Published online Nov 14, 2014. doi: 10.3748/wjg.v20.i42.15715
Revised: April 22, 2014
Accepted: May 12, 2014
Published online: November 14, 2014
Processing time: 291 Days and 15 Hours
AIM: To investigate esophageal Helicobacter pylori (H. pylori) colonization on esophageal injury caused by reflux and the related mechanisms.
METHODS: An esophagitis model, with acid and bile reflux, was surgically produced in male rats. The rats were randomly divided into either: (1) an esophagogastroduodenal anastomosis (EGDA) group; (2) an EGDA with H. pylori infection group; (3) a pseudo-operation with H. pylori infection group; or (4) a pseudo-operation group. All rats were kept for 36 wk. Based on the location of H. pylori colonization, the EGDA rats with H. pylori infection were subdivided into those with concomitant esophageal H. pylori colonization or those with only gastric H. pylori colonization. The esophageal injuries were evaluated grossly and microscopically. The expressions of CDX2 and MUC2 were determined by real-time polymerase chain reaction (RT-PCR) and immunohistochemistry. Ki-67 antigen expression was determined by immunohistochemistry. The mRNA levels of cyclin D1, c-Myc, Bax and Bcl-2 were determined by RT-PCR. Cell apoptosis was evaluated using the TdT-mediated dUTP nick-end labeling method.
RESULTS: Esophagitis, Barrett’s esophagus (BE), and esophageal adenocarcinoma (EAC) developed in rats that underwent EGDA. When comparing rats with EGDA and concomitant esophageal H. pylori colonization to EGDA-only rats, the severity of injury (87.9 ± 5.2 vs 77.2 ± 8.6, macroscopically, 92.5 ± 8.0 vs 83.8 ± 5.5, microscopically, both P < 0.05) and the incidences of BE (80.0% vs 33.3%, P = 0.055) and EAC (60.0% vs 11.1%, P < 0.05) were increased. These increases were associated with upregulation of CDX2 and MUC2 mRNA (10.1 ± 5.4 vs 3.0 ± 2.9, 8.4 ± 4.6 vs 2.0 ± 3.2, respectively, Ps < 0.01) and protein (8.1 ± 2.3 vs 3.3 ± 3.1, 7.3 ± 4.0 vs 1.8 ± 2.7, respectively, all P < 0.05). The expression of Ki-67 (8.9 ± 0.7 vs 6.0 ± 1.7, P < 0.01) and the presence of apoptotic cells (8.3 ± 1.1 vs 5.3 ± 1.7, P < 0.01) were also increased significantly in rats with EGDA and concomitant esophageal H. pylori colonization compared with rats with EGDA only. The mRNA levels of cyclin D1 (5.8 ± 1.9 vs 3.4 ± 1.3, P < 0.01), c-Myc (6.4 ± 1.7 vs 3.7 ± 1.2, P < 0.01), and Bax (8.6 ± 1.6 vs 5.1 ± 1.3, P < 0.01) were significantly increased, whereas the mRNA level of Bcl-2 (0.6 ± 0.3 vs 0.8 ± 0.3, P < 0.01) was significantly reduced in rats with EGDA and concomitant esophageal H. pylori colonization compared with rats with EGDA only.
CONCLUSION: Esophageal H. pylori colonization increases esophagitis severity, and facilitates the development of BE and EAC with the augmentation of cell proliferation and apoptosis in esophageal mucosa.
Core tip: The relationship between gastroesophageal reflux disease and Helicobacter pylori (H. pylori) is controversial. This study demonstrates that esophageal H. pylori colonization can aggravate esophageal injury and promote the incidence of Barrett’s esophagus and esophageal adenocarcinoma. Gastric H. pylori colonization did not aggravate esophageal mucosal lesions in rats with mixed reflux. However, esophageal H. pylori infection was associated with increased cell proliferation and apoptosis in the esophagi of rats with mixed reflux. Loss of balance between cell proliferation and apoptosis may be important in H. pylori-induced esophageal malignancy.
-
Citation: Chu YX, Wang WH, Dai Y, Teng GG, Wang SJ. Esophageal
Helicobacter pylori colonization aggravates esophageal injury caused by reflux. World J Gastroenterol 2014; 20(42): 15715-15726 - URL: https://www.wjgnet.com/1007-9327/full/v20/i42/15715.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i42.15715
Esophageal adenocarcinoma (EAC) has received considerable attention in recent years due to the rapid increase in incidence rate and poor prognosis[1]. As a result, the number of studies attempting to elucidate the mechanisms of this increasingly prevalent cancer has increased. Barrett’s esophagus (BE) is the result of intestinal metaplasia, where the normal esophageal squamous epithelium is replaced by simple columnar intestinal-type epithelium. BE is generally recognized as the major precursor for EAC. The estimated risk of BE patients developing EAC is 30-125 times higher than that of the general population[2]. Although various factors have been suggested to cause BE development, the pathogenesis of the disease remains unknown.
Chronic gastroesophageal reflux with bile plays a crucial role in the development of BE[3]. Acid and bile, the main components of gastroesophageal refluxate, act synergistically to induce mucosal injury[4]. Both components have been suggested to promote intestinal-type differentiation in esophageal keratinocytes by inducing the expression of the caudal type homeobox 2 (cdx2) gene, an intestine-specific transcription factor involved in intestinal differentiation. Aberrant expression of this gene is associated with intestinal metaplasia and tumorigenesis[5]. Homeodomain protein CDX2 regulates goblet-specific mucin 2 (muc2) gene expression resulting in the differentiation of intestinal epithelium[6]. Experimental models of gastroesophageal reflux in rodents provide a basis for understanding the molecular events, which characterize the metaplasia-dysplasia-adenocarcinoma process in the esophagus.
