Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15756
Revised: May 16, 2014
Accepted: June 14, 2014
Published online: November 14, 2014
Processing time: 239 Days and 17.7 Hours
AIM: To investigate the agreement and prognostic value of different measures of covert hepatic encephalopathy (CHE).
METHODS: One-hundred-and-thirty-two cirrhotic outpatients underwent electroencephalography (EEG), paper-and-pencil psychometry (PHES) and critical flicker frequency, scored on the original/modified (CFFo/CFFm) thresholds. Eighty-four patients underwent Doppler-ultrasound to diagnose/exclude portal-systemic shunt. Seventy-nine were followed-up for 11 ± 7 mo in relation to the occurrence of hepatic encephalopathy (HE)-related hospitalisations.
RESULTS: On the day of study, 36% had grade I HE, 42% abnormal EEG, 33% abnormal PHES and 31/21% abnormal CFFo/CFFm. Significant associations were observed between combinations of test abnormalities; however, agreement was poor (Cohen’s κ < 0.4). The prevalence of EEG, PHES and CFFo/CFFm abnormalities was significantly higher in patients with grade I overt HE. The prevalence of EEG and CFFm abnormalities was higher in patients with shunt. The prevalence of EEG abnormalities was significantly higher in patients with a history of HE. During follow-up, 10 patients died, 10 were transplanted and 29 had HE-related hospitalisations. Grade I HE (P = 0.004), abnormal EEG (P = 0.008) and abnormal PHES (P = 0.04) at baseline all predicted the subsequent occurrence of HE; CFF did not.
CONCLUSION: CHE diagnosis probably requires a combination of clinical, neurophysiological and neuropsychological indices.
Core tip: Covert hepatic encephalopathy is a heterogeneous entity, which should probably be sought for by a combination of clinical, neurophysiological and neuropsychological indices. Grade I hepatic encephalopathy (HE), as diagnosed by an experienced clinician, holds prognostic relevance and is associated with a higher degree of hepatic failure. Thus, while its use as an outcome for clinical trials is not recommended, the abolition of Grade I HE seems premature.
- Citation: Montagnese S, Balistreri E, Schiff S, De Rui M, Angeli P, Zanus G, Cillo U, Bombonato G, Bolognesi M, Sacerdoti D, Gatta A, Merkel C, Amodio P. Covert hepatic encephalopathy: Agreement and predictive validity of different indices. World J Gastroenterol 2014; 20(42): 15756-15762
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15756.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15756
The diagnosis of hepatic encephalopathy (HE) is problematic. Clinical scales have been criticized because of limited specificity and considerable inter-operator variability, especially in relation to mild HE[1]. Similarly, there is no real agreement as to which psychometric, neurophysiological, and/or psychophysical tests should be utilized[2].
Recently, the term covert hepatic encephalopathy (CHE) has been proposed. CHE encompasses both minimal HE (i.e., abnormalities detected on neuropsychological, neurophysiological and/or psychophysical testing) and grade I overt HE, based on the West Haven classification[3]. The idea behind the proposal is that the clinical diagnosis of grade II overt HE is less controversial than that of grade I. Thus, grouping any alteration which is milder than grade II may help contain disagreement between centres and facilitate comparisons in multicentre clinical trials. However, CHE has been agreed upon but not formally defined or assessed. In addition, while its potential advantages are clear in relation to the top end of the HE severity spectrum (≥ HE grade II), there is more uncertainty as to which test/test combination should be utilized to distinguish unimpaired patients from patients with CHE (bottom end of the HE severity spectrum).
The aim of the present study was to investigate the agreement and prognostic validity of clinical, psychometric, neurophysiological and psychophysical indices of CHE, also in relation to ammonia levels, portal-systemic shunt, HE history and the development of HE-related hospitalisations over time.
The patient population comprised 132 consecutive outpatients with cirrhosis (94 men; age: 58 ± 11 years) from 1 June 2009 to 1 November 2011. The diagnosis of cirrhosis and its etiology were determined by use of clinical, laboratory, radiological, and, where needed, histological variables. The functional severity of the liver disease was assessed using the Child-Pugh grading system[4] and model for end-stage liver disease (MELD)[5]. Patients were excluded if they were < 20 years or > 80 years of age, could not comply with the study procedures, had misused alcohol in the preceding 6 mo, had a history of significant head injury, cardiovascular/cerebrovascular disease or significant neurological/psychiatric co-morbidity, were taking psychoactive drugs, had symptoms or signs of infection or had overt HE ≥ grade II according to the West Haven criteria.
Neuropsychiatric assessment was conducted in one morning session after breakfast. All patients underwent clinical assessment, EEG recording, PHES and CFF, in the aforementioned order.
Clinical assessment: Each patient’s mental status was assessed by an experienced physician (MS or AmP). The assessment included a detailed and comprehensive medical history, full neurological examination; exclusion of concomitant neurological disorders or other metabolic encephalopathies; and a clinical grading of the neuropsychiatric abnormalities according to the West Haven criteria[6]. Patients were finally qualified as having/not having grade I overt HE and were excluded from the study if they had overt HE ≥ grade II.
Neurophysiology: EEGs were recorded for 10 minutes, eyes-closed, in a condition of relaxed wakefulness, using a 21-electrode EEG cap. Electrodes were placed according to the International 10-20 system; the ground electrode was Fpz; the reference electrode was Oz; impedance was kept below 5 kΩ. Each channel had its own analogue-to-digital converter; the resolution was 0.19 μV/bit (Brainquick 3200, Micromed, Italy equipment). One continuous 80-100 s period of artifact-free EEG tracing was selected for subsequent spectral analysis by Fast Fourier Transform. The following spectral parameters were calculated on the P3-P4 derivation: the mean dominant frequency (MDF), which is an estimate of the background frequency of the EEG, and the relative power of the spectral bands delta (1-3.5 Hz), theta (4-8 Hz), alpha (8.5-13 Hz) and beta (13.5-25.5 Hz). EEGs were classified as normal/abnormal based on the spectral criteria proposed by Van der Rijt et al[7] and subsequently modified by Amodio et al[8].
Psychometry: Psychometric performance was assessed, under standardized conditions, using number connection tests A and B, the digit symbol subtest of the Wechsler adult intelligence scale, and line tracing and serial dotting tests[9]. Individual psychometric test results were scored in relation to age- and education-adjusted Italian norms[10]. Psychometric performance was classified as impaired if the sum of the integer scores of each test computed from age- and education-adjusted Z values (integer score = -3 for Z≤ -3, -2 for -3 < Z≤ -2, -1 for -2 < Z≤ -1, 0 for -1 < Z < 1, 1 for Z≥ 1), known as psychometric hepatic encephalopathy score (PHES), was ≤ -4[9,10]. The mean psychometric Z score (MPZS) was also calculated, as suggested by Amodio et al[10].
Psychophysiology: Critical flicker frequency (CFF) is the highest frequency in cycles per second at which the flicker of a flickering light source can be detected; at frequencies above the cutoff the light source appears to be continuous. CFF was measured with a Hepatonorm analyzer and two alternative thresholds for abnormality were utilised: the original one (o) proposed by Kircheis et al[11] (abnormal < 39; CFFo) and the modified one (m) proposed by Romero-Gómes et al[12] (abnormal < 38; CFFm).
Fasting venous ammonia was measured in the emergency laboratory immediately after blood had been drawn in an iced tube.
Doppler ultrasound evaluations were obtained in 84/132 patients (64%) by three equally experienced operators (BG, BM, SD), using one ultrasound machine (ATL 5000, Philips) with a 5 MHz convex probe provided by a colour- and pulsed-Doppler device. Patients were qualified as having portal-systemic shunts if convoluted, anechoic channels were detected, and venous flow confirmed by colour-Doppler[13].
Information about previous episodes of overt HE (clinical records plus patients’/relatives’ reports) was obtained in 120 patients, of which 76 (63%) had a positive history. Finally, 79/132 patients (60%) patients were followed prospectively for 11 ± 7 mo, in relation to the occurrence of death/transplantation and HE-related hospitalisations.
One patient (male 50, Child-Pugh score B7, MELD 11) was studied prior to, and on three occasions after placement of a transjugular intrahepatic portal-systemic shunt (TIPS); one patient (male 70, A6, MELD 9) was studied immediately and 18 wk after TIPS placement; two patients (male 63, B9, MELD 14 and male 69, B7, MELD 9) were studied during and after the resolution of an episode of grade II overt HE.
The protocol was approved by the Hospital of Padua Ethics Committee. All participating subjects provided written, informed consent. The study was conducted according to the Declaration of Helsinki (Hong Kong Amendment) and European Good Clinical Practice guidelines.
Differences between groups were examined using Mann-Whitney U or Student t-test, as appropriate. Differences between multiple groups were examined using ANOVA (post-hoc: Tukey test) or Kruskal-Wallis ANOVA, as appropriate. Categorized indices were compared by Pearson’s χ2. Agreement between categorized indices was assessed by Cohen’s kappa coefficient (poor: 0 < κ < 0.4; fair: 0.4 < κ < 0.6; good: 0.6 < κ < 0.8; excellent: 0.8 < κ < 1). The predictive validity of different variables on the occurrence of HE-related hospitalizations was assessed using the Kaplan-Meier cumulative survival method; patients hospitalized because of HE were qualified as complete cases.
The etiology of cirrhosis was viral (hepatitis C or B) in 54 (41%) patients, alcohol in 49 (37%), mixed (viral plus alcohol) in 19 (14%), cryptogenic in three (2%), autoimmune and Wilson’s disease in two each (1.5%), and nonalcoholic steatohepatitis, primary biliary cirrhosis and haemochromatosis in one each (1%). Functionally, 44 patients (33%) were qualified as Child-Pugh class A, 62 (47%) B, and 26 (20%) C. The average MELD score was 13 ± 5.
On the day of study, 47 (36%) patients had grade I HE, 56 (42%) had abnormal EEG, 44 (33%) abnormal PHES and 41/28 (31/21%) abnormal CFFo/CFFm. Significant associations were observed between combinations of test abnormalities; however, agreement was poor (Tables 1 and 2). Similarly, significant correlations were observed between neurophysiological and neuropsychological/psychophysical indices analysed as continuous variables (MDF vs MPZS: r = 0.51, P < 0.0001; MDF vs CFF: r = 0.20, P < 0.05; MPZS vs CFF: not significant).
| PHES | CFFo | CFFm | ||||
| Normal | Abnormal | Normal | Abnormal | Normal | Abnormal | |
| EEG normal | 58 | 18 | 57 | 19 | 64 | 12 |
| EEG abnormal | 30 | 26 | 34 | 22 | 40 | 16 |
| Association | χ2 = 7.5, P = 0.006 | χ2 = 3.0, P = 0.08 | χ2 = 3.1, P = 0.08 | |||
| Cohen ĸ | 0.23 | 0.15 | 0.14 | |||
| CFFo | CFFm | |||
| Normal | Abnormal | Normal | Abnormal | |
| PHES normal | 69 | 19 | 75 | 13 |
| PHES abnormal | 22 | 22 | 29 | 15 |
| Association | χ2 = 11.0, P = 0.001 | χ2 = 6.5, P = 0.01 | ||
| Cohen ĸ | 0.3 | 0.21 | ||
Of the 47 patients with grade I HE, 34 (72%) had abnormal EEG, 30 (64%) abnormal PHES and 20/14 (43/30%) abnormal CFFo/CFFm. Such prevalence was significantly higher compared to that of patients with no grade I HE for all three types of indices (EEG: 72% vs 26%, P < 0.0001; PHES: 64% vs 16%, P < 0.0001; CFFo/CFFm 43% vs 25%, P = 0.03, 30% vs 16%, P = 0.07). Similarly, when quantitative neuropsychiatric indices were analysed as continuous variables, patients with grade I HE had slower EEG and worse PHES and CFF performances than their counterparts with no grade I HE (Table 3).
| EEG | Psychometry | CFF | ||||||
| MDF (Hz) | δ | θ | α | β | PHES | MPZS | ||
| No grade I HE | 10.3 ± 1.8 | 7% ± 6% | 26% ± 17% | 45% ± 17% | 21% ± 13% | -0.9 ± 2.9 | -0.2 ± 0.9 | 41.9 ± 5.4 |
| (n = 85) | ||||||||
| Grade I HE | 8.2 ± 1.9b | 15% ± 16%b | 43% ± 17%b | 29% ± 18%b | 12% ± 8%b | -5.3 ± 4.0b | -1.5 ± 1.1b | 39.9 ± 5.3a |
| (n = 47) | ||||||||
| No portal-systemic shunt | 10.1 ± 1.8 | 5% ± 3% | 29% ± 23% | 44% ± 18% | 21% ± 13% | -1.3 ± 2.8 | -0.3 ± 0.8 | 42.8 ± 5.1 |
| (n = 24) | ||||||||
| Portal-systemic shunt | 9.3 ± 2.2 | 11% ± 12%d | 35% ± 19% | 36% ± 19% | 18% ± 13% | -2.6 ± 3.8 | -0.7 ± 1.1 | 41.2 ± 5.7 |
| (n = 60) | ||||||||
| No HE history | 10.2 ± 1.7 | 6% ± 7% | 25% ± 17% | 48% ± 18% | 21% ± 12% | -1.5 ± 3.6 | -0.3 ± 1.1 | 41.9 ± 5.9 |
| (n = 44) | ||||||||
| HE history | 9.1 ± 2.1f | 12% ± 12%f | 37% ± 19%f | 35% ± 19%f | 17% ± 12% | -2.9 ± 4.0 | -0.8 ± 1.1e | 40.6 ± 5.3 |
| (n = 76) | ||||||||
Trend/significant differences in ammonia levels were observed in patients with/without grade I HE (91 ± 62 μmol/L vs 67 ± 52 μmol/L, P = 0.07) and patients with abnormal/normal EEG (101 ± 60 μmol/L vs 52 ± 42 μmol/L, P < 0.001). In contrast, ammonia levels were comparable in patients with/without PHES (86 ± 64 μmol/L vs 70 ± 52 μmol/L, P > 0.05) or CFFo/CFFm abnormalities (81 ± 62 μmol/L vs 74 ± 55 μmol/L, P > 0.05, 94 ± 67 μmol/L vs 71 ± 53 μmol/L, P > 0.05).
Of the 85 patients with no grade I HE, 22 (26%) had abnormal EEG, 14 (16%) abnormal PHES and 21/14 (25/16%) abnormal CFFo/CFFm. Depending on the CFF threshold utilised 47/51 (55/60%) patients had no test abnormalities (unimpaired). No significant associations were observed between combinations of test abnormalities (abnormal EEG vs abnormal PHES or CFFo/CFFm; abnormal PHES vs abnormal CFFo/CFFm). However, significant correlations were observed between neurophysiological and neuropsychological indices analysed as continuous variables (MDF vs MPZS: r = 0.30, P < 0.01; MDF and MPZS vs CFF: not significant).
Of the 84 patients who underwent Doppler ultrasound, 60 (71%) were qualified as having portal-systemic shunts. Trends were observed for a higher prevalence of EEG (50% vs 29%, P = 0.08) and CFFm abnormalities (25% vs 4%, P = 0.03) in patients with portal-systemic shunt; in contrast, the prevalence of PHES and CFFo abnormalities was comparable in the two groups. When quantitative neuropsychiatric indices were analysed as continuous variables, patients with portal-systemic shunt had slower EEG (delta power: 11% ± 12% vs 5% ± 3%, P = 0.02), while PHES and CFFo/CFFm performances were comparable in the two groups (Table 3).
Of the 120 patients in whom HE history was obtained, 76 (63%) had a positive history. The prevalence of EEG abnormalities was significantly higher in patients with a positive history (51% vs 27%, P = 0.01) while those of PHES and CFFo/CFFm abnormalities were comparable in the two groups. When quantitative neuropsychiatric indices were taken as continuous variables, patients with a history of HE had slower EEG (P < 0.01 for most spectral indices, Table 2) and worse PHES scores (-2.9% ± 3.9% vs -1.5% ± 3.5%, P = 0.05) than their counterparts with negative history, while CFF was comparable in the two groups (Table 3).
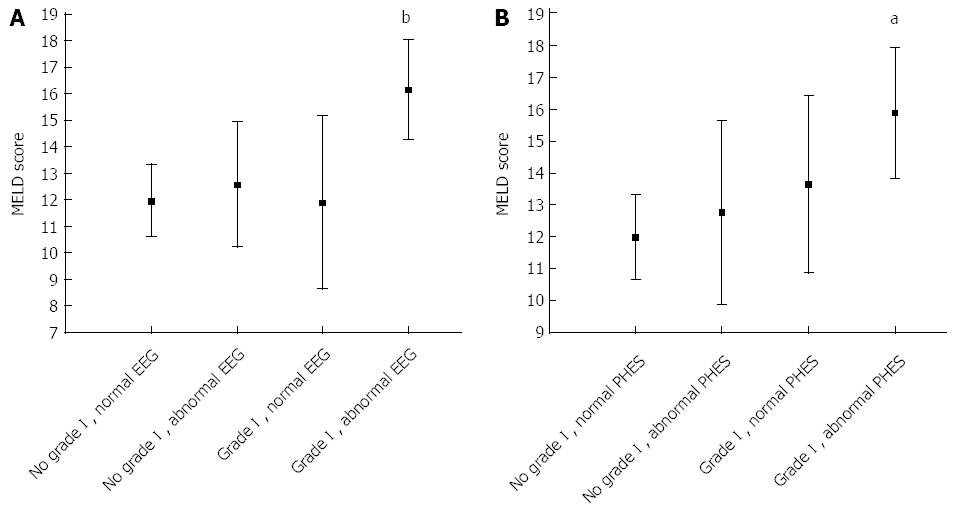
During follow-up, 10 patients died, 10 were transplanted and 29 had HE-related hospitalisation. The presence of grade I HE (P = 0.004), abnormal EEG (P = 0.008) and abnormal PHES (P = 0.04) at baseline all predicted the subsequent occurrence of HE; CFFo/CFFm did not. The presence of grade I HE had independent, additional prognostic value compared to tests alone as patients with grade I HE (with or without EEG/PHES abnormalities) had worse prognosis than those with EEG/PHES abnormalities who appeared clinically normal (Figure 1). In addition, only patients with a combination of EEG/PHES abnormality and grade I HE had significantly higher MELD scores than unimpaired patients (Figure 2).
In the four patients studied on repeat occasions, both the EEG and the PHES reflected changes in clinical conditions and modified treatment regimes, at least to some degree, while the CFF did not (Table 4).
| Patient | Condition | Time | EEG | Psychometry | CFF | |||||
| MDF (Hz) | δ | θ | α | β | PHES | MPZS | (Hz) | |||
| 1 male 55, B7, MELD 11 | Baseline | 0 | 10.8 | 8% | 19% | 49% | 24% | 5 | 1.6 | 41 |
| 1 | Post-TIPS, overt grade I | + 11 mo | 9.6 | 3% | 34% | 49% | 14% | 5 | 1.6 | 49 |
| 1 | Post-TIPS, treated | + 19 mo | 10.5 | 3% | 15% | 64% | 18% | 2 | 0.9 | 46 |
| 1 | Post-TIPS, treated | + 23 mo | 11.5 | 4% | 11% | 59% | 27% | 4 | 1.2 | 46 |
| 2 male 70, A6, MELD 9 | Post-TIPS, overt grade I | 0 | 7.9 | 7% | 64% | 20% | 9% | 0 | -0.2 | 46 |
| 2 | Post-TIPS, treated | + 18 wk | 10.7 | 10% | 18% | 44% | 28% | -1 | -0.1 | 39 |
| 3 male 63, B9, MELD 14 | Overt grade II | 0 | 4.8 | 42% | 50% | 6% | 2% | -6 | -1.8 | 42 |
| 3 | Treated | + 11 wk | 8.2 | 4% | 67% | 21% | 7% | -1 | -0.5 | 57 |
| 4 male 69, B7, MELD 9 | Overt grade II | 0 | 5.4 | 45% | 38% | 10% | 7% | -9 | -2.4 | 37 |
| 4 | Treated | + 6 wk | 10.8 | 4% | 21% | 51% | 24% | -3 | -0.7 | 39 |
The results of this study indicate that CHE is a heterogeneous syndrome.
The presence of grade I HE, diagnosed by an experienced hepatologist based on the original West Haven criteria[6], was associated with previous and subsequent HE episodes, with higher ammonia levels and with more profound alterations in psychometric, neurophysiological and psychophysical indices. In addition, patients with grade I HE and EEG/PHES abnormalities had higher MELD scores than unimpaired patients, while those with EEG/PHES abnormalities alone did not. This suggests that even mild neuropsychiatric alterations should be sought for on clinical examination, despite difficulties in their formal definition and in comparing them over time and/or across centres[1]. The fact that patients with grade I HE had higher MELD scores and were at higher risk of HE development makes it unlikely that their being qualified as grade I HE, even in the absence of PHES and/or EEG abnormalities, was a result of clinical misclassification.
The agreement between abnormalities in psychometric, neurophysiological and psychophysical indices was poor. In patients without grade I HE, abnormalities in these indices were not even associated with one another. These observations are probably related to a number of factors. Firstly, psychometric, neurophysiological and psychophysical tools measure different aspects of brain functioning, which do not necessarily change simultaneously, or even in parallel, especially in patients with mild/no clinical signs of HE[14]. It has already been highlighted that psychometric, neurophysiological and psychophysical tests can all be expected to be normal in very well compensated cirrhotics, and abnormal in those with severe, overt HE[2]. However, in intermediate states, their behavior is probably more erratic, and more sensitive to confounding factors[15]. Secondly, thresholds for abnormality of these indices for patients with cirrhosis have been derived in different ways, often on relatively small populations, and may need improving. For example, the original spectral EEG thresholds were derived by comparison of patients graded on the West Haven criteria[7]. In contrast, PHES thresholds were defined as deviations from normal reference values, adjusted for age and education, and then assessed in patients with cirrhosis with no/varying degree of clinical abnormality[9]. The hypothesis that threshold adjustment may be needed is supported by the fact that while limited associations were observed between test abnormalities, there were significant correlations between continuous psychometric, neurophysiological and psychophysical indices. Thirdly and finally, changes in psychometric, neurophysiological and psychophysical indices may reflect different pathogenic mechanisms. For example, EEG slowing has been shown to reflect high ammonia and indole levels, while abnormalities in PHES performance seem more closely associated with raised inflammatory markers[16].
Based on the above considerations, and the observation that grade I HE, the EEG and the PHES all predicted subsequent HE-related hospitalizations, their association may represent a reasonable screening system for CHE. Similarly, the EEG and the PHES could be utilized to monitor patients over time, as they were also shown to reflect, albeit on a small number of representative cases, changes in clinical conditions and therapeutic regimes. In addition, their association would ensure that both hyperammonaemia and inflammation, which are considered major pathogenic mechanisms in relation to HE development, are “covered”[17].
The fact that CFF, based either on the original or the modified threshold, had less obvious relationships with clinical status, a history and the subsequent development of HE is only partially in contrast with previously published data. In the original paper by Kircheis et al[11], considerable overlap was observed between CFFo performances of patients qualified as having no, subclinical and grade I HE; these classes would now all be part of CHE. At odds with our results, in the study by Romero-Gómez et al[12], correlations were observed between CFF and PHES performance in patients with minimal HE, and CFF at baseline predicted the development of HE over time. However, in the same study the agreement between PHES and CFFm abnormalities was poor, as in our study. This was also the case in a study by Dhiman et al[18], who also suggested that CFF, contrary to previous observations, might be strongly influenced by age. This was recently confirmed by Goldbecker et al[19] who, in line with our results, also showed limited sensitivity of CFF in confirming the diagnosis of overt HE.
In conclusion, our results suggest that CHE is a heterogeneous entity, which should probably be screened for by a combination of clinical, neurophysiological and neuropsychological indices. The presence of clinically detectable signs of HE, albeit mild, holds prognostic relevance. Thus the definition of a more reproducible, less operator-dependent diagnostic system for grade I HE seems worthy of further research efforts.
The diagnosis of hepatic encephalopathy relies on clinical, neurophysiological, psychometric and psychophysical indices. However, their respective relevance, agreement and predictive validity are unknown.
The nomenclature/grading of hepatic encephalopathy have been recently modified.
The current study contributes to a better definition of covert hepatic encephalopathy.
To current study provides the first set of data on the usefulness of different neuropsychiatric tools for the diagnosis of covert hepatic encephalopathy.
Covert hepatic encephalopathy is a newly introduced class of disease severity, which encompasses the minimal and mild overt forms of the disease.
In this article, authors analyse the agreement between clinical assessment, psychometric (PHES), neuropsycological (EEG) and psycophysical test (original and modified critical flicker frequency) in cirrhotic patients with covert hepatic encephalopathy (minimal and grade I overt hepatic encephalopathy). The authors conclude that covert hepatic encephalopathy is a heterogeneous entity, which should probably be evaluated by a combination of clinical, neurophysiological and neuropsychological tests. The study also evaluates the prognostic capacity of these tools, finding that the presence of grade I hepatic encephalopathy, abnormal EEG or abnormal PHES predict the development of a new episode of hepatic encephalopathy.
P- Reviewer: Simon-Talero M S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
| 1. | Kircheis G, Fleig WE, Görtelmeyer R, Grafe S, Häussinger D. Assessment of low-grade hepatic encephalopathy: a critical analysis. J Hepatol. 2007;47:642-650. [PubMed] |
| 2. | Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT. Hepatic encephalopathy--definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology. 2002;35:716-721. [PubMed] |
| 3. | Bajaj JS, Cordoba J, Mullen KD, Amodio P, Shawcross DL, Butterworth RF, Morgan MY. Review article: the design of clinical trials in hepatic encephalopathy--an International Society for Hepatic Encephalopathy and Nitrogen Metabolism (ISHEN) consensus statement. Aliment Pharmacol Ther. 2011;33:739-747. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 281] [Cited by in RCA: 261] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 4. | Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R. Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. 1973;60:646-649. [PubMed] |
| 5. | Kamath PS, Wiesner RH, Malinchoc M, Kremers W, Therneau TM, Kosberg CL, D’Amico G, Dickson ER, Kim WR. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464-470. [PubMed] |
| 6. | Conn HO, Leevy CM, Vlahcevic ZR, Rodgers JB, Maddrey WC, Seeff L, Levy LL. Comparison of lactulose and neomycin in the treatment of chronic portal-systemic encephalopathy. A double blind controlled trial. Gastroenterology. 1977;72:573-583. [PubMed] |
| 7. | Van der Rijt CC, Schalm SW, De Groot GH, De Vlieger M. Objective measurement of hepatic encephalopathy by means of automated EEG analysis. Electroencephalogr Clin Neurophysiol. 1984;57:423-426. [PubMed] |
| 8. | Amodio P, Marchetti P, Del Piccolo F, de Tourtchaninoff M, Varghese P, Zuliani C, Campo G, Gatta A, Guérit JM. Spectral versus visual EEG analysis in mild hepatic encephalopathy. Clin Neurophysiol. 1999;110:1334-1344. [PubMed] |
| 9. | Weissenborn K, Ennen JC, Schomerus H, Rückert N, Hecker H. Neuropsychological characterization of hepatic encephalopathy. J Hepatol. 2001;34:768-773. [PubMed] |
| 10. | Amodio P, Campagna F, Olianas S, Iannizzi P, Mapelli D, Penzo M, Angeli P, Gatta A. Detection of minimal hepatic encephalopathy: normalization and optimization of the Psychometric Hepatic Encephalopathy Score. A neuropsychological and quantified EEG study. J Hepatol. 2008;49:346-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 136] [Cited by in RCA: 149] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 11. | Kircheis G, Wettstein M, Timmermann L, Schnitzler A, Häussinger D. Critical flicker frequency for quantification of low-grade hepatic encephalopathy. Hepatology. 2002;35:357-366. [PubMed] |
| 12. | Romero-Gómez M, Córdoba J, Jover R, del Olmo JA, Ramírez M, Rey R, de Madaria E, Montoliu C, Nuñez D, Flavia M. Value of the critical flicker frequency in patients with minimal hepatic encephalopathy. Hepatology. 2007;45:879-885. [PubMed] |
| 13. | Berzigotti A, Merkel C, Magalotti D, Tiani C, Gaiani S, Sacerdoti D, Zoli M. New abdominal collaterals at ultrasound: a clue of progression of portal hypertension. Dig Liver Dis. 2008;40:62-67. [PubMed] |
| 14. | Ardila A, Bernal B. What can be localized in the brain? Toward a “factor” theory on brain organization of cognition. Int J Neurosci. 2007;117:935-969. [PubMed] |
| 15. | Montagnese S, Schiff S, De Rui M, Crossey MM, Amodio P, Taylor-Robinson SD. Neuropsychological tools in hepatology: a survival guide for the clinician. J Viral Hepat. 2012;19:307-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Montagnese S, Biancardi A, Schiff S, Carraro P, Carlà V, Mannaioni G, Moroni F, Tono N, Angeli P, Gatta A. Different biochemical correlates for different neuropsychiatric abnormalities in patients with cirrhosis. Hepatology. 2011;53:558-566. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 17. | Shawcross D, Jalan R. The pathophysiologic basis of hepatic encephalopathy: central role for ammonia and inflammation. Cell Mol Life Sci. 2005;62:2295-2304. [PubMed] |
| 18. | Dhiman RK, Kurmi R, Thumburu KK, Venkataramarao SH, Agarwal R, Duseja A, Chawla Y. Diagnosis and prognostic significance of minimal hepatic encephalopathy in patients with cirrhosis of liver. Dig Dis Sci. 2010;55:2381-2390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 146] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 19. | Goldbecker A, Weissenborn K, Hamidi Shahrezaei G, Afshar K, Rümke S, Barg-Hock H, Strassburg CP, Hecker H, Tryc AB. Comparison of the most favoured methods for the diagnosis of hepatic encephalopathy in liver transplantation candidates. Gut. 2013;62:1497-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 69] [Article Influence: 5.8] [Reference Citation Analysis (0)] |