | 10 (1-33) | 12 (1-29) | 0.3768 |
| Patients with ≥ 12 lymph nodes resected | 12 (46) | 20 (43) | 49 (51) | 0.6600 |
| Involvement of radial margin | < 0.0001 | |||
| No | 26 (100) | 44 (94) | 62 (64) | |
| Yes | 0 | 0 | 15 (16) | |
| Not assessed | 0 | 3 (6) | 20 (21) |
Significant differences were found in the levels of CEA at diagnosis among patients with different types of responses (P < 0.05). There were also significant differences among patients with complete, intermediate and poor responses in days spent in the hospital and involvement of the radial margin (negative by definition in ypT0N0 patients; Ps < 0.05). Age-, sex-, presurgical staging- and presurgical hemoglobin-adjusted analyses identified CEA as the sole predictor of complete response achievement (used as a continuous variable, OR = 0.82, 95%CI: 0.68-0.99, P = 0.0362).
None of the complete-responder patients received adjuvant chemotherapy according to the study protocol, though 11/47 (23%) patients with an intermediate response did. However, a significantly larger proportion (47/97; 48%) of patients in the poor-responder group received adjuvant chemotherapy (P < 0.001).
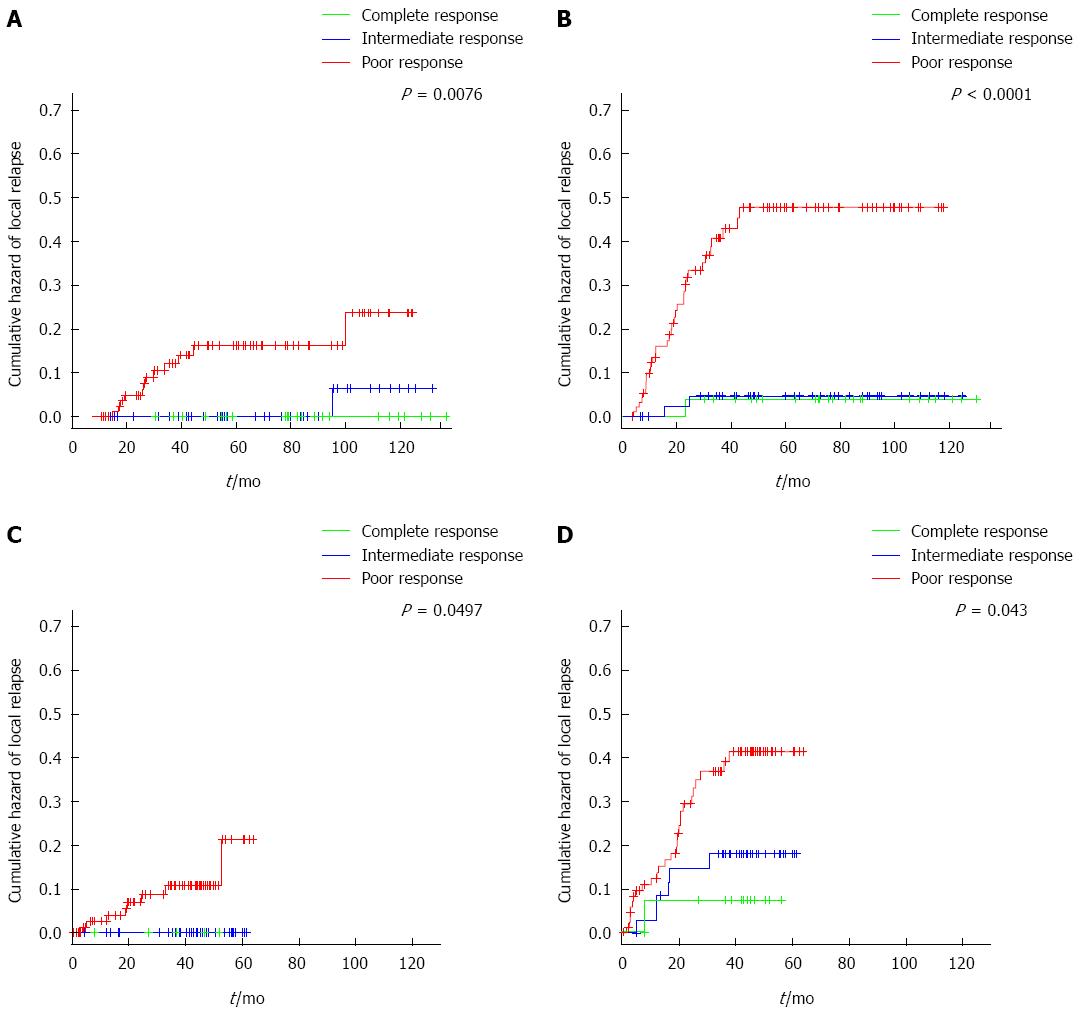
Recurrences were observed in 46/170 patients (27%). In the group of poor-responders there were 42/97 (43%) relapses (30 distant only, 9 local only, and 2 local and distant relapses). In the group of intermediate-responders, there were two distant relapses and one local relapse (3/47; 6% relapse). One of 26 (4%) patients with a complete response developed metastases and none presented local recurrence. The patient with complete response developed an isolated liver metastasis 15 mo after primary resection, which was salvaged with a right hepatectomy. Systemic recurrence occurred most frequently in the liver (11%), followed