Helicobacter pylori (H. pylori) has been widely recognized as an important causative factor in the development of gastric adenocarcinoma. Studies indicate that H. pylori may colonize in the lower esophagus with gastric metaplasia. The prevalence of H. pylori infection in the lower esophagus varies from 20%-70%[7,8]. A preliminary study on the association between H. pylori infection and gastroesophageal reflux disease (GERD) suggested that the colonization of H. pylori occurs in the lower esophagus. Hence, the bacteria potentially aggravate esophageal mucosa injury and increase the incidence of BE and EAC with the upregulation of CDX2[9]. However, that particular study lacked an additional group of rats with both chronic reflux and esophageal H. pylori infection. Data analyses combined all the groups of rats with chronic reflux and esophageal H. pylori infection, as well as rats treated with celecoxib. The implicit mechanisms for the increase in esophageal injury and the subsequent progression from esophagitis to BE and EAC associated with H. pylori colonization are unknown.
The imbalance between cell proliferation and apoptosis is common in neoplasia development. Ki-67 expression, a marker of cell proliferation, is increased in Barrett’s epithelium and adenocarcinoma[10]. Cyclin D1, a regulating factor of the cell cycle, and c-Myc, a transcriptional enhancer, are overexpressed in gastric tumorigenesis induced by H. pylori infection[11,12]. Cell apoptosis is a complex process regulated by various apoptotic proteins, such Bcl-2 and Bcl2-associated X protein (Bax). While Bax promotes apoptosis, Bcl-2 inhibits the process. Both Bax and Bcl-2 have been shown to be associated with the development of gastric adenocarcinoma caused by H. pylori infection[13,14]. However, the alteration of cell proliferation and apoptosis in the esophagus in response to H. pylori infection has not been investigated.
The aims of the present study were to verify our previous findings by using a group of rats with chronic reflux and esophageal H. pylori colonization, and to investigate the possible mechanisms in the sequence of inflammation-metaplasia-adenocarcinoma in response to H. pylori colonization of the esophagus. A rat model of chronic acid and bile reflux with H. pylori infection was successfully established. As a result, the severity of esophagitis, the incidence of BE and EAC, the index of proliferation and apoptosis of esophageal mucosa, and the expression of possible regulators could be determined.
Sixty-five specific-pathogen-free male Sprague-Dawley rats (8-wk-old) were purchased from Vital River Laboratory Animal Technology Co. Ltd. (Beijing, China). The rats were allowed to acclimate for one week with standard solid laboratory chow and distilled water, in an animal room with controlled temperature, and a 12 h light-dark cycle. The rats were randomly divided into four groups: (1) an esophagogastroduodenal anastomosis (EGDA) group (n = 15); (2) an EGDA-H. pylori infection group (n = 34); (3) a pseudo-operation with H. pylori infection group (pseudo-operation with H. pylori gastric gavage; n = 8); and (4) a pseudo-operation group [pseudo-operation with brain heart infusion (BHI) gastric gavage; n = 8]. In our previous study, the mortality of rats that underwent EGDA was 40%-50%, and the prevalence of esophageal H. pylori colonization was 64.3%[9]. As a result, more rats were assigned to both the EGDA and EGDA-H. pylori infection groups. Based on the H. pylori colonization site, the rats in the EGDA-H. pylori infection group were further divided into two subgroups: EGDA with concomitant esophageal H. pylori colonization and EGDA with gastric H. pylori colonization. EGDA was performed as described previously to create a rat model with acid and bile reflux[15]. Briefly, a side-to-side anastomosis was made between the gastroesophageal junction and the middle duodenum on the anti-mesenteric border with an accurate mucosa-to-mucosa position. In the two pseudo-operation groups, the rats’ abdomens were opened, the bowels were flipped, and the abdomens were sutured. H. pylori infection was established by inoculating the H. pylori strain SS1 intragastrically two weeks after EGDA. The study was approved by the Animal Care Committee of Peking University First Hospital (Beijing, China).
Rats were anesthetized 36 wk after surgery with chloral hydrate (0.3 mg/kg, intraperitoneal). The esophagus was removed 0.2 cm above the anastomotic stoma. The macroscopic score of esophageal mucosal injury was evaluated according to the presence of: (1) hyperemia; (2) edema; (3) erosion; (4) ulcer; (5) intramural; or (6) intraluminal hemorrhage[16]. Once removed, the esophagus was longitudinally cut into three segments. One segment was fixed in 40 g/L formaldehyde, and the other two were kept in liquid nitrogen for subsequent mRNA extraction. Gastric specimens were taken and opened along the greater curvature. The anterior and posterior wall of the stomach, including the body and the antrum, were cut roughly into two 3 mm × 10 mm segments along the greater curvature. One segment was used for a rapid urease test and the other was immediately immersed in 10% buffered formalin and embedded in paraffin. Blood samples were collected from the abdominal aorta and centrifuged for 20 min at 3500 rpm. The supernatants were aliquoted and stored at -70 °C until further use.
Paraffin sections (4 μm thick) were stained with hematoxylin and eosin (HE) to examine inflammation of the esophagus and the presence of BE and EAC. BE was defined as the presence of columnar epithelium with intestinal-type goblet cells[17]. EAC was diagnosed when dysplastic columnar epithelial cells invaded the basement membrane[18]. The microscopic scores of the esophageal mucosal injury were evaluated as previously described[16]. High-iron diamine-Alcian blue-Periodic acid-Schiff (HID-AB-PAS) staining was performed according to the HID and AB-PAS kit protocols (Shijiheli Co. Ltd., Beijing, China). For immunohistochemical staining, paraffin sections were immersed in xylene and hydrated using a graded alcohol series. Antigen retrieval was performed by immersing the sections in 0.01 mol/L citrate buffer (pH 6.0) and heating in a microwave oven at 80 W for 20 min. After a 10 min peroxide-block treatment (BioGenex Laboratories, Fremont, CA, United States) to eliminate endogenous peroxidase activity, the sections were blocked with power block (BioGenex Laboratories) for 10 min in a humidified box at room temperature to eliminate non-specific binding. Sections were incubated overnight at 4 °C with primary antibodies: rabbit anti-rat Ki-67 monoclonal antibody (Abcam, Cambridge, United Kingdom), mouse anti-rat CDX2 monoclonal antibody (Cell Signaling Technology Inc., Beverly, MA, United States), or rabbit anti-rat MUC2 polyclonal antibody (Santa Cruz Biotechnology, Dallas, TX, United States). The sections were rinsed in phosphate-buffered saline (PBS) and incubated with super enhancer (BioGenex Laboratories) for 20 min, followed by Polymer-HRP (BioGenex Laboratories) for 40 min. Staining was developed with diaminobenzidine (BioGenex Laboratories) under a light microscope. Sections were counterstained with hematoxylin. Full-tissue section slides were scored using an “H-score approach”[19]. The percentage of cells positively stained was scored as: a = 0% (score 0); 1%-20% (score 1); 21%-40% (score 2); 41%-60% (score 3); 61%-80% (score 4); or 81%-100% (score 5). The intensity of staining was categorized as i = absent (score 0); faint (score 1); moderate (score 2); or strong (score 3). High power magnification (400 ×) was used to count the number of positive cells. The final score for the counted cells was calculated by multiplying a by i with a minimum possible value of 0 and a maximum of 15.
Total RNA was extracted from frozen esophageal tissues. The esophageal tissue was cleaved with 1 mL TRIzol® (Life Technologies, Carlsbad, CA, United States), followed by 200 μL of chloroform. The mixture was then shaken vigorously and centrifuged at 12000 rpm for 15 min at 4 °C. The supernatant was mixed with 500 μL of isopropanol and centrifuged again at 12000 rpm for 15 min at 4 °C. The RNA pellet was washed with 75% ethanol, dried, resuspended in sterile water, and quantified using a NanoDrop spectrophotometer (ND-1000V3.5.2 software, NanoDrop Technologies, Wilmington, DE, United States). Reverse transcription of mRNA to cDNA was performed using a High Capacity cDNA Reverse Transcription Kit (Life Technologies). The total reaction volumes were 20 μL, and consisted of 10 μL of 2 × master mix and 10 μL of RNA sample (2 μg total RNA). Reverse transcription was performed in a thermal cycler (Eppendorf, Hamburg, Germany) at 25 °C for 10 min, 37 °C for 120 min, and 85 °C for 5 min. The cDNAs were aliquoted and stored at -70 °C. Real-time polymerase chain reaction (RT-PCR) was performed using the Applied Biosystems 7500 Sequence Detection System (Applied Biosystems of Thermo Fisher Scientific, Waltham, MA, United States). Primers were designed by Primer Express 3.0 software (Applied Biosystems) and were as follows: Cdx2, forward, 5’- CCATGAGGAGCACGGACACT-3’ and reverse, 5’-TTCTGCCCTCTGT CCTCGAT-3’; Muc2, forward, 5’-CGTGCCACGGCAAGGT-3’ and reverse, 5’-AGTGTCACAGG AGCAGGAGTC-3’; C-Myc, forward, 5’-GCGTTATTTGAAGCCTGAATTTCC-3’ and reverse, 5’-CCTGTTAGCGAAGCTCACGTTG-3’; Ccnd1 (cyclin D1), forward, 5’-CTA ATGTAAAGCCAGCCGCAATG-3’ and reverse, 5’-TGGACACAGCAGCCCTCAAG -3’; Bcl-2, forward, 5’-GGGATGCCTTTGTGGAACTATATG-3’ and reverse, 5’-TGAGCAGCGTCTTCAGAGACA-3’; Bax, forward, 5’-GCGATGAACTGGACAA CAACAT-3’ and reverse, 5’-TAGCAAAGTAGAAAAGGGCAACC-3’; and Actb (β-actin; housekeeping gene), forward, 5’-GCCTCACTGTCCACCTTCCA-3’ and reverse, 5’-GTCCGCCTAGAAGCATTTGC-3’. RT-PCR was performed in a final volume of 15 μL containing 1 μL of cDNA, 7.5 μL of SYBR Green RT-PCR Master Mix (Applied Biosystems), 1 μL of forward and reverse primers, and 5.5 μL of sterile water. The reactions were carried out according to the following parameters: denaturation at 95 °C for 10 min, followed by 40 cycles at 95 °C for 15 s, 60 °C for 1 min, and a further melting curve step at 95 °C for 15 s, 60 °C for 30 s, and 95 °C for 15 s. All reactions were performed in triplicate. The PCR products were quantified using the 2-ΔΔCT comparative method.
H. pylori strain SS1 obtained from the National Collection of Type Cultures (Colindale, London, United Kingdom) was cultured in a microaerobic humidified atmosphere on a Columbia agar (Oxoid, Hampshire, United Kingdom) plate with 80 mL/L defibrinogen goat blood (Baote Ltd., Beijing, China) at 37 °C. After 48 h incubation, the bacteria were harvested in BHI broth (Oxoid, Hampshire, United Kingdom) and adjusted to 1 × 108 CFU/mL. EGDA rats were inoculated intragastrically for two weeks with 1 mL of H. pylori suspension every other day for a total of three times.
Warthin-Starry silver staining, a rapid urease test (RUT), and a serological test were used to confirm H. pylori infection. Paraffin-embedded esophageal and gastric sections were processed according to the Warthin-Starry silver staining protocol with H. pylori appearing dark-brown to black. For the RUT, samples from rat stomach and the lower esophagus were placed in a tube filled with RUT reagent and monitored for color change (from yellow to red) for up to 24 h. For the serological test, an antigen-specific antibody in serum was detected by an enzyme-linked immunosorbent assay (CagA-Hp-IgG ELISA kit, Jingying Co. Ltd., Shanghai, China)[9,20]. Samples with optical density (A) readings 2.1-fold greater than uninfected rats were considered positive. Rats with positive Warthin-Starry silver staining, or with positive RUT and anti-H. pylori serological tests were considered positive for H. pylori infection.
Apoptosis was determined by the TdT-mediated dUTP nick-end labeling (TUNEL) method according to the protocol of the In Situ Cell Death Detection kit (Roche, Basel, Germany). Sections were de-waxed with xylene and hydrated, and then placed in 0.1 M citrate buffer (pH 6.0) in a microwave oven at 80 W for 20 min. After rinsing with PBS, sections were mixed with TUNEL mixture (enzyme solution and label solution) and incubated at 37 °C for 60 min in a dark humidified box. Following this incubation, the converter-peroxidase was added and sections were incubated at 37 °C for 30 min. Staining was developed with diaminobenzidine, and sections were counterstained with hematoxylin. TUNEL scoring was the same as that used for immunohistochemistry.
All quantitative values are expressed as mean ± SD. Statistically significant differences between groups were determined using χ2, Fisher’s exact, or nonparametric Wilcoxon and Mann-Whitney U tests. Data management and statistical analyses were performed using SPSS software 16.0 (SPSS Inc., Chicago, IL, United States). All analyses were considered significant when the P value was less than 0.05.
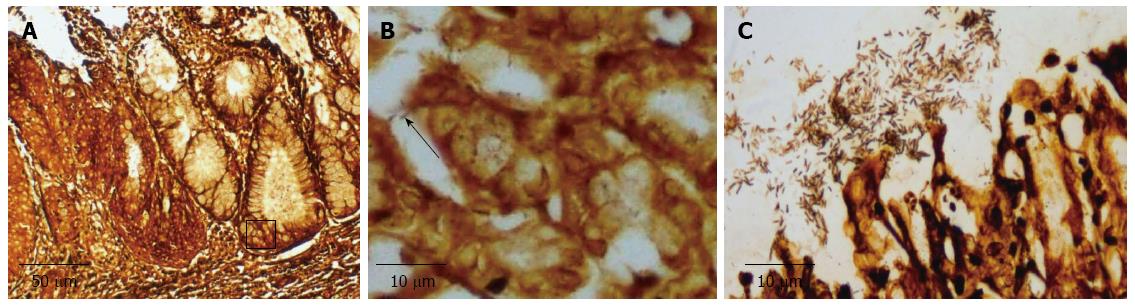
Within eight weeks of surgery, 21 rats died: six in the EGDA group, and 15 in the EGDA-H. pylori infection group. Of these, ten were due to anastomotic infarction, seven to malnutrition caused by vomiting, and four were due to undetermined reasons. Forty-four rats survived 36 wk after operation and completed the study: eight were in the pseudo-operation group, eight were in the pseudo-operation-H. pylori infection group, nine were in the EGDA group, and 19 were in the EGDA-H. pylori infection group. Gastric colonization of H. pylori was detected in all H. pylori inoculated rats. Of the 19 rats in the EGDA-H. pylori infection group, 10 were found to have concomitant H. pylori colonization in the esophagus (Figure 1).
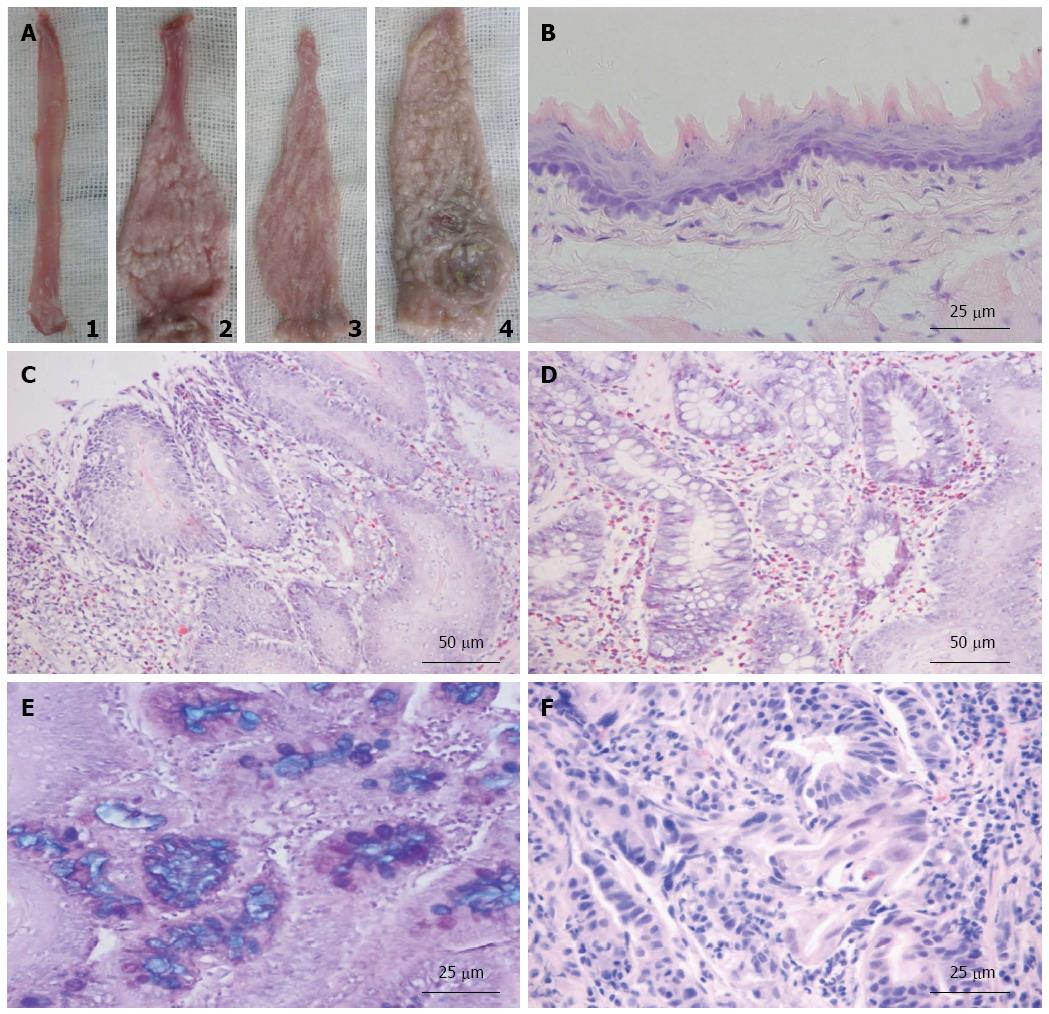
The esophagi in the two pseudo-operated groups appeared smooth and light pink (Figure 2A). The middle and lower esophagi of EGDA rats were markedly dilated and thickened with hyperkeratinization. Epithelial sloughing and ulcerations were found in large areas of the lower esophagi in EGDA rats. Esophagitis was identified as either a “white cobblestone” appearance or a “white tree bark” appearance (Figure 2A).
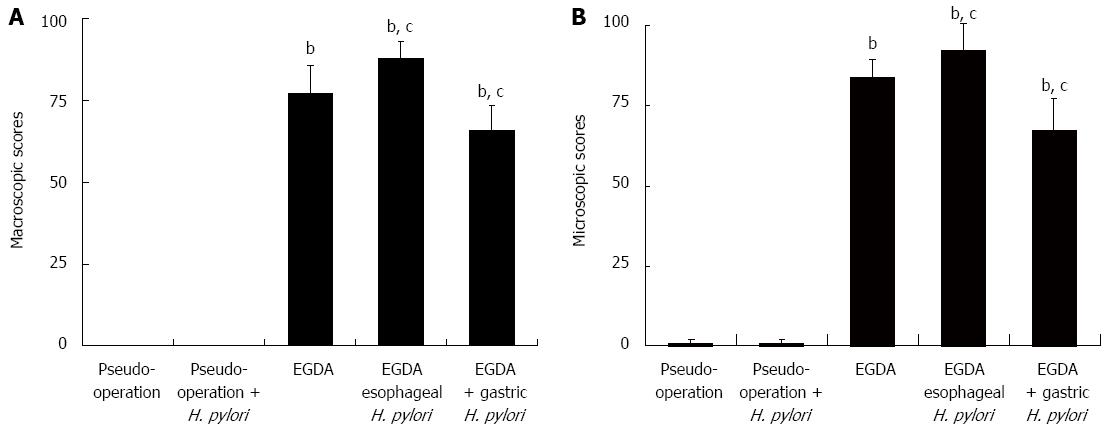
Under the microscope, normal rat esophagus displayed stratified keratinized squamous epithelium (Figure 2B). The esophagi of rats in the two pseudo-operation groups did not reveal any abnormalities. However, mucosal injury and typical appearance of reflux esophagitis were found in the middle and lower esophagi of rats that underwent EGDA. An increase in the thickness of squamous epithelium, with basal cell hyperplasia and elongation of lamina propria papillae, was found in the EGDA rat esophagi (Figure 2C). Other pathologic findings included the infiltration of inflammatory cells, squamous epithelium deletion, columnar epithelium replacement, intestinal metaplasia, dysplasia, and even EAC (Figure 2D-F). The scores of esophageal mucosal injury were increased in EGDA rats compared to rats without EGDA (P < 0.05). There were significant reductions in the scores of esophageal mucosal injury in EGDA rats with only gastric H. pylori colonization compared to EGDA rats (P < 0.05). The EGDA rats with concomitant esophageal H. pylori colonization had higher mucosal injury scores in the lower esophagus than EGDA rats (P < 0.05) (Figure 3).
None of the rats in the two pseudo-operation groups suffered from BE or EAC. There was no statistical difference in the incidence of BE and EAC in EGDA rats compared to rats in the two pseudo-operation groups (Table 1). Concomitant esophageal H. pylori colonization accompanied by chronic reflux increased the incidence of BE and EAC (P < 0.05). However, the frequency of BE and EAC remained unchanged between the EGDA rats with only gastric H. pylori colonization and the EGDA rats (Table 1).
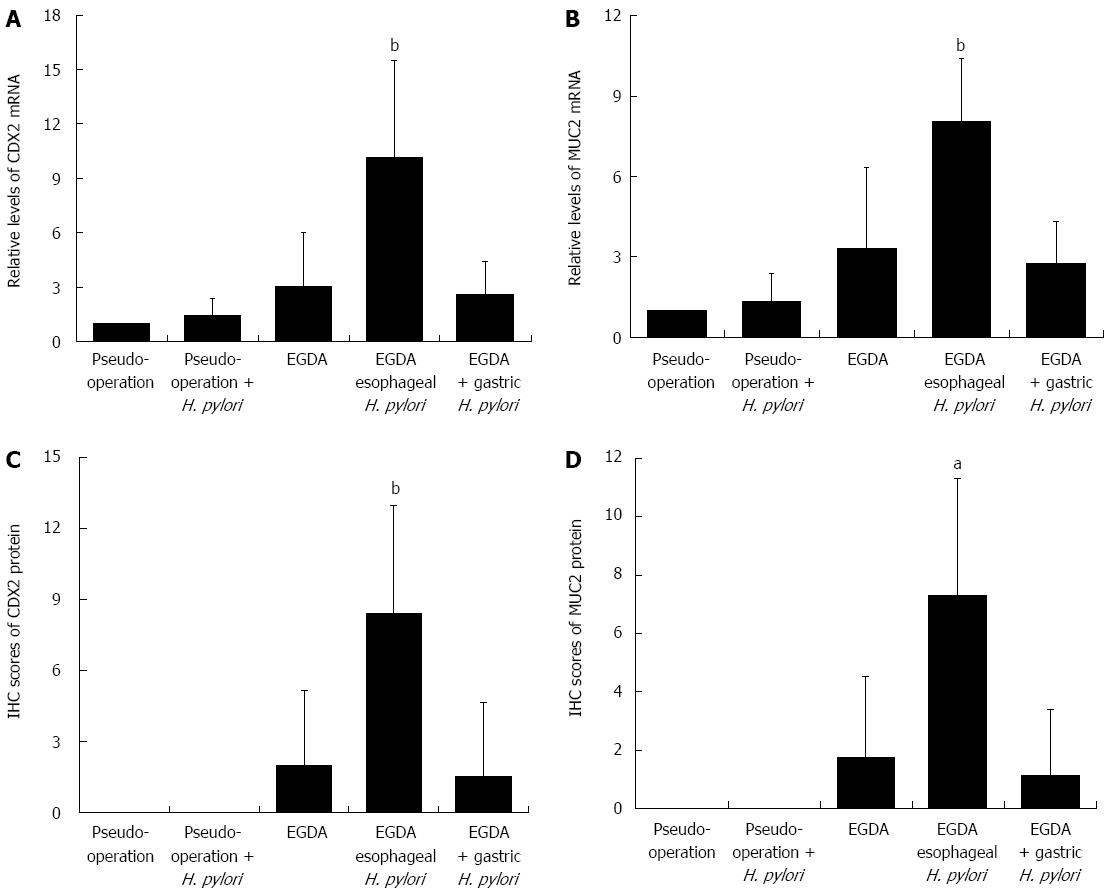
In an effort to determine the role of CDX2 and its downstream molecules, the expression of the markers of intestinal metaplasia was evaluated. Chronic reflux caused by EGDA induced the expression of CDX2 and MUC2. Overexpression of CDX2 and MUC2 in the esophagus was found in EGDA rats with concomitant esophageal H. pylori colonization, as compared to EGDA rats (P < 0.05) (Figure 4).
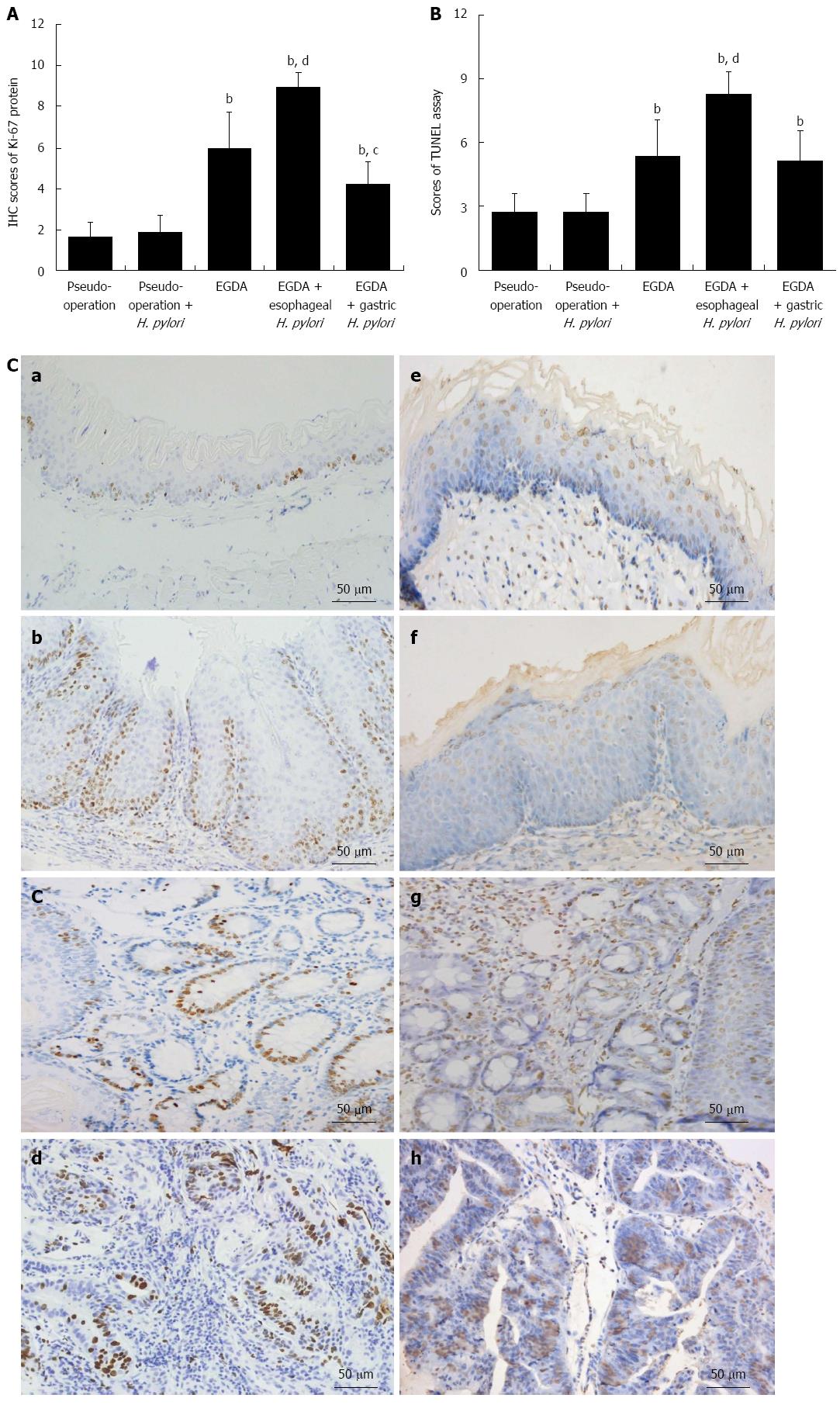
Loss of balance between cell proliferation and apoptosis is common in the development of neoplasia. Ki-67 expression was analyzed to evaluate the extent of cell proliferation, while TUNEL staining was used to indicate apoptosis in esophageal mucosa. In both normal esophageal mucosa and mucosa with esophagitis, basal layer cells in squamous epithelium expressed Ki-67. The TUNEL-positive cells were horny layer cells located in squamous epithelium. Ki-67 expression and TUNEL-positive cells were also detected in metaplastic glands and mesenchymal cells in Barrett’s mucosa and EAC.
Sporadic expression of Ki-67 was found in esophageal squamous epithelial cells in the two pseudo-operation groups. Similarly, there were few TUNEL-positive cells in the esophagi of rats from the two pseudo-operation groups. In contrast, the expression of Ki-67 protein and the number of TUNEL-positive cells increased in the esophagi of rats that underwent EGDA (P < 0.05). In EGDA rats with concomitant esophageal H. pylori colonization, Ki-67 expression was elevated, and an increased number of esophageal apoptotic cells was detected (P < 0.05). The expression of Ki-67 in the esophagus was significantly decreased (P < 0.05), whereas the numbers of apoptotic cells remained unchanged in EGDA rats with gastric H. pylori colonization compared to EGDA rats (Figure 5).
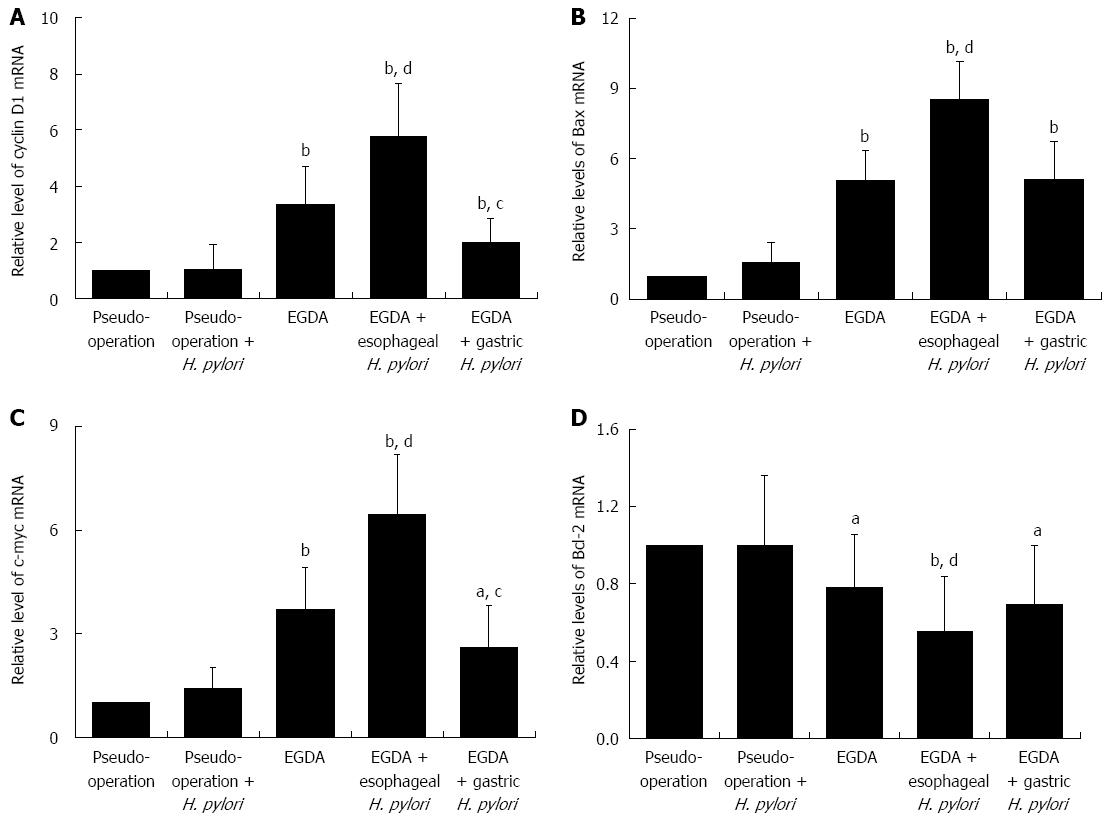
To elucidate the regulatory mechanisms for the imbalance between cell proliferation and apoptosis, the mRNA expression of associated genes was investigated. There was no statistical difference in the mRNA expression of cyclin D1 and c-Myc in the esophagi of the two pseudo-operation groups, however, both were significantly induced in EGDA rats (both P < 0.05). Further increases in the expression of cyclin D1 and c-Myc mRNAs were evident in EGDA rats with concomitant esophageal H. pylori colonization (P < 0.01). The mRNA expression of cyclin D1 and c-Myc was reduced in EGDA rats with only gastric H. pylori colonization compared to EGDA rats (P < 0.05).
There were no significant differences in the mRNA expression of Bax or Bcl-2 in the esophagi of rats in the pseudo-operation and pseudo-operation-H. pylori infection groups. However, Bax mRNA in the esophagus increased significantly, whereas Bcl-2 mRNA was reduced in EGDA rats compared to the rats of the two pseudo-operation groups (Ps < 0.05). Bax mRNA was further induced, and Bcl-2 reduced, in the esophagi of EGDA rats with concomitant esophageal H. pylori colonization compared to EGDA rats (both P < 0.01). There was no significant difference in the expression of Bax or Bcl-2 mRNA between EGDA rats and EGDA rats with only gastric H. pylori colonization (Figure 6).
H. pylori has been shown to increase the risk of precancerous lesions in the stomach; however, the role H. pylori infection plays in the esophagus remains controversial. Epidemiologic studies have shown lower prevalence of H. pylori infection in patients with GERD, BE, and EAC[21]. Fallone et al[22] reported a correlation between H. pylori infection and a reduction in the severity of reflux esophagitis. These findings suggest an inverse association between H. pylori infection and the risk of esophagitis, as well as BE and EAC. However, Henihan et al[7] found that severe esophageal mucosal injury can result from H. pylori colonization of the esophagus.
Until recently, no research differentiated the importance of different H. pylori colonization sites on the development of GERD. We established a rat model with chronic acid and bile reflux using EGDA. To elucidate the effects of H. pylori infection on reflux esophagitis and development of BE and EAC, EGDA rats were infected with H. pylori. Gastric colonization of H. pylori was detected in all rats inoculated with H. pylori. In H. pylori-inoculated rats without reflux, no H. pylori colonization or related lesions were found in the esophagus. These findings suggest that H. pylori colonization of the stomach has no influence on the esophageal mucosa of rats in the absence of reflux. Acid and bile reflux, due to EGDA, led to injury of the lower esophagus, and initiated the replacement of squamous epithelium by columnar epithelium in the esophagus. H. pylori colonization was found in Barrett epithelium of the lower esophagus of some rats that underwent EGDA. The inflammation scores of esophageal injury were not aggravated in EGDA rats with gastric H. pylori colonization compared to EGDA rats. However, the severity of esophagitis was exacerbated and the incidence of BE and EAC increased in EGDA rats with concomitant esophageal H. pylori colonization. The ability of H. pylori infection to affect the esophagus may depend on the type of infection site. Esophageal H. pylori infection may play an important role as an aggressive factor and initiate a pathogenic process.
Previous studies have indicated that H. pylori can colonize the gastric-type epithelium of the lower esophagus[7]. In the present study, esophageal squamous epithelium damaged by chronic acid and bile reflux might be replaced by Barrett’s epithelium, providing the prerequisite for H. pylori colonization. Long-term exposure of squamous epithelium to acid and bile produced by EGDA caused the development of columnar epithelium metaplasia through de novo metaplasia or proximal migration of duodenal epithelium[23]. The high incidence of columnar epithelium in the lower esophagus provided the high possibility of H. pylori colonization observed in the present study. Up to 52.6% (10/19) of rats inoculated with H. pylori strain SS1 after EGDA, were found to have H. pylori colonization in the lower esophageal mucosa with gastric metaplasia. The high H. pylori colonization rate of the esophagus was associated with the strong ability of SS1 strain to colonize[24], the chronic reflux of gastric contents that contained a large amount of the bacteria, and the high incidence of columnar epithelium replacement in the lower esophagus caused by EGDA. The results of the present study are consistent with our previous findings which showed that esophageal H. pylori colonization increased the severity of reflux esophagitis and augmented the risk for the development of BE and EAC.
CDX2 is expressed in the epithelium of the small intestine and colon[25] and is generally accepted as one of the key factors in the induction of intestinal metaplasia and the formation of intestinal-type carcinoma[5]. In rat models, acid and bile can induce CDX2 expression, an early event in lesions caused by reflux, and considered to be a key step in EAC morphogenesis[4,26]. H. pylori induces cag pathogenicity island-dependent mRNA expression of CDX2 and MUC2[27]. In our study, H. pylori further increased the expression of CDX2, while its downstream target, MUC2, was induced by mixed reflux leading to the development of BE and EAC. The findings of this study suggest that the combined presence of injurious refluxate and H. pylori infection synergistically activate ectopic expression of CDX2, which in turn initiates the development of the intestinal phenotype.
Chronic acid and bile reflux produces severe esophageal injury and initiates metaplasia, dysplasia, and even EAC[4]. In the present study, esophageal H. pylori colonization further promoted the process. It is known that both H. pylori infection and bile exposure induce intestinal metaplasia of gastric mucosa and the development of gastric adenocarcinoma[28-30]. Therefore, we speculated that infection of H. pylori in the lower esophagus might initiate a pathologic process, as in the stomach, and facilitate the development of BE, and even EAC. The high incidence rates of BE and EAC found in this study were associated with the synergistic effect of mixed reflux caused by EGDA and H. pylori infