Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15736
Revised: June 10, 2014
Accepted: July 22, 2014
Published online: November 14, 2014
Processing time: 234 Days and 23.4 Hours
AIM: To investigate the possible role of chitinase 3-like-1 (CHI3L1) in the progression of colitis-associated carcinoma (CAC).
METHODS: Thirty-four Balb/c mice were randomly assigned to five groups, including the control, CAC control, CAC + caffeine, colitis control and colitis + caffeine. Three animals were sacrificed every two weeks for blinded macroscopic inspection, histological analysis, and total RNA extraction. An immunofluorescent assay was performed using specimens from the colitis control and colitis + caffeine groups to investigate whether the protective effect of caffeine was associated with less oxidative DNA damage. In vitro, HT29 cells pre-stimulated with different concentrations of recombinant CHI3L1 protein and H2O2 were loaded with the DCFH-DA fluorescent probe to determine the effect of CHI3L1 on intracellular reactive oxygen species production.
RESULTS: CHI3L1 mRNA was increased during the progression of colon carcinogenesis. Tumors were mostly located in the distal end of the colon where the expression of CHI3L1 was higher than in the proximal colon. Caffeine-treated mice developed fewer tumors and milder inflammation than untreated mice. CHI3L1 protein increased reactive oxygen species in HT29 cells when exposed to H2O2.
CONCLUSION: Caffeine reduces tumor incidence by decreasing oxidative DNA damage. CHI3L1 may contribute to CAC by increasing reactive oxygen species production.
Core tip: Increased expression of chitinase 3-like-1 (CHI3L1) mRNA was found in ulcerative colitis patients with dysplasia. It is not known whether CHI3L1 contributes to inflammation-driven carcinogenesis. Our study showed increased expression of CHI3L1 during the progression of carcinogenesis. Caffeine protected against severe inflammation and neoplasms by acting as a scavenger of reactive oxygen species (ROS), which may be partly attributed to CHI3L1 inhibition. In vitro data showed that CHI3L1 increased ROS production in colonic epithelial cells under conditions of oxidative stress. Our study has made a modest advance in the exploration of CHI3L1 in colitis-associated carcinoma.
- Citation: Ma JY, Li RH, Huang K, Tan G, Li C, Zhi FC. Increased expression and possible role of chitinase 3-like-1 in a colitis-associated carcinoma model. World J Gastroenterol 2014; 20(42): 15736-15744
- URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15736.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15736
Cancer risk is a lifelong major fear in patients who suffer from inflammatory bowel diseases (IBD)[1]. In general, the risk of colorectal cancer (CRC) increases 8 or 10 years after the establishment of IBD diagnosis. Depending on the study and country, the risk of developing CRC in patients with ulcerative colitis (UC) fluctuates between 0.9-fold and 8.8-fold[2]. Compared with sporadic CRC, cancer in IBD is macroscopically heterogeneous and tends to be poorly delimited, irregular, and multifocal, which results in challenges for gastroenterologists associated with the detection of dysplasia by endoscopy[3]. Therefore, there is an urgent need to detect neoplastic lesions at an early stage.
The chitinase 3-like-1 (CHI3L1) molecule was discovered 10 years ago and is a biomarker for chronic inflammation and some types of carcinoma including IBD and colitis-associated carcinoma (CAC). CHI3L1 does not seem to be a specific biomarker for CAC. However, the pathogenic effect of CHI3L1 on IBD has gradually been recognized by more and more researchers. Serum CHI3L1 is elevated in UC and Crohn’s disease (CD) patients and increases with increasing disease activity and the degree of stricture formation[4,5]. CHI3L1 mRNA level was up-regulated in active UC and the involved region in CD compared with inactive UC and the uninvolved region in CD[6]. Chitin is the most abundant polysaccharide in microorganisms. CHI3L1 does not possess any catalytic activity, but is able to interact with chitin, and contributes to the exacerbation of acute colitis due to enhancement of bacterial adhesion and invasion of colonic epithelial cells through the chitin-binding protein[7,8]. Furthermore, CHI3L1 induces NF-κB activation and subsequent pro-inflammatory cytokine production such as TNF-α and IL-8 in colon cell lines (CECs). A recent study reported a significant increase in the expression of CHI3L1 in non-dysplastic mucosa from patients with IBD and remote dysplasia/cancer, compared to patients with IBD without dysplasia and healthy controls[9]. As dysplasia can be multifocal, the proximal CHI3L1-overexpressed mucosa is likely to have a high risk of developing dysplasia/cancer. Combining the evidence that CHI3L1 promotes acute inflammation and CAC arises in inflammation, we hypothesize that CHI3L1 may be an aggressive promoter for initiating and/or accelerating oncogenic transformation in chronic inflamed mucosa.
In the present study, we used the classic Azoxymethane (AOM)/dextran sulfate sodium (DSS) model to induce tumors in mice. We documented, for the first time, increased expression of CHI3L1 during the progression of carcinogenesis. As the development of acute colitis can be reduced with caffeine treatment due to the down-regulation of CHI3L1 expression[10], we evaluated whether caffeine can protect against chronic inflammation flare and reduce carcinogenic events. Because caffeine is an anti-oxidation agent and a CHI3L1 inhibitor, and oxidative stress is a well-accepted mechanism underlying the pathophysiology of IBD[11,12], we examined whether CHI3L1 is related to oxidative damage.