by the lung (10%), peritoneum (4%) and lymph nodes (3%). Figure 2 shows the cumulative hazards of local and distant relapse. Local relapses are seen late in follow-up in both the intermediate- and poor-responder groups, which were significantly different among groups (P = 0.0112). The cumulative hazard of distant relapse rose steadily in the group of poor-responders and stabilized after 43 mo of follow-up. Three distant relapses were seen in the group of intermediate-responders in the first 25 mo of follow-up.
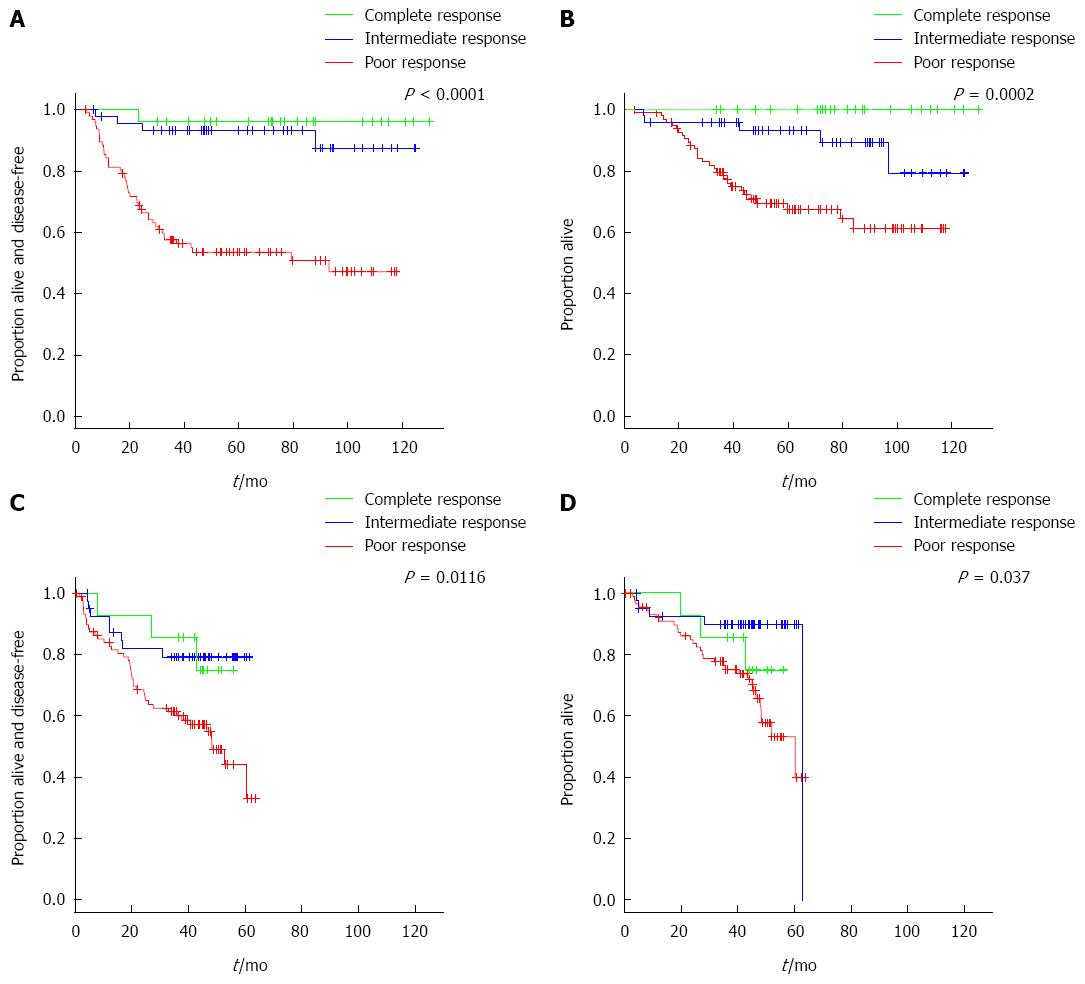
Four patients (4/73; 5%) from the complete- and intermediate-responders groups died from causes not related to rectal cancer, compared with eight (8/97; 8%) patients in the poor-responders group. At a median follow-up of 58.3 mo, five-year DFS was 96 (95%CI: 89-100), 93 (95%CI: 86-100) and 54% (95%CI: 44-65) in the complete, intermediate and poor response groups, respectively (P < 0.0001) (Figure 3A). Five-year OS was 100, 93 (95%CI: 86-100) and 67% (95%CI: 58-78) in the complete, intermediate and poor response groups, respectively (P = 0.0002) (Figure 3B).
In the multivariate analysis, type of response was the main predictive factor for DFS. Taking the poor responders as the reference category (since it is the most frequent type of response), patients with complete response had a HR for DFS of 0.07 (95%CI: 0.01-0.54) and patients with an intermediate response had an HR of 0.16 (95%CI: 0.06-0.46). Baseline CEA level and days of admission following surgery were also predictive for DFS (Table 2). Patients with intermediate response also presented better OS in the multivariate analysis when compared with patients with poor response, with an HR of 0.30 (95%CI: 0.11-0.78) (Table 3).
| Variable | Main cohort | External reference cohort | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Type of response after CRT | ||||
| ypT0N0 | 0.07 (0.01-0.54) | 0.0100 | 0.51 (0.15-1.7) | 0.2700 |
| ypT1-2N0 | 0.16 (0.06-0.46) | 0.0007 | 0.41 (0.18-0.94) | 0.0360 |
| ypT3-4/N1-2 | Reference | Reference | ||
| CEA | 1.02 (1.01-1.03) | 0.0010 | 1.02 (1.01-1.03) | 0.0072 |
| Pre-surgery clinical stage | ||||
| T2-3N0 | Reference | Reference | ||
| T4 and/or N1 | 1.19 (0.82-1.73) | 0.3500 | 1.66 (0.85-3.26) | 0.1400 |
| Admission stay after surgery1, d | 1.02 (1.01-1.02) | 0.0020 | ||
| Variable | Main cohort | External reference cohort | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Chemotherapy1 | 1.09 (0.55-2.16) | 0.810 | ||
| Type of response after CRT | ||||
| ypT0N0 | NE | 0.61 (0.19-2.02) | 0.420 | |
| ypT1-2N0 | 0.30 (0.11-0.78) | 0.014 | 0.32 (0.13-0.83) | 0.019 |
| ypT3-4/N1-2 | Reference | Reference | ||
| CEA | 1.00 (0.97-1.03) | 0.780 | ||
| Presurgical clinical stage | ||||
| T2-3N0 | Reference | |||
| T4 and/or N1 | 1.89 (0.95-3.76) | 0.069 | ||
One hundred and forty five patients were evaluated from the NCT01500993 study. Seventy-three and 72 patients were treated with fluorouracil- or capecitabine-based regimes, respectively. There were no relevant differences from the main cohort in terms of age, presurgical hemoglobin, CEA or clinical staging. The proportion of patients achieving a complete response was lower in the external reference cohort (10%), and the proportion of patients with a poor response was slightly higher (62%). A multivariate analysis of age, sex, CEA, clinical stage and presurgical hemoglobin did not identify any of these factors as predictors of response. Of note, in both cohorts, clinical stage was unrelated to the type of response achieved (χ2: P = 0.36 for the main cohort and P = 0.61 for the external reference cohort).
Three patients did not receive adjuvant chemotherapy, one in the complete-responder group and two in the poor-responder group. This is the principal difference from the main cohort, where complete-responders were not treated with adjuvant chemotherapy. Median follow up was 43.7 mo (range: 0.5-63.7 mo). No patient with complete or intermediate response suffered a local relapse during the study follow-up. Three-year local relapse-free survival was 90% (87% in the main cohort) (Figure 2). Rate of distant relapse was also different by type of response achieved, with a three-year distant relapse-free survival of 93%, 84% and 68% for complete-, intermediate- and poor-responders, respectively (P = 0.0430). In the main cohort these percentages are 97%, 96% and 67% (P < 0.0001).
The degrees of response to CRT and CEA level were predictors of DFS (Table 2) and degrees of response to CRT and presurgical clinical stage were predictors of OS (Table 3). There were three deaths in the group of complete-responders in the external reference cohort. One patient died due to distant spread of the disease, another due to myocardial infarction without evidence of relapse, and the third death was due to septic shock following elective surgery of the primary tumor. Three-year OS was 86%, 90% and 75% in the complete-, intermediate- and poor-responders, respectively (P = 0.0370) (Figure 3). The magnitude of the prediction of OS by degree of response was similar to the main cohort (HR = 0.32, 95%CI: 0.13-0.83, for intermediate- vs poor-responders) (Table 3). The magnitude of the prediction of DFS by degree of response, although statistically significant, was lower (Table 3). Both DFS and OS were worse by type of response achieved in the external reference cohort compared with the main cohort (Figure 3).
This is the largest prospective rectal cancer cohort used to date to evaluate the withholding of adjuvant therapy for complete pathologic responders after standard CRT and laparoscopic resection. Our long-term oncologic results are comparable with those obtained in our external reference cohort and with previous studies[16-18].
We provide evidence that patients achieving ypT0N0 fare extremely well, despite adjuvant treatment not being administered. Complete-responders in the main cohort presented even better DFS and OS than those ypT0N0 patients in the external reference cohort, where adjuvant chemotherapy was recommended. Important baseline prognostic factors (i.e., age, clinical stage, CEA, hemoglobin) did not differ between both cohorts. The main cohort represents an unselected population, whereas the external reference cohort was taken from a clinical trial, which tends to include fitter patients.
The most widely used and reproducible system for evaluating CRT efficacy is down-staging. Although different down-staging classifications have being proposed[18-20], the most commonly used method separates ypT0-2N0 and ypT3-4 or N+. As most patients treated with preoperative CRT are staged by endoscopic ultrasound or magnetic resonance imaging as cT3, and T3 (involvement of mesorectum) is optimally defined with both techniques[21], it seems reasonable to use this down-staging classification. Another advantage is that this method is widely reproducible among pathologists and includes pathologic nodal information. Several studies have confirmed the prognostic value of this specific down-staging[16,20,22,23]. We observed differences in DFS and OS in the main and external reference cohorts. The degree of pathologic response was more discriminative in the main cohort, and prognosis was better in each strata. This could be due to random variability or to better pathologic assessment in the main cohort. Better classification of the patients would encompass a stage migration and improvement of the prognosis in every stratum. Other reasons for differences in the percentage of complete pathologic responses is that time from termination of CRT and surgery in the external reference cohort was usually four weeks, whereas in the main cohort it was five to eight weeks.
Although methodologically complex, oncologists should pursue the identification of dynamic strategies of treatment for rectal cancer where initial response to CRT could guide subsequent adjuvant therapies and surveillance policies. This can be achieved either by high-quality observational data and proper analytic methods[24] or with randomized clinical trials. Clinical trials studying adjuvant chemotherapy should consider that pathologic down-staging after neoadjuvant CRT separates patients with different prognoses and endorses proper stratification (e.g., ypT1-2N0 vs ypT3-4 or N+). The timing and magnitude of risk of local and distant recurrence shown here may also help to guide postsurgical surveillance strategies in these patients. Our results suggest that local relapse surveillance can be more flexible in patients with good response since the risk is very low.
Adjuvant chemotherapy following neoadjuvant CRT has not been proven beneficial in randomized clinical trials[4,25], though an unplanned sub-analysis of a European Organization for Research and Treatment of Cancer Radiation Oncology Group trial suggested a benefit in ypT0-2 patients[26]. An important limitation of this study is that only a subset (78%) of the originally randomized patients was included, which introduces the risk of a selection bias[27] not solved by the original randomization, turning the study into an observational one and thus subject to bias due to unmeasured confounders. Only one prospective clinical trial (SCRIPT) is currently evaluating the value of adjuvant therapy with a control arm without therapy. The other trial (CHRONICLE) was closed before schedule due to low accrual.
Although we suggest that patients with ypT0N0 should not be treated with adjuvant chemotherapy, this statement should be taken with caution for two reasons. First, the results are based on a single third-level oncologic institution, where expert radiologists, surgeons, gastroenterologists and oncologists coordinate to provide state-of-the art oncologic care and surveillance to patients, and these results may lack the external validation required for extrapolation to other institutions. Second, our study has a limited number of patients. However, our study has a long follow-up, is the first to evaluate the natural history of patients after CRT without adjuvant therapy, and included all patients with > cT3, mid and low rectal tumors younger than 85 years, reflecting a non-selected population of patients.
In conclusion, we have shown that the natural behavior of ypT0N0 patients is optimal when treated in a tertiary care center and that adjuvant chemotherapy could be of low therapeutic value. Our results suggest that withholding adjuvant chemotherapy from those patients achieving ypT0N0 after standard neoadjuvant CRT and TME, if treated by an experienced multidisciplinary team, might be a reasonable option.
We thank Katherine Williams for her English editorial assistance.
Preoperative chemoradiation is the standard of care for localized rectal cancer. The role of further adjuvant chemotherapy for those patients achieving a complete response is a gray area.
Current lines of research in rectal cancer aim to tailor treatment to the least invasive approach possible while maintaining the best possible outcomes. Patient selection is key in this process. Elements that may help inform patient selection include genetics, pathway analysis, tumor stage/localization together with patients’ comorbidities and overall health status. Evaluation of the response of the tumor to therapy as performed in the neoadjuvant setting can act as an additional tool for patient selection.
Authors provide evidence about the natural history of patients with localized rectal cancer achieving a complete response after preoperative chemoradiation. The inherent good prognosis of these patients will have implication on clinical trial design and care of patients.
The results of this study provide evidence for a clinical trial that might consider the absence of adjuvant treatment for those patients achieving a pathologically complete response after chemoradiation as a control arm. They also provide comfort to those patients and physicians that decide upon withholding adjuvant chemotherapy in such scenarios.
Laparoscopic resection: minimally invasive surgery using small incision in the abdomen. Total mesorectal excision: excision of the fat and fascia surrounding the rectum along with the rectum itself en bloc. Chemoradiotherapy: combination of radiotherapy and chemotherapy, which improves local control in rectal cancer, when administered preoperatively. Pathologic complete response: absence of malignant cells in the pathologic specimen after chemoradiotherapy.
The manuscript is a retrospective database analysis of rectal cancer patients who received neoadjuvant treatment. Those who experienced complete remission were not given adjuvant treatment and showed a very good outcome. A validation cohort is provided. In general, the study is well performed, the manuscript well written and easy to follow.
P- Reviewer: Carboni F, Langner C, Steele SR, Wang JY S- Editor: Gou SX L- Editor: AmEditor E- Editor: Liu XM
| 1. | Sauer R, Becker H, Hohenberger W, Rödel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731-1740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4342] [Cited by in RCA: 4451] [Article Influence: 212.0] [Reference Citation Analysis (1)] |
| 2. | Sebag-Montefiore D, Stephens RJ, Steele R, Monson J, Grieve R, Khanna S, Quirke P, Couture J, de Metz C, Myint AS. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811-820. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1219] [Cited by in RCA: 1109] [Article Influence: 69.3] [Reference Citation Analysis (2)] |
| 3. | Roh MS, Colangelo LH, O’Connell MJ, Yothers G, Deutsch M, Allegra CJ, Kahlenberg MS, Baez-Diaz L, Ursiny CS, Petrelli NJ. Preoperative multimodality therapy improves disease-free survival in patients with carcinoma of the rectum: NSABP R-03. J Clin Oncol. 2009;27:5124-5130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 627] [Cited by in RCA: 699] [Article Influence: 43.7] [Reference Citation Analysis (0)] |
| 4. | Bosset JF, Collette L, Calais G, Mineur L, Maingon P, Radosevic-Jelic L, Daban A, Bardet E, Beny A, Ollier JC. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114-1123. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1993] [Cited by in RCA: 2037] [Article Influence: 107.2] [Reference Citation Analysis (0)] |
| 5. | Gérard JP, Conroy T, Bonnetain F, Bouché O, Chapet O, Closon-Dejardin MT, Untereiner M, Leduc B, Francois E, Maurel J. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620-4625. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1251] [Cited by in RCA: 1272] [Article Influence: 66.9] [Reference Citation Analysis (0)] |
| 6. | Bujko K, Nowacki MP, Nasierowska-Guttmejer A, Michalski W, Bebenek M, Kryj M. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215-1223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 870] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 7. | Braendengen M, Tveit KM, Berglund A, Birkemeyer E, Frykholm G, Påhlman L, Wiig JN, Byström P, Bujko K, Glimelius B. Randomized phase III study comparing preoperative radiotherapy with chemoradiotherapy in nonresectable rectal cancer. J Clin Oncol. 2008;26:3687-3694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 342] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 8. | NCCN. Rectal cancer. NCCN Clinical Practical Guidelines in Oncology 2012 [Internet]. Available from: http://www.nccn.org.professionals/physician_gls/PDF/rectal.pdf. |
| 9. | Glimelius B, Tiret E, Cervantes A, Arnold D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24 Suppl 6:vi81-vi88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 338] [Cited by in RCA: 351] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 10. | Bujko K, Glynne-Jones R, Bujko M. Does adjuvant fluoropyrimidine-based chemotherapy provide a benefit for patients with resected rectal cancer who have already received neoadjuvant radiochemotherapy? A systematic review of randomised trials. Ann Oncol. 2010;21:1743-1750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 130] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 11. | Capirci C, Valentini V, Cionini L, De Paoli A, Rodel C, Glynne-Jones R, Coco C, Romano M, Mantello G, Palazzi S. Prognostic value of pathologic complete response after neoadjuvant therapy in locally advanced rectal cancer: long-term analysis of 566 ypCR patients. Int J Radiat Oncol Biol Phys. 2008;72:99-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 346] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 12. | Maas M, Nelemans PJ, Valentini V, Das P, Rödel C, Kuo LJ, Calvo FA, García-Aguilar J, Glynne-Jones R, Haustermans K. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010;11:835-844. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 1452] [Article Influence: 96.8] [Reference Citation Analysis (0)] |
| 13. | Hofheinz RD, Wenz F, Post S, Matzdorff A, Laechelt S, Hartmann JT, Müller L, Link H, Moehler M, Kettner E. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol. 2012;13:579-588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 350] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 14. | Ugidos L, Delgado S, Conill C, Ginés A, Gallego R, Ayuso JR, Miquel R, Tosca M, de Lacy A, Castells A. Phase I trial of neoadjuvant chemoradiotherapy (CRT) with capecitabine and weekly irinotecan followed by laparoscopic total mesorectal excision (LTME) in rectal cancer patients. Invest New Drugs. 2009;27:262-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Fernández-Martos C, Pericay C, Aparicio J, Salud A, Safont M, Massuti B, Vera R, Escudero P, Maurel J, Marcuello E. Phase II, randomized study of concomitant chemoradiotherapy followed by surgery and adjuvant capecitabine plus oxaliplatin (CAPOX) compared with induction CAPOX followed by concomitant chemoradiotherapy and surgery in magnetic resonance imaging-defined, locally advanced rectal cancer: Grupo cancer de recto 3 study. J Clin Oncol. 2010;28:859-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 331] [Article Influence: 22.1] [Reference Citation Analysis (0)] |
| 16. | Valentini V, van Stiphout RG, Lammering G, Gambacorta MA, Barba MC, Bebenek M, Bonnetain F, Bosset JF, Bujko K, Cionini L. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J Clin Oncol. 2011;29:3163-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 411] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 17. | Park IJ, You YN, Agarwal A, Skibber JM, Rodriguez-Bigas MA, Eng C, Feig BW, Das P, Krishnan S, Crane CH. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol. 2012;30:1770-1776. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 325] [Cited by in RCA: 382] [Article Influence: 29.4] [Reference Citation Analysis (0)] |
| 18. | Lee JH, Kim SH, Kim JG, Cho HM, Shim BY. Preoperative chemoradiotherapy (CRT) followed by laparoscopic surgery for rectal cancer: predictors of the tumor response and the long-term oncologic outcomes. Int J Radiat Oncol Biol Phys. 2011;81:431-438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Wolthuis AM, Penninckx F, Haustermans K, Ectors N, Van Cutsem E, D’Hoore A. Outcome standards for an organ preservation strategy in stage II and III rectal adenocarcinoma after neoadjuvant chemoradiation. Ann Surg Oncol. 2011;18:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Kaminsky-Forrett MC, Conroy T, Luporsi E, Peiffert D, Lapeyre M, Boissel P, Guillemin F, Bey P. Prognostic implications of downstaging following preoperative radiation therapy for operable T3-T4 rectal cancer. Int J Radiat Oncol Biol Phys. 1998;42:935-941. [PubMed] |
| 21. | Fernández-Esparrach G, Ayuso-Colella JR, Sendino O, Pagés M, Cuatrecasas M, Pellisé M, Maurel J, Ayuso-Colella C, González-Suárez B, Llach J. EUS and magnetic resonance imaging in the staging of rectal cancer: a prospective and comparative study. Gastrointest Endosc. 2011;74:347-354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 22. | Janjan NA, Crane CN, Feig BW, Cleary K, Dubrow R, Curley SA, Ellis LM, Vauthey J, Lenzi R, Lynch P. Prospective trial of preoperative concomitant boost radiotherapy with continuous infusion 5-fluorouracil for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys. 2000;47:713-718. [PubMed] |
| 23. | Mohiuddin M, Hayne M, Regine WF, Hanna N, Hagihara PF, McGrath P, Marks GM. Prognostic significance of postchemoradiation stage following preoperative chemotherapy and radiation for advanced/recurrent rectal cancers. Int J Radiat Oncol Biol Phys. 2000;48:1075-1080. [PubMed] |
| 24. | Hernán MA, Lanoy E, Costagliola D, Robins JM. Comparison of dynamic treatment regimes via inverse probability weighting. Basic Clin Pharmacol Toxicol. 2006;98:237-242. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 188] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 25. | Bosset JF, Calais G, Mineur L, Maingon P, Stojanovic-Rundic S, Bensadoun RJ, Bardet E, Beny A, Ollier JC, Bolla M. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 552] [Article Influence: 50.2] [Reference Citation Analysis (0)] |
| 26. | Collette L, Bosset JF, den Dulk M, Nguyen F, Mineur L, Maingon P, Radosevic-Jelic L, Piérart M, Calais G. Patients with curative resection of cT3-4 rectal cancer after preoperative radiotherapy or radiochemotherapy: does anybody benefit from adjuvant fluorouracil-based chemotherapy? A trial of the European Organisation for Research and Treatment of Cancer Radiation Oncology Group. J Clin Oncol. 2007;25:4379-4386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 331] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 27. | Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615-625. [PubMed] |