Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15780
Revised: March 10, 2014
Accepted: April 28, 2014
Published online: November 14, 2014
Processing time: 321 Days and 10.8 Hours
AIM: To characterize clinical, laboratorial, and histological profile of pediatric autoimmune gastritis in the setting of unexplained iron deficiency anemia investigation.
METHODS: A descriptive, observational study including pediatric patients with a diagnosis of autoimmune gastritis (positive parietal cell antibody and gastric corpus atrophy) established in a 6 year period (2006-2011) in the setting of refractory iron deficiency anemia (refractoriness to oral iron therapy for at least 6 mo and requirement for intravenous iron therapy) investigation, after exclusion of other potentially contributing causes of anemia. Helicobacter pylori (H. pylori) infection and anti-secretory therapy were also excluded. Data were retrospectively collected from clinical files, including: demographic data (age, gender, and ethnic background), past medical history, gastrointestinal symptoms, familial history, laboratorial evaluation (Hb, serum ferritin, serum gastrin, pepsinogen I/ pepsinogen II, B12 vitamin, intrinsic factor autoantibodies, thyroid autoantibodies, and anti-transglutaminase antibodies), and endoscopic and histological findings (HE, Periodic Acid-Schiff/Alcian blue, gastrin, chromogranin A and immunochemistry analysis for CD3, CD20 and CD68). Descriptive statistical analysis was performed (mean, median, and standard deviation).
RESULTS: We report a case-series concerning 3 girls and 2 boys with a mean age of 13.6 ± 2.8 years (3 Caucasian and 2 African). One girl had type I diabetes. Familial history was positive in 4/5 cases, respectively for autoimmune thyroiditis (2/5), sarcoidosis (1/5) and multiple myeloma (1/5). Laboratorial evaluation on admission included: Hb: 9.5 ± 0.7 g/dL; serum ferritin: 4.0 ± 0.9 ng/mL; serum gastrin: 393 ± 286 pg/mL; low pepsinogen I/ pepsinogen II ratio in 1/5 patients; normal vitamin B12 levels (analyzed in 3 patients). Endoscopy findings included: duodenal nodularity (2/5) and gastric fold softening (2/5), and histological evaluation showed corpus atrophic gastritis with lymphocytic infiltration (5/5), patchy oxyntic gland mononuclear cell infiltration (5/5), intestinal and/or pseudo-pyloric metaplasia in corpus mucosa (4/5), and enterochromaffin cell hyperplasia (4/5). Immunochemistry for gastrin on corpus biopsies was negative in all cases. Duodenal histology was normal. All biopsies were negative for H. pylori (Giemsa staining and cultural examination).
CONCLUSION: We highlight autoimmune gastritis as a diagnosis to be considered when investigating refractory iron deficiency anemia in children, particularly in the setting of a personal/familial history of autoimmune disease, as well as the diagnostic contribution of a careful immunohistological evaluation.
Core tip: Autoimmune gastritis (AIG) is a rare entity at young age. Although classically associated with pernicious anemia in adulthood, its presentation as iron-deficiency anemia (IDA) has been recently reported, particularly in younger patients. Our study aimed to further contribute to a better characterization of clinical and histological expression of AIG at pediatric age, highlighting IDA as a precocious hematological manifestation and the diagnostic contribution of a careful immunohistological evaluation. Furthermore, in the setting of personal and/or familial history of autoimmune disease, the importance of taking AIG into account and including parietal cell antibodies in the autoimmune screening panel is emphasized.
- Citation: Gonçalves C, Oliveira ME, Palha AM, Ferrão A, Morais A, Lopes AI. Autoimmune gastritis presenting as iron deficiency anemia in childhood. World J Gastroenterol 2014; 20(42): 15780-15786
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15780.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15780
Autoimmune gastritis (AIG) is an inflammatory condition of the stomach, typically restricted to the corpus and characterized by the presence of autoantibodies against the proton pump H+/K+ adenosine triphosphatase (present in gastric parietal cells), and to a lesser extent to intrinsic factor[1-3]. The immunopathogenic basis for this process seems to involve the activation of parietal cell-specific T helper type 1 CD4-T cells[4,5]. Macroscopically, gastric mucosa becomes thinner and the folds soften. Histologically it is characterized by the loss of gastric glandular structures in the oxyntic mucosa, which are inappropriately replaced by glands[6]. Histological features result in achlorhydria, low serum pepsinogen I, and hypergastrinemia. Additionally, a proliferation of enterochromaffin-like cells (ECL) occurs due to trophic stimulus induced by hypergastrinemia[4,7,8].
AIG is a well-known cause of pernicious anemia in middle-aged and elderly adults, and is usually expressed by cobalamin deficiency and megaloblastic anemia. Its role in iron deficiency anemia (IDA) (a recognized complication of achlorhydria) has recently been evaluated, and seems to be more prevalent in young patients with AIG compared to older patients in whom pernicious anemia is the most prevalent hematologic condition[1,9,10]. Hershko et al[10] reported a significant frequency of AIG in adults with IDA without gastrointestinal symptoms and a progressive increase in mean corpuscular volume with age[9].
AIG accounts for up to 10% of cases of gastritis in adults[11] and it has an estimated overall prevalence closer to 20% in the general population, as assessed by the serological biomarker of parietal cell antibody[12]. However, its true incidence worldwide remains unclear, because it is usually asymptomatic before clinical presentation as pernicious anemia in adulthood.
In children, AIG is considered a very rare condition[13,14]. There are only a few reports of AIG in pediatric patients[8,15-18] and, in such cases, it is rarely associated with IDA[16]. In fact, in the two series that have been published to date, gastric autoimmunity has been incidentally disclosed in the setting of type 1 diabetes[18] and thyroiditis[19].
The present study describes 5 pediatric cases of AIG diagnosed during the work-up evaluation of IDA, emphasizing the important contribution of gastric corpus histopathology findings to a definitive diagnosis.
We performed a descriptive, observational case-series study of five cases of pediatric AIG retrospectively collected from clinical files covering a 6-year period (2006-2011).
Diagnosis was suggested during investigation of IDA [Hemoglobin (Hb) < 2 SD for age and sex and serum ferritin < 15 ng/mdL], refractoriness to oral iron therapy for at least 6 mo, and requirement for intravenous iron therapy. Upper endoscopy confirmed the presence of corpus atrophic gastritis and positive anti-parietal cell autoantibodies (PCA). At least three gastric biopsies were collected from each patient (gastric fundus, corpus, and antrum). Duodenal mucosa biopsies were also obtained. Cultural examination for Helicobacter pylori (H. pylori), according to standard methodology[20], was performed in all cases.
Formalin-fixed (10%), paraffin-embedded tissues from antrum and corpus biopsies were processed according to conventional histological technique. Serial sections (4 μm) were stained with hematoxylin-eosin (HE), Giemsa staining for H. pylori, and Periodic acid- Schiff/Alcian blue staining at pH = 2.5 to confirm the presence of intestinal and pseudopyloric metaplasia (antral-like mucosa obtained from anatomically-collected corpus mucosa). Degree of active and chronic inflammation was scored on a scale of 0 to 3 (0 = none, 1 = mild, 2 = moderate, and 3 = intense) according to the updated Sidney system[21]. As gastrin cells are absent from corpus mucosa, gastrin immunostaining was performed in all cases (indirect method with polymer detection system peroxidase/DAB) were performed for gastrin (polyclonal antibody, 1:1800 dilution, A0568, DAKO®), to ensure that the biopsied tissue was obtained from corpus. ECL-cell hyperplasia in the gastric corpus was also evaluated in all cases using chromogranin A staining (polyclonal antibody, 1:350 dilution, Invitrogen®). Results were scored as normal, linear, or nodular hyperplasia, using the modified Solcia classification[22]. According to this classification, linear ECL-cell hyperplasia is characterized by a linear sequence of at least five ECL-cells lying inside the basement membrane of glands. The diagnosis requires at least two such lines per linear millimeter of mucosa; micronodular ECL-cell hyperplasia is defined by the presence of micronodular clusters of five or more ECL-cells not exceeding 150 μm in size.
To characterize mucosal inflammatory infiltrate, corpus biopsy immunostaining with anti-CD3 antibody (polyclonal A0452, DAKO®), anti-CD20 antibody (Clone L26, M0755, DAKO®), anti-CD68 antibody (Clone PG-M1, A0452, DAKO®), and anti-gastrin (polyclonal, A0568, DAKO®) was performed. All positively-marked cells were counted (epithelium, crypt, and lamina propria) at 40x magnification; results were expressed as number of immunostained cells per field. Sections were independently evaluated by two experienced pathologists. Clinical data concerned age, gender, ethnic background, past medical history, familial history, and laboratorial data at admission. These included iron status evaluation (Hb, ferritin), serum B12 vitamin levels, serum gastrin, serum pepsinogen I, pepsinogen II, respective ratio (PGI/PGII), and autoimmune profile [PCA, intrinsic factor auto-antibodies (IFA), anti-thyroglobulin and/ or anti-thyroid peroxidase autoantibodies (TPO/Tg-Ab) and anti-transglutaminase auto-antibodies (TgA)]. H. pylori serology was further included. Other potentially contributing causes of anemia were excluded, namely gastrointestinal blood loss, nutritional deficiency, menstrual losses, inflammatory bowel disease, and celiac disease. No patient was under any pharmacological treatment, including anti-secretory therapy.
Descriptive statistical analysis was performed (mean, median, SD, minimum, and maximum).
The five patients included in this report had a mean age of 13.6 ± 2.8 years and a male:female ratio of 2:3. Three patients were Caucasian and two were of African origin. Regarding past medical history, one girl had type I diabetes and the rest were previously healthy. Familial history was positive for autoimmune thyroiditis (two mothers), multiple myeloma (MM) and sarcoidosis (one mother), breast cancer (two mothers), and type I diabetes (two uncles of one child). No patient had major gastrointestinal symptoms (Table 1).
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Mean ± SD | |
| Patient Data | ||||||
| Age (yr) | 14.75 | 16 | 14.1 | 16.1 | 7.25 | 13.6 ± 2.87 |
| Gender | M | F | M | F | F | - |
| Ethnicity | African | African | Caucasian | Caucasian | Caucasian | - |
| Personal History | - | - | Type I Diabetes | - | - | - |
| Familial History | Mother (sarcoidosis, multiple myeloma) | Mother (breast cancer). Uncles (type I diabetes) | Mother (breast cancer) | Mother (autoimmune thyroiditis) | Mother (autoimmune thyroiditis) | - |
| Symptoms | Dyspepsia | Dyspepsia | - | - | - | - |
| Laboratorial Data | ||||||
| Hemoglobin (g/dL) | 9.1 | 10.7 | 9.7 | 9.9 | 8.5 | 9.56 ± 0.71 |
| Serum ferritin (ng/mL) | 4.5 | 5.6 | 4.1 | 2.9 | 3.,2 | 4.06 ± 0.96 |
| (Ref: 7-140) | ||||||
| Vitamin B12 (pg/mL) | - | 509 | 236 | 618 | - | 454 ± 160 |
| (Ref: 200-835) | ||||||
| Gastrin (pg/mL) | 106 | 479 | 548 | 32 | 800 | 393 ± 286 |
| (Ref: < 100) | ||||||
| PGI/ PGII | 1.68 | 0.9 | 2.07 | 1.09 | 1.04 | 1.36 ± 0.46 |
| (Ref: > 1.0) | ||||||
| PCA | (+) | (+) | (+) | (+) | (+) | - |
| IFA | (-) | (-) | (-) | (-) | (-) | - |
| TPO/Tg-Ab | (-) | (-) | (-) | (+) | (-) | - |
Laboratorial values on admission included: mean Hb = 9.5 ± 0.7 g/dL; mean serum ferritin = 4.1 ± 0.9 ng/dL; mean serum gastrin = 393 ± 286 pg/mL (4/5 had hypergastrinemia) and the PGI/PGII ratio below 1 in only one patient. Serum B12 vitamin levels were normal in the three patients in whom it was performed (Table 1). Laboratory values were otherwise normal including liver and kidney function tests.
All patients bar one had negative IgG for H. pylori. By definition, all children had positive PCA; none had positive IFA. TPO/Tg-Ab were positive in the sole patient with autoimmune thyroiditis and none had positive TgA (Table 1).
Esophagogastroduodenoscopy showed slight nodular duodenitis in two patients and fold softening in the other two patients.
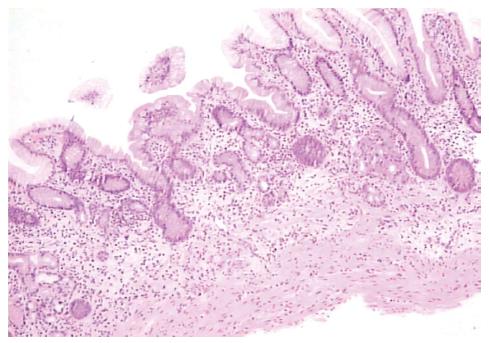
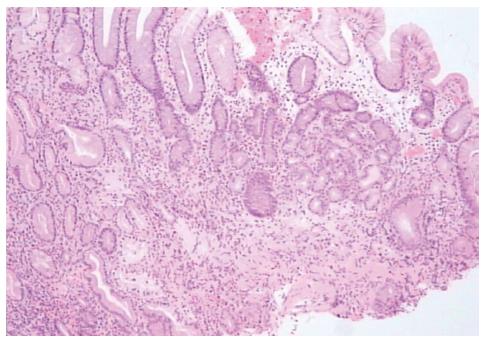
Histological findings included the presence of a diffuse and moderate to intense mononuclear infiltrate composed of lymphocytes and plasma cells in the lamina propria (Figure 1, Table 2). In all cases, patchy oxyntic gland mononuclear cell infiltration was evident, with moderate to severe atrophic gastritis in four patients (Figure 2) and mild atrophic gastritis in one (younger patient). In corpus mucosa, intestinal and pseudopyloric metaplasia were observed in one and three patients, respectively. All biopsies were negative for H. pylori, both in Giemsa staining and cultural examination.
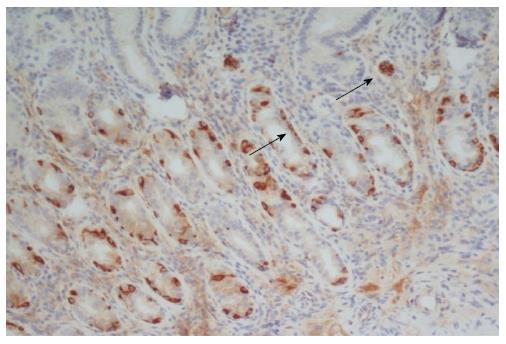
Characterization of corpus mucosa inflammatory infiltrate showed that it was mainly composed of T lymphocytes (CD3+) rather than B lymphocytes (CD20+) or macrophages (CD68+). Gastrin immunostaining of corpus biopsies was negative in all cases, indicating that the biopsies concerned the oxyntic mucosa (data not shown). Furthermore, chromogranin A staining at corpus showed linear ECL-cell hyperplasia in 4/5 patients and micronodular ECL-cell hyperplasia in 3/5 cases (Figure 3). Duodenal biopsies showed no major abnormalities in any of the patients.
AIG is recognized as a very rare condition in childhood[13,14], but due to its asymptomatic course in the majority of the patients, it may remain underdiagnosed at a young age. In this report, we studied five youths with a diagnosis of AIG presenting as IDA. Though this association has recently been highlighted in the adult population, it has rarely been reported in pediatric patients.
IDA is a common condition in childhood[23], and its refractoriness to oral iron therapy should suggest a range of gastrointestinal pathologies, including celiac disease. This entity was excluded in our patients. Although iron absorption is also known to be impaired in the setting of gastric hypo/achlorhydria, gastric atrophy is usually not considered a frequent etiological factor for IDA in the pediatric age group. In adult patients with IDA and no major gastrointestinal symptoms, it has been previously reported that 20% had gastric atrophy associated with hypergastrinemia and positive PCA[24]. Moreover, Hershko et al[25] reported that AIG is 4-6 times more frequent in patients with unexplained IDA than celiac disease. Although pernicious anemia is considered the classic hematological presentation of AIG, further studies have highlighted IDA as a precocious manifestation of this gastric condition[10,11]. Hershko et al[25] compared patients with macrocytic anemia and patients with IDA to show that the former were much younger, and the mean globular volume of erythrocytes, ferritin, and gastrin increases with age as cobalamin levels decrease[9]. In our report, all patients bar one (a seven year old girl) were adolescents and diagnosed as having AIG during investigation for refractory IDA, thus reinforcing the hypothesis of IDA as an early manifestation of AIG. On the other hand, it can’t be assumed that AIG is the only etiologic factor, as IDA in fertile woman may incur menstrual loss[10,26]. Although in the present study intravenous iron therapy and follow-up data were not included, it is well recognized that periodical replacement of iron stores in these patients is necessary.
Although serum gastrin levels were increased in three of our patients in the present study, the PGI/PGII ratio was low in only one patient. The different disease course time, as well as lesser histological involvement, should be taken into account in patients of a young age compared to those in adulthood.
Lahner et al[27] have recently reported, PCA and IFA sensitivity of 81.5% and 37%, respectively, in adults. It is not surprising that all our patients had positive PCA and negative IFA, as it has been argued that the positivity of IFA increases with age and the duration of the disease[28], and eventually some patients will develop IFA at later stages.
It has been clearly established that a CD4+ T cell response to the H/K ATPase beta-subunit, in particular, is essential for the initiation of autoimmune gastritis[29]. Thus, the immunopathology of autoimmune gastritis is due to a disruption of the mucosal immune response, potentially involving Treg cells, rather than a direct depletion of the end-stage parietal and zymogenic cells[29]. According to the frequent association of AIG with other autoimmune diseases, we report the case of one girl with a previous diagnosis of type 1 diabetes and another case with positive TPO/Tg-Ab without clinical manifestations. There was also a strong familial history of autoimmunity in three patients (type 1 diabetes in the uncles of one girl and autoimmune thyroiditis in the mothers of two girls). Previous studies showed that type 1 diabetes[30,31] and autoimmune thyroiditis[19,32,33] may be associated with positive PCA and AIG at young ages.
Segni et al[19] reported an incidence of PCA in patients with autoimmune thyroid disease reaching 21%, with atrophic gastritis in 5/18 patients submitted to endoscopy. Interestingly, all our patients had both positive PCA and corpus atrophic gastritis. This underlines the need to screen patients with autoimmune conditions for AIG.
Concerning histopathological features, we noticed that in all of our patients (with one exception) there was a moderate to severe gastritis with intense inflammatory infiltrate (T lymphocytes predominance, as expected), intestinal and/or pseudo-pyloric metaplasia, and ECL micronodular or linear hyperplasia. Micronodular hyperplasia is generally combined with linear hyperplasia and is typically found in patients with autoimmune chronic atrophic gastritis in association with higher levels of serum gastrin[22].
The importance of a firm histological diagnosis of AIG should be emphasized, which requires a representative number of gastric mucosal samples (antrum, corpus, and fundus) due to the focal nature of the process[4,34]. It is also essential to topographically localize mucosal biopsies in order to ensure that the gastritis process is localized to the corpus and fundus (negative immunohistochemistry for G cells).
The association of H. pylori with AIG is a controversial issue. Although AIG is not classically associated with H. pylori active infection, this agent had been advocated by some authors as a trigger for the development of the autoimmune process in the gastric mucosa[35-37].
According with this, no culture was positive and only one of our patients had positive IgG serology for H. pylori, which was compatible with past infection. These findings are consistent with previous reports that did not find an association between AIG and H. pylori infection[38]. Finally, concerning the issue of cancer risk, endoscopic surveillance of AIG every 5 years has been suggested[39].
In conclusion, our study aimed to further contribute to a better characterization of the clinical expression of AIG at a pediatric age, as well as highlighting IDA as a precocious hematological manifestation and the diagnostic contribution of a careful immunohistological evaluation. Furthermore, in the setting of personal and/or familial history of autoimmune disease, the importance of taking AIG into account and including PCA in the autoimmune screening panel is emphasized.
Autoimmune gastritis is a very rare entity in patients of a young age. Although classically associated with pernicious anemia in adulthood, recent studies have reported its presentation as an iron-deficiency anemia.
Pernicious megaloblastic anemia is a well-known complication of autoimmune gastritis. The association of this digestive disease with iron deficiency anemia in young patients and the mean globular volume evolution throughout life in autoimmune gastritis patients has currently been highlighted.
Autoimmune gastritis is classically seen as a disease of the elderly, manifesting as pernicious anemia. This study describes five cases of this disease in children and adolescents, which was diagnosed in the setting of refractory iron deficiency anemia.
Refractory iron deficiency anemia could be a manifestation of autoimmune gastritis in young patients and this diagnosis should be considered, after the exclusion of more frequent etiologies, particularly in the setting of a personal or familial history of other autoimmune diseases.
Autoimmune gastritis - atrophic gastritis restricted to the corpus associated with the presence of autoantibodies against the parietal cell proton pump H+/K+ adenosine triphosphatase. Refractory Iron Deficiency Anemia - Anemia (Hb < 2 SD for age and sex) due to iron deficiency (serum ferritin < 15 ng/mL), refractory to oral iron therapy for at least 6 mo and a requirement for intravenous iron therapy.
The inflammatory infiltrate in the gastric mucosa should be identified by immunohistology with antibody markers minimally for T cells, B cells, and macrophages. The characteristics of the gastric glandular atrophy should be documented by immunostaining for parietal cells by antibody to these cells in order to assess for any loss of these cells from the gastric mucosa; the hypergastrinemia supports the loss of these cells. The presence of metaplasia should be assessed by immunostaining for mucin.
P- Reviewer: Lee JC, Saniabadi AR, Toh BH S- Editor: Qi Y L- Editor: Rutherford A E- Editor: Ma S
| 1. | Toh BH, van Driel IR, Gleeson PA. Pernicious anemia. N Engl J Med. 1997;337:1441-1448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 418] [Cited by in RCA: 349] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 2. | Taylor KB, Roitt IM, Doniach D, Couchman KG, Shapland C. Autoimmune phenomena in pernicious anaemia: gastric antibodies. Br Med J. 1962;2:1347-1352. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 307] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 3. | Taylor KB. Inhibition of intrinsic factor by pernicious anaemia sera. Lancet. 1959;2:106-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 90] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 4. | Faller G, Kirchner T. Immunological and morphogenic basis of gastric mucosa atrophy and metaplasia. Virchows Arch. 2005;446:1-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 5. | Hogan TV, Ang DK, Gleeson PA, van Driel IR. Extrathymic mechanisms of T cell tolerance: lessons from autoimmune gastritis. J Autoimmun. 2008;31:268-273. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Solcia E, Capella C, Fiocca R, Cornaggia M, Rindi G, Villani L, Bosi F, Ambrosiani L. Exocrine and endocrine epithelial changes in type A and B chronic gastritis. Helicobacter pylori, Gastritis and Peptic Ulcer. Berlin: Springer-Verlag 1990; 245-58. [DOI] [Full Text] |
| 7. | Lehy T, Roucayrol AM, Mignon M. Histomorphological characteristics of gastric mucosa in patients with Zollinger-Ellison syndrome or autoimmune gastric atrophy: role of gastrin and atrophying gastritis. Microsc Res Tech. 2000;48:327-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Russell AC, Black JO, Schwartz DA, Correa H, Rosen MJ. 15-year-old girl with metaplastic atrophic gastritis and enterochromaffin-like cell hyperplasia. J Pediatr Gastroenterol Nutr. 2012;55:e148-e151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 9. | Hershko C, Ronson A, Souroujon M, Maschler I, Heyd J, Patz J. Variable hematologic presentation of autoimmune gastritis: age-related progression from iron deficiency to cobalamin depletion. Blood. 2006;107:1673-1679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 145] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 10. | Hershko C, Hoffbrand AV, Keret D, Souroujon M, Maschler I, Monselise Y, Lahad A. Role of autoimmune gastritis, Helicobacter pylori and celiac disease in refractory or unexplained iron deficiency anemia. Haematologica. 2005;90:585-595. [PubMed] |
| 11. | Irvine WJ, Cullen DR, Mawhinney H. Natural history of autoimmune achlorhydric atrophic gastritis. A 1-15-year follow-up study. Lancet. 1974;2:482-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 52] [Article Influence: 1.0] [Reference Citation Analysis (1)] |
| 12. | Zhang Y, Weck MN, Schöttker B, Rothenbacher D, Brenner H. Gastric parietal cell antibodies, Helicobacter pylori infection, and chronic atrophic gastritis: evidence from a large population-based study in Germany. Cancer Epidemiol Biomarkers Prev. 2013;22:821-826. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 13. | Houston GA, Files JC, Morrison FS. Race, age, and pernicious anemia. South Med J. 1985;78:69-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 14. | Adamu MA, Weck MN, Gao L, Brenner H. Incidence of chronic atrophic gastritis: systematic review and meta-analysis of follow-up studies. Eur J Epidemiol. 2010;25:439-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 15. | Greenwood DL, Crock P, Braye S, Davidson P, Sentry JW. Autoimmune gastritis and parietal cell reactivity in two children with abnormal intestinal permeability. Eur J Pediatr. 2008;167:917-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 16. | Katz S, Berernheim J, Kaufman Z, Lazar L, Erez I, Wolach B. Pernicious anemia and adenocarcinoma of the stomach in an adolescent: clinical presentation and histopathology. J Pediatr Surg. 1997;32:1384-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 15] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Guilloteau M, Bertrand Y, Lachaux A, Mialou V, Le Gall C, Girard S. [Pernicious anemia: a teenager with an unusual cause of iron-deficiency anemia]. Gastroenterol Clin Biol. 2007;31:1155-1156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Landin-Olsson M, Karlsson A, Dahlquist G, Blom L, Lernmark A, Sundkvist G. Islet cell and other organ-specific autoantibodies in all children developing type 1 (insulin-dependent) diabetes mellitus in Sweden during one year and in matched control children. Diabetologia. 1989;32:387-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 98] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Segni M, Borrelli O, Pucarelli I, Delle Fave G, Pasquino AM, Annibale B. Early manifestations of gastric autoimmunity in patients with juvenile autoimmune thyroid diseases. J Clin Endocrinol Metab. 2004;89:4944-4948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 20. | Queiroz DM, Mendes EN, Rocha GA. Indicator medium for isolation of Campylobacter pylori. J Clin Microbiol. 1987;25:2378-2379. [PubMed] |
| 21. | Dixon MF, Genta RM, Yardley JH, Correa P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20:1161-1181. [PubMed] |
| 22. | Solcia E, Fiocca R, Villani L, Luinetti O, Capella C. Hyperplastic, dysplastic, and neoplastic enterochromaffin-like-cell proliferations of the gastric mucosa. Classification and histogenesis. Am J Surg Pathol. 1995;19 Suppl 1:S1-S7. [PubMed] |
| 23. | Wu AC, Lesperance L, Bernstein H. Screening for Iron Deficiency. Ped Rev. 2002;23:171-178. [RCA] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 63] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 24. | Dickey W, Kenny BD, McMillan SA, Porter KG, McConnell JB. Gastric as well as duodenal biopsies may be useful in the investigation of iron deficiency anaemia. Scand J Gastroenterol. 1997;32:469-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 65] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Hershko C, Patz J, Ronson A. The anemia of achylia gastrica revisited. Blood Cells Mol Dis. 2007;39:178-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 25] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Marignani M, Delle Fave G, Mecarocci S, Bordi C, Angeletti S, D’Ambra G, Aprile MR, Corleto VD, Monarca B, Annibale B. High prevalence of atrophic body gastritis in patients with unexplained microcytic and macrocytic anemia: a prospective screening study. Am J Gastroenterol. 1999;94:766-772. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 40] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 27. | Lahner E, Norman GL, Severi C, Encabo S, Shums Z, Vannella L, Delle Fave G, Annibale B. Reassessment of intrinsic factor and parietal cell autoantibodies in atrophic gastritis with respect to cobalamin deficiency. Am J Gastroenterol. 2009;104:2071-2079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 130] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Carmel R. Reassessment of the relative prevalences of antibodies to gastric parietal cell and to intrinsic factor in patients with pernicious anaemia: influence of patient age and race. Clin Exp Immunol. 1992;89:74-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 62] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 29. | van Driel IR, Baxter AG, Laurie KL, Zwar TD, La Gruta NL, Judd LM, Scarff KL, Silveira PA, Gleeson PA. Immunopathogenesis, loss of T cell tolerance and genetics of autoimmune gastritis. Autoimmun Rev. 2002;1:290-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 30. | De Block CE, De Leeuw IH, Van Gaal LF. Autoimmune gastritis in type 1 diabetes: a clinically oriented review. J Clin Endocrinol Metab. 2008;93:363-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 130] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 31. | De Block CE, De Leeuw IH, Bogers JJ, Pelckmans PA, Ieven MM, Van Marck EA, Van Acker KL, Van Gaal LF. Autoimmune gastropathy in type 1 diabetic patients with parietal cell antibodies: histological and clinical findings. Diabetes Care. 2003;26:82-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Centanni M, Marignani M, Gargano L, Corleto VD, Casini A, Delle Fave G, Andreoli M, Annibale B. Atrophic body gastritis in patients with autoimmune thyroid disease: an underdiagnosed association. Arch Intern Med. 1999;159:1726-1730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 93] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 33. | Tozzoli R, Kodermaz G, Perosa AR, Tampoia M, Zucano A, Antico A, Bizzaro N. Autoantibodies to parietal cells as predictors of atrophic body gastritis: a five-year prospective study in patients with autoimmune thyroid diseases. Autoimmun Rev. 2010;10:80-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 34. | Ricuarte O, Gutierrez O, Cardona H, Kim JG, Graham DY, El-Zimaity HM. Atrophic gastritis in young children and adolescents. J Clin Pathol. 2005;58:1189-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 65] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 35. | Appelmelk BJ, Faller G, Claeys D, Kirchner T, Vandenbroucke-Grauls CM. Bugs on trial: the case of Helicobacter pylori and autoimmunity. Immunol Today. 1998;19:296-299. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | D’Elios MM, Appelmelk BJ, Amedei A, Bergman MP, Del Prete G. Gastric autoimmunity: the role of Helicobacter pylori and molecular mimicry. Trends Mol Med. 2004;10:316-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 109] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 37. | Amedei A, Bergman MP, Appelmelk BJ, Azzurri A, Benagiano M, Tamburini C, van der Zee R, Telford JL, Vandenbroucke-Grauls CM, D’Elios MM. Molecular mimicry between Helicobacter pylori antigens and H+, K+ --adenosine triphosphatase in human gastric autoimmunity. J Exp Med. 2003;198:1147-1156. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 202] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 38. | De Block CE, De Leeuw IH, Bogers JJ, Pelckmans PA, Ieven MM, Van Marck EA, Van Hoof V, Máday E, Van Acker KL, Van Gaal LF. Helicobacter pylori, parietal cell antibodies and autoimmune gastropathy in type 1 diabetes mellitus. Aliment Pharmacol Ther. 2002;16:281-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 39. | Armbrecht U, Stockbrügger RW, Rode J, Menon GG, Cotton PB. Development of gastric dysplasia in pernicious anaemia: a clinical and endoscopic follow up study of 80 patients. Gut. 1990;31:1105-1109. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 38] [Article Influence: 1.1] [Reference Citation Analysis (0)] |