All animal experiments were approved by the Committee on Animal Experimentation of Sun Yat-Sen University and performed in compliance with the University’s Guidelines for the Care and Use of Laboratory Animals.
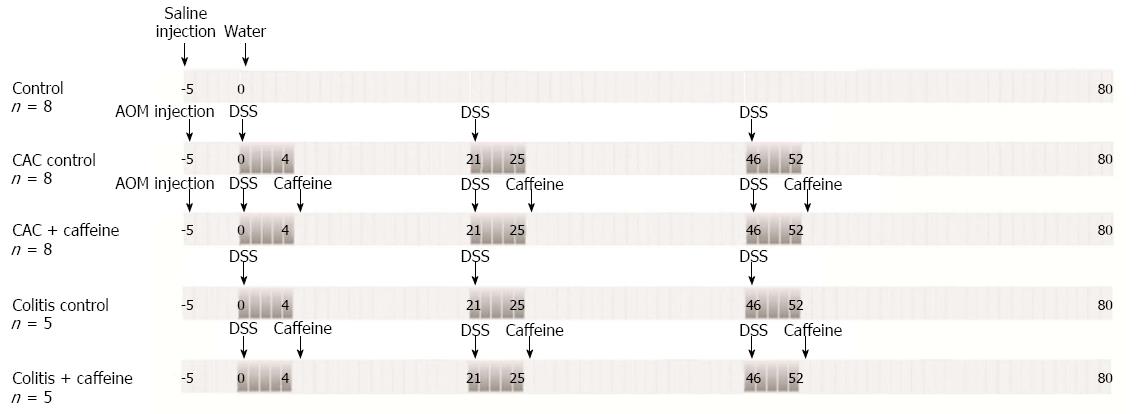
Forty nine 6-8-wk old male WT Balb/c mice were housed under specific pathogen-free conditions at Sun Yat-Sen University with free access to food and water during the course of the experiments. The studies contained three control groups: animals given saline by intraperitoneal injection and water without DSS (MW 36000-50000 D, MP Biomedicals, Santa Ana, CA, United States) which served as the control, mice given 3 cycles of DSS alone which served as the colitis control, and mice given DSS followed by an injection of AOM (Sigma-Aldrich, St. Louis, MO, United States) which served as the CAC control. Caffeine was administered orally in the period between DSS cycles in the CAC + caffeine and colitis + caffeine groups. These mice had free access to 2.5 mmol/L caffeine (Aladdin Reagent, Shanghai, China) dissolved in the drinking water. 2.5 mmol/L (approximately 19.419 mg/kg per mouse and equivalent to 2-3 cups of coffee) was an appropriate concentration according to previous in vivo and in vitro studies by Lee et al[10]. The protocol is illustrated in Figure 1. Three animals were sacrificed every two weeks for blinded macroscopic inspection, histological analysis, and total RNA extraction.
After careful dissection, the colon was divided equally into five segments from the proximal to the distal end. A third of each segment was fixed in 10% formalin, dehydrated in increasing concentrations of ethanol and embedded in paraffin. The other segments were preserved at -80 °C for subsequent RNA extraction. Tissue sections (3 μm) were stained using hematoxylin/eosin (HE) and examined under the microscope (Olympus BX51 microscope, Tokyo, Japan). For immunohistochemistry analysis, tissue sections were incubated in citrate buffer (0.01 mol/L, pH 6.0) at 95 °C-100 °C for 10 min in a water bath for antigen retrieval. The sections were allowed to cool to room temperature. The sections were then incubated in 3% H2O2 for 10 min and in 3% bovine serum albumin for 30 min at room temperature followed by overnight incubation at 4 °C with rabbit anti-YKL-40 antibody (1:70, PL Laboratories, British Columbia, Canada). The sections were then washed with PBS and incubated with peroxidase-marked secondary antibody for 1 h at room temperature. The immunoreaction product was detected using 3,3’-diaminobenzidinetetrahydrochloride (DAB) (ZSGB-Bio, Beijing, China) and examined under a microscope.
RNA was isolated using RNAiso Plus reagent (TaKaRa, Dalian, China), and RNA (1 μg) was reverse transcribed for synthesis of the first cDNA strand according to the manufacturer’s protocol. Real-time quantitative (Q)-PCR analyses were performed with the SYBR Green I Master and the LC480 thermal cycler quantitative PCR machine (Roche Diagnostics, Switzerland). Pairs of primers for mouse CHI3L1 (forward: 5’-GTACAAGCTGGTCTGCTACT-3’, reverse: 5’-GTTGGAGGCAATCTCGGAAA-3’) and mouse β-actin (forward: 5’-GTGGGCCGCTCTAGGCACCA-3’, reverse: 5’-CGGTTGGCCTTAGGGTTCAGGGG-3’) were used[6].
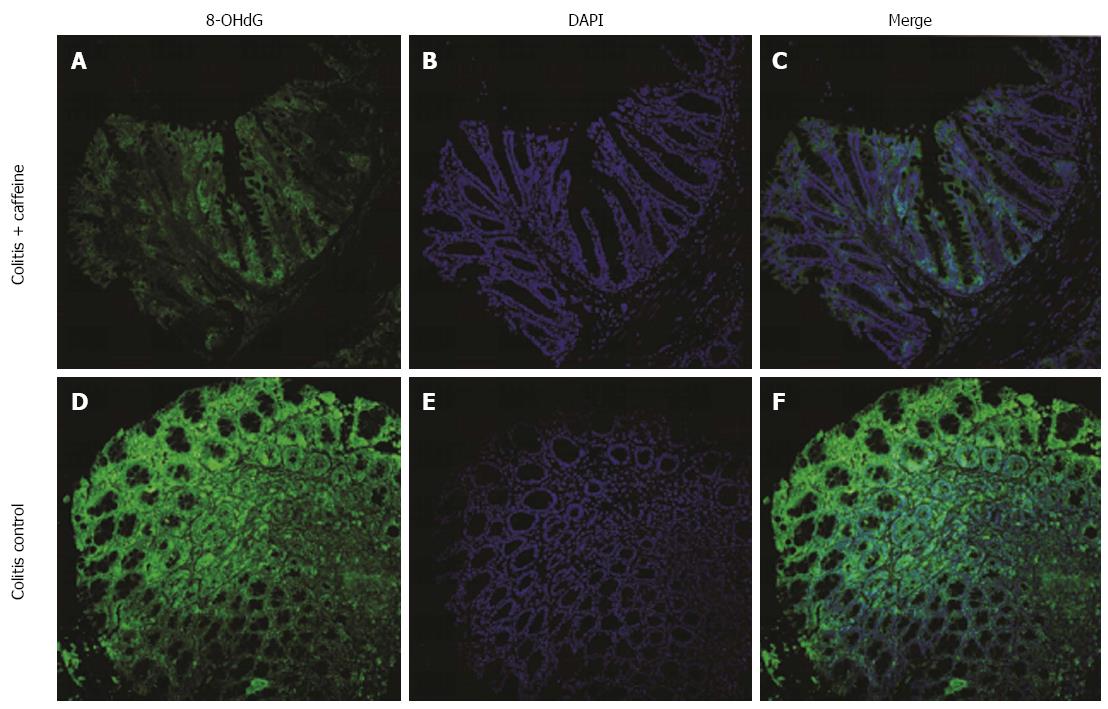
8-hydroxyguanine (8-OHdG), a marker of oxidative damage to RNA and DNA[13], was used to test whether reduced abnormal DNA and RNA products in colon specimens was one of the mechanisms involved in the protective effect of caffeine. Briefly, deparaffinized sections were rehydrated using routine methods. After antigen retrieval and blocking non-specific antigen binding sites, the primary antibody was applied (8-OHdG antibody, 1:80, Santa Cruz, CA, United States). The mixture was then incubated overnight in a moist environment at 4 °C. Next, the slides were washed three times with PBS for 5 min each wash, and goat-FITC secondary antibody was applied (1:100, Santa Cruz, CA, United States) in dark conditions. The sections were counter-stained with 4′,6-diamidino-2-phenylindole (DAPI, 1:20, Beyotime, Shanghai, China). The immunoreaction product was detected under the microscope at an excitation wavelength of 488 nm and an emission wavelength of 510 nm.
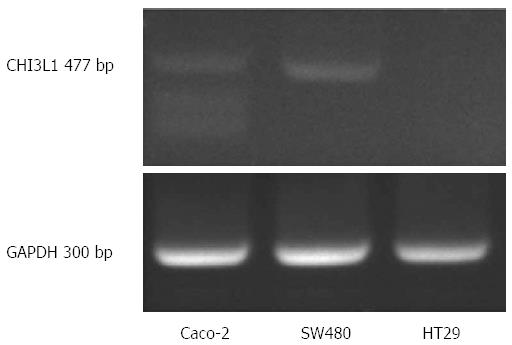
SW480, Caco-2 and HT29 cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, United States) and cultured in Dulbecco’s modified Eagle’s medium (Gibco), supplemented with 10% (v/v) fetal calf serum at 37 °C in 5% CO2. HT-29 cells do not express CHI3L1 constitutively and were therefore chosen for our study (Figure 2).
HT29 cells were cultured to 80% confluence in 6-well plates. HT29 cells were stimulated with different concentrations of recombinant human CHI3L1 protein (SB Inc. Beijing, China) for 24 h (0, 40, 80 ng/mL) followed by 200 μmmol/L H2O2 for 1.5 h. After that, HT29 cells were loaded with 10 μmmol/L of 2, 7-dichlorodihydrofluoresceindiacetate (DCHF-DA, Sigma-Aldrich, St. Louis, MO, United States) for 20 min and washed 3 times with PBS to avoid high background fluorescence[14]. Wells without stimulation were also loaded with fluorescent probe and served as controls. Fluorescence intensity was measured by a LSR Fotessa flow cytometer (BD, NJ, United States) at an excitation wavelength of 488 nm and an emission wavelength of 510 nm.
Values of the measured parameters were expressed as mean ± SD. Mauchly’s test of sphericity, student’s t-test or Welch’s test was used to detect significant differences between the groups. A value of P < 0.05 was considered statistically significant. All statistical analyses were processed using SPSS 19.0 (IBM, Armonk, NY, United States).
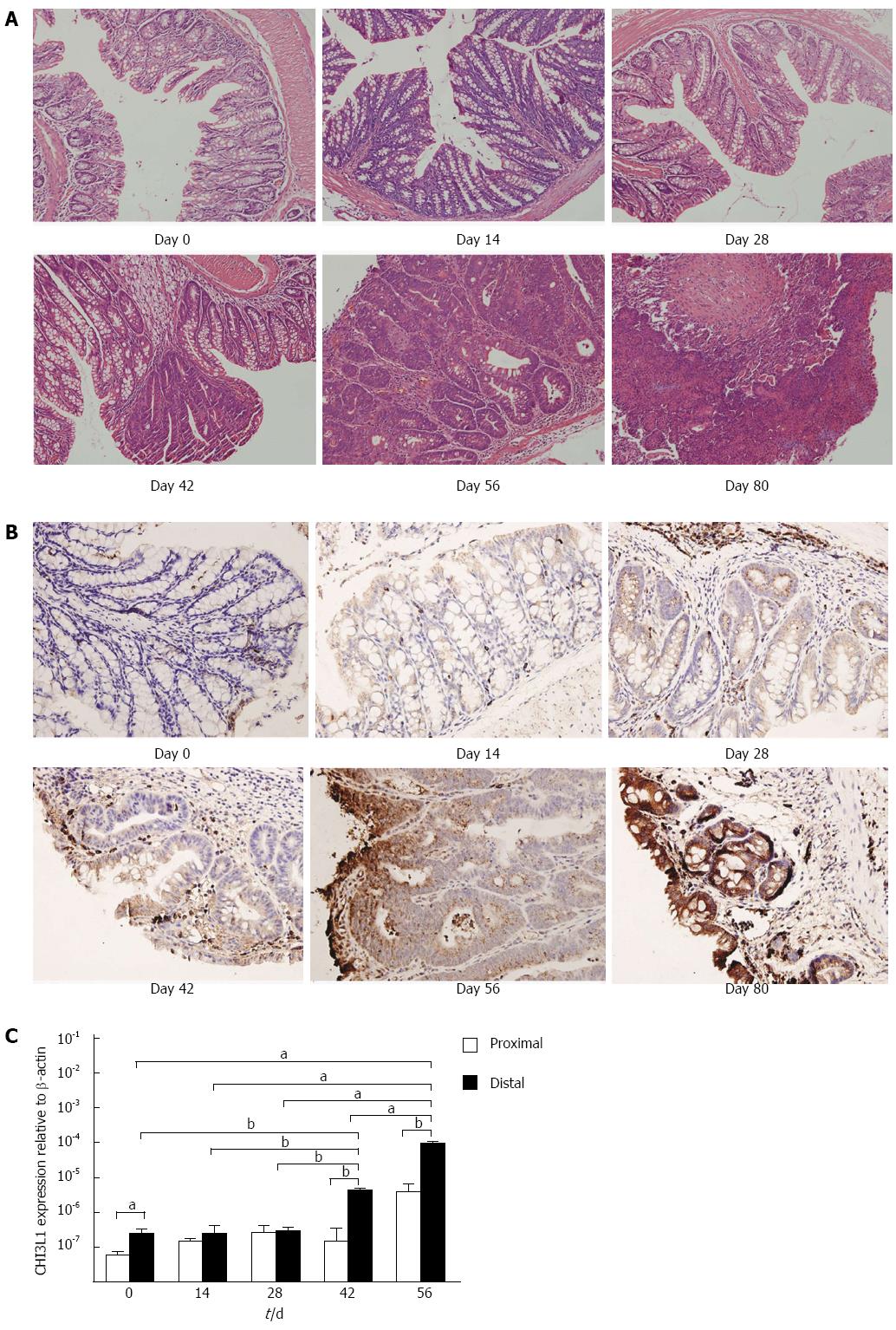
To monitor the histological changes during the time course of carcinogenesis, we sacrificed mice at day 0, 14, 28, 42, 56, and 80, respectively. Carcinoma in situ appeared at day 42, and advanced during the following days (Figure 3A). To confirm the hypothesis that CHI3L1 is associated with the promotion of carcinogenesis, we determined CHI3L1 protein expression by immunohistochemical analysis in the colons of AOM/DSS treated mice at the indicated time intervals. If CHI3L1 plays an essential role in neoplasia, its colonic expression should be altered during the course of carcinogenesis. As expected, CHI3L1 was faintly expressed in normal mucosa. Following injection of the carcinogen and subsequent DSS intake, CHI3L1 expression was up-regulated in neoplastic cells, crypt cells, and in cells infiltrating the lamina propria and submucosal regions (Figure 3B). Similarly, this pattern was observed at the mRNA level (Figure 3C). Furthermore, the inflection point was between days 35 and 42, which was consistent with the above microscopic changes.
According to a previous report, tumors are usually located in the middle to distal colon in mice[15]. This prompted us to investigate whether there was a difference in the level of CHI3L1 expression between the distal and proximal colon. CHI3L1 expression in the proximal colon was lower at day 42 and day 56 than in the distal colon (P < 0.01, Figure 3C), which indicated that the difference was more obvious after carcinoma in situ appeared.
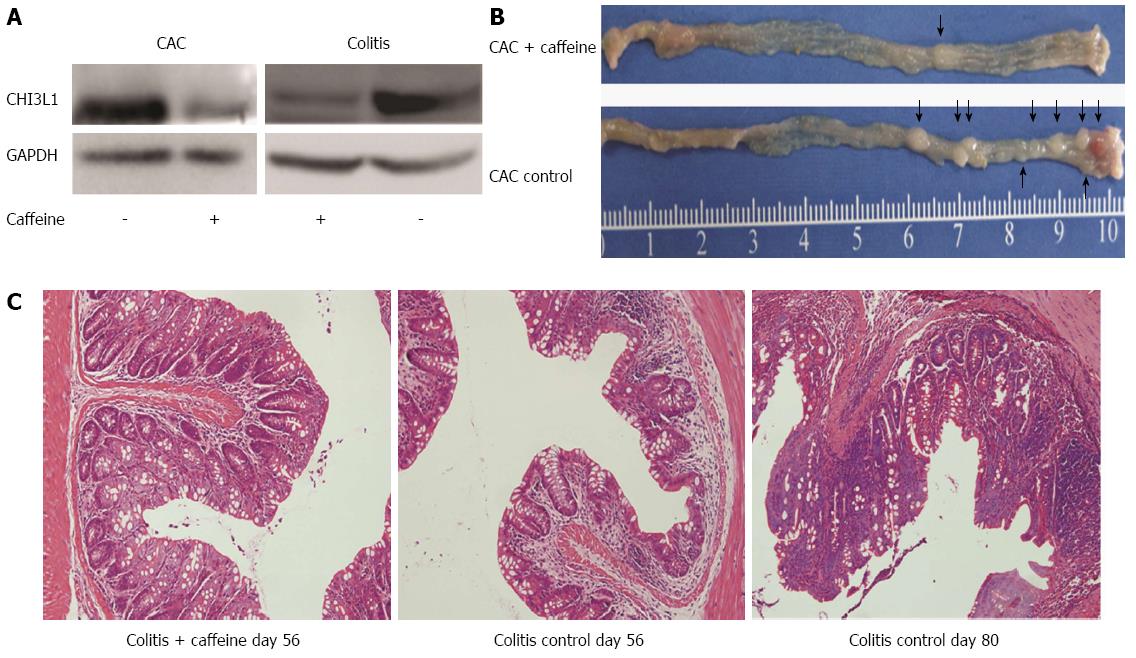
Western blot was performed to confirm the efficacy of caffeine. Compared with the untreated groups, the groups treated with caffeine showed decreased CHI3L1 expression (Figure 4A). Of note, there were apparent differences in the macroscopic and microscopic appearance of the colon in treated and untreated mice in the CAC group and colitis group (Figure 4B, C). The tumor incidence rate was 25% in the CAC + caffeine group (2/8) compared with 75% in the CAC control group (6/8). The specimen from the colitis control group at day 56 showed a loss of crypts of two-thirds and marked inflammatory cell infiltration. In contrast, the caffeine treated group showed relatively mild inflammation. Two mice in the colitis control group spontaneously developed carcinoma at day 80 (2/5), without AOM acceleration (Figure 4C). None of the mice in the colitis + caffeine group developed tumors (0/5).
Oxidative DNA damage leads to genetic mutations which have been linked to colitis-colorectal cancer transition[16]. As caffeine is a well-known antioxidant agent, we wondered whether this mechanism could explain the above protective effects. To confirm this hypothesis, we compared 8-OHdG expression in the colitis control group and the colitis + caffeine group. Mice treated with caffeine exhibited attenuated oxidative DNA damage (Figure 5).
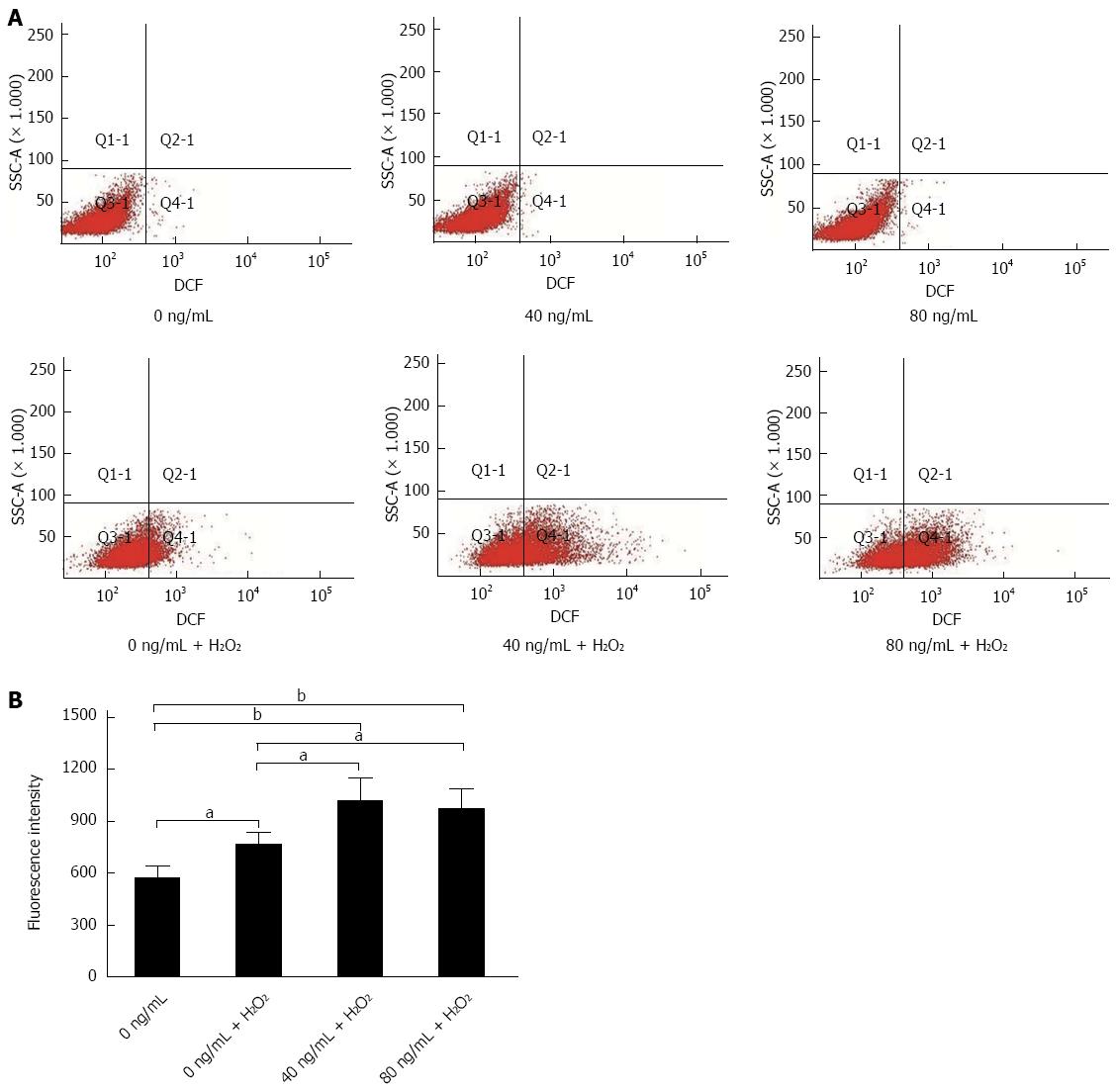
Caffeine can inhibit CHI3L1, thus we determined whether CHI3L1 influenced intracellular reactive oxygen species (ROS) production. As CHI3L1 is a type of secretory protein, we added recombinant protein to the cells to mimic exocrine secretion[17]. Koutroubakis et al[4] reported that mean serum YKL-40 levels were 102.6 ± 82.7 ng/mL in UC patients. Therefore, concentrations of 0 ng/mL, 40 ng/mL and 80 ng/mL were used in this study. H2O2 is usually used to initiate oxidative stress conditions in vitro and 200 μmol/L is an appropriate concentration to effectively enhance ROS level and maintain 92.0%-97.9% cell viability[18]. An oxidation-sensitive fluorescent probe, DCFH-DA, was used to detect intracellular oxidants. The results showed that recombinant CHI3L1 protein alone did not increase the intracellular level of ROS. However, when exposed to H2O2, HT29 cells supplemented with recombinant CHI3L1 protein achieved a higher loading concentration of fluorescence probe. CHI3L1 protein at 40 and 80 ng/mL significantly increased the level of ROS under oxidative stress conditions (P < 0.05, P < 0.05). However, a statistically significant dose-dependent association was not observed (Figure 6A, B).
Several classic acute murine models of UC including both gene deficiency (IL-10 knockout, T-cell receptor α-chain knockout, B-cell-deficient TCRα double knockout) and chemical induction methods (dextran sodium sulfate colitis) have shown increased expression of CHI3L1 mRNA[6]. However, to date, no inflammation-induced carcinogenesis model has been developed to show the change in CHI3L1 expression level during the progression of malignancy. This study showed that the expression of CHI3L1 is specifically induced during the course of colonic inflammation and ultimately carcinoma. Furthermore, expression in the distal colon was higher than that in the proximal colon, suggesting that CHI3L1 may be responsible for the development of tumors. Previous research has also correlated poor prognosis in colorectal cancer with increased CHI3L1 expression[19]. However, increased expression level alone is not enough to support the hypothesis that CHI3L1 contributes to inflammation-induced neoplasia. Therefore, we investigated whether a CHI3L1 inhibitor had a protective effect on the colon. Tumor number reflects tumor initiation, whereas a difference in tumor size typically provides evidence of the factors involved in tumor progression[20]. Caffeine-treated mice developed fewer and smaller tumors, suggesting that caffeine is effective in controlling inflammation-induced tumor initiation and tumor progression.
A previous study described a reduction in acute colitis due to caffeine, and correlated this with bacterial interaction[10]. Considering that anti-oxidation is a well-known function of caffeine, we examined the possibility that this may be one of the underlying mechanisms in addition to the inhibition of bacterial translocation. As expected, mice not treated with caffeine demonstrated severe oxidation-induced DNA damage, as evidenced by significant expression of 8-OHdG in colon samples. As caffeine is an inhibitor of CHI3L1, we investigated whether CHI3L1 was involved in increased oxidative stress. Various concentrations of CHI3L1 protein alone did not increase intracellular ROS in HT29 cells. However, when challenged with H2O2, CHI3L1-treated HT29 cells exhibited an enhanced level of ROS. This effect was obvious when 40 ng/mL CH3L1 protein was added. Although a statistically significant concentration-dependent effect was not observed, 40 ng/mL and 80 ng/mL CHI3L1 increased the level of ROS compared to the control group. This may be explained by the unstable nature of ROS, as they are very reactive[21-23]. However, the tendency was clear. Previous research has shown that a chitin derivative, chitosan oligosaccharide, can preserve the intestinal epithelial barrier integrity through anti-oxidative and anti-apoptotic mechanisms, which is consistent with the findings in our study[24]. Thus, it can be concluded that CHI3L1 makes the colon cell line more susceptible to oxidative stress and caffeine is a potent inflammation suppressor by decreasing oxidative products which lead to tumor initiation. Further in vivo studies should be carried out to determine whether CHI3L1 influences the balance between oxidative stress genes and antioxidant defense genes.
Besides chitosan oligosaccharide, chitin microparticles have also been reported to ameliorate DSS-induced acute colitis by modulating cytokine balance and the microbial environment in the colon[25]. However, it is difficult to satisfy the technological requirements for large scale preparation of chitin products of certain purity, diameter and concentration for medical use. Compared with chitin particles and chitosan oligosaccharide, relatively low extraction/production costs and good tolerance levels make caffeine an ideal option to target chitinase-mediated IBD pathogenesis. Other pan-family 18 chitinase inhibitors including allosamidin and methylxanthine derivatives (e.g., theophylline and pentoxifylline) are also attractive candidates requiring further research[26]. CHI3L1 antibody has already been proved to control bacterial adhesion and invasion in intestinal mucosa[6]. However, further investigation is required to determine the possible allergy and anti-antibody effect.
In conclusion, our study as presented has made a modest advance in the exploration of CHI3L1 in colitis-associated carcinoma. One limitation of our work is that caffeine is not a specific inhibitor of CHI3L1. The protective effects cannot solely, but may partly, be attributed to CHI3L1 inhibition. CHI3L1 gene deficient mice may provide strong support for the tumor promoter role of CHI3L1. Together with the finding that caffeine protects against severe inflammation and neoplasms by acting as a scavenger of ROS and the in vitro data indicating that CHI3L1 is involved in oxidative stress, it can be concluded that CHI3L1 may participate in inflammation-driven carcinogenesis by elevating ROS levels. Further research should be conducted to elucidate the unknown mechanisms involved in the induction of CHI3L1 in CAC and the feasibility of CHI3L1 as a therapeutic target of inflammatory bowel disease.
We are grateful to pathologist Chu-Di Chen for assistance in histological analysis.
Patients with long-standing ulcerative colitis have an increased risk of developing colorectal cancer, although the oncogenes involved are largely unknown.
Chitinase 3-like-1 (CHI3L1), one of the mammalian members of the chitinase family, was reported to contribute to the exacerbation of acute colitis in a mouse model. The role of CHI3L1 in colorectal cancer in the presence of inflammatory bowel disease is unknown. In this study, the authors demonstrated that CHI3L1 was over-expressed during the course of carcinogenesis and its possible promoter role in colitis-associated carcinoma was assessed.
Recent reports have highlighted the therapeutic effect of caffeine, as a pan-family 18 chitinase inhibitor, in ameliorating acute colitis by suppressing bacterial adhesion. This is the first study to report that caffeine is also able to reduce chronic inflammation and tumor incidence by scavenging reactive oxygen species (ROS). Furthermore, this in vitro studies suggested that CHI3L1 increased intracellular ROS production following H2O2 exposure. The authors conclude that CHI3L1 may participate in inflammation-driven carcinogenesis by elevating ROS levels.
This study is a preliminary exploration into the expression and possible mechanism of CHI3L1 in colitis-associated carcinoma. Blocking CHI3L1 expression may be a promising therapeutic option in the treatment of patients with inflammatory bowel disease.
Oxidative stress is defined as an imbalance between the generation of ROS and decreased antioxidant defense systems. ROS produced in inflammatory reactions lead to DNA damage and genetic alterations, which pave the way for chronic inflammatory bowel disease (IBD)-related colorectal cancer.
The work shows for the first time the association of increased expression of CHI3L1 with an experimental model of colitis-associated colorectal cancer. It also shows a temporal and spatial (more distal) relationship with the intensification of the expression of a candidate marker, underscoring its possible potential to indicate malignancy. The impact of CHI3L1 on intracellular ROS production provides an insight of the oncogeneic role of this gene into the process of IBD-related carcinogenesis.
P- Reviewer: Bian ZX, Kuo SM, Sipahi AM S- Editor: Ma YJ L- Editor: Webster JR E- Editor: Ma S
| 1. | Beaugerie L. Inflammatory bowel disease therapies and cancer risk: where are we and where are we going? Gut. 2012;61:476-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 2. | Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer Res. 2009;29:2727-2737. [PubMed] |
| 3. | Harpaz N, Polydorides AD. Colorectal dysplasia in chronic inflammatory bowel disease: pathology, clinical implications, and pathogenesis. Arch Pathol Lab Med. 2010;134:876-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 4. | Koutroubakis IE, Petinaki E, Dimoulios P, Vardas E, Roussomoustakaki M, Maniatis AN, Kouroumalis EA. Increased serum levels of YKL-40 in patients with inflammatory bowel disease. Int J Colorectal Dis. 2003;18:254-259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Erzin Y, Uzun H, Karatas A, Celik AF. Serum YKL-40 as a marker of disease activity and stricture formation in patients with Crohn’s disease. J Gastroenterol Hepatol. 2008;23:e357-e362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Mizoguchi E. Chitinase 3-like-1 exacerbates intestinal inflammation by enhancing bacterial adhesion and invasion in colonic epithelial cells. Gastroenterology. 2006;130:398-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 146] [Cited by in RCA: 172] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | Tran HT, Barnich N, Mizoguchi E. Potential role of chitinases and chitin-binding proteins in host-microbial interactions during the development of intestinal inflammation. Histol Histopathol. 2011;26:1453-1464. [PubMed] |
| 8. | Kawada M, Chen CC, Arihiro A, Nagatani K, Watanabe T, Mizoguchi E. Chitinase 3-like-1 enhances bacterial adhesion to colonic epithelial cells through the interaction with bacterial chitin-binding protein. Lab Invest. 2008;88:883-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 78] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 9. | Chen CC, Pekow J, Llado V, Kanneganti M, Lau CW, Mizoguchi A, Mino-Kenudson M, Bissonnette M, Mizoguchi E. Chitinase 3-like-1 expression in colonic epithelial cells as a potentially novel marker for colitis-associated neoplasia. Am J Pathol. 2011;179:1494-1503. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 10. | Lee IA, Low D, Kamba A, Llado V, Mizoguchi E. Oral caffeine administration ameliorates acute colitis by suppressing chitinase 3-like 1 expression in intestinal epithelial cells. J Gastroenterol. 2014;49:1206-1216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 11. | Keshavarzian A, Fusunyan RD, Jacyno M, Winship D, MacDermott RP, Sanderson IR. Increased interleukin-8 (IL-8) in rectal dialysate from patients with ulcerative colitis: evidence for a biological role for IL-8 in inflammation of the colon. Am J Gastroenterol. 1999;94:704-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 77] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Azer SA. Overview of molecular pathways in inflammatory bowel disease associated with colorectal cancer development. Eur J Gastroenterol Hepatol. 2013;25:271-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 13. | Symonds DA, Merchenthaler I, Flaws JA. Methoxychlor and estradiol induce oxidative stress DNA damage in the mouse ovarian surface epithelium. Toxicol Sci. 2008;105:182-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 29] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Hempel SL, Buettner GR, O’Malley YQ, Wessels DA, Flaherty DM. Dihydrofluorescein diacetate is superior for detecting intracellular oxidants: comparison with 2’,7’-dichlorodihydrofluorescein diacetate, 5(and 6)-carboxy-2’,7’-dichlorodihydrofluorescein diacetate, and dihydrorhodamine 123. Free Radic Biol Med. 1999;27:146-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 593] [Cited by in RCA: 588] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 15. | Suzuki R, Kohno H, Sugie S, Nakagama H, Tanaka T. Strain differences in the susceptibility to azoxymethane and dextran sodium sulfate-induced colon carcinogenesis in mice. Carcinogenesis. 2006;27:162-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 176] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 16. | Amrouche-Mekkioui I, Djerdjouri B. N-acetylcysteine improves redox status, mitochondrial dysfunction, mucin-depleted crypts and epithelial hyperplasia in dextran sulfate sodium-induced oxidative colitis in mice. Eur J Pharmacol. 2012;691:209-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 65] [Article Influence: 5.0] [Reference Citation Analysis (1)] |
| 17. | Shao R, Hamel K, Petersen L, Cao QJ, Arenas RB, Bigelow C, Bentley B, Yan W. YKL-40, a secreted glycoprotein, promotes tumor angiogenesis. Oncogene. 2009;28:4456-4468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 191] [Cited by in RCA: 233] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 18. | Shoji H, Oguchi S, Shinohara K, Shimizu T, Yamashiro Y. Effects of iron-unsaturated human lactoferrin on hydrogen peroxide-induced oxidative damage in intestinal epithelial cells. Pediatr Res. 2007;61:89-92. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 19. | Cintin C, Johansen JS, Christensen IJ, Price PA, Sørensen S, Nielsen HJ. High serum YKL-40 level after surgery for colorectal carcinoma is related to short survival. Cancer. 2002;95:267-274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 101] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 20. | Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998-2004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 557] [Article Influence: 30.9] [Reference Citation Analysis (0)] |
| 21. | Cash TP, Pan Y, Simon MC. Reactive oxygen species and cellular oxygen sensing. Free Radic Biol Med. 2007;43:1219-1225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 143] [Cited by in RCA: 136] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 22. | Gomes EC, Silva AN, de Oliveira MR. Oxidants, antioxidants, and the beneficial roles of exercise-induced production of reactive species. Oxid Med Cell Longev. 2012;2012:756132. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 158] [Cited by in RCA: 175] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 23. | Hawkins CL, Morgan PE, Davies MJ. Quantification of protein modification by oxidants. Free Radic Biol Med. 2009;46:965-988. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 348] [Cited by in RCA: 355] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 24. | Yousef M, Pichyangkura R, Soodvilai S, Chatsudthipong V, Muanprasat C. Chitosan oligosaccharide as potential therapy of inflammatory bowel disease: therapeutic efficacy and possible mechanisms of action. Pharmacol Res. 2012;66:66-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 137] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 25. | Nagatani K, Wang S, Llado V, Lau CW, Li Z, Mizoguchi A, Nagler CR, Shibata Y, Reinecker HC, Mora JR. Chitin microparticles for the control of intestinal inflammation. Inflamm Bowel Dis. 2012;18:1698-1710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 26. | Eurich K, Segawa M, Toei-Shimizu S, Mizoguchi E. Potential role of chitinase 3-like-1 in inflammation-associated carcinogenic changes of epithelial cells. World J Gastroenterol. 2009;15:5249-5259. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 62] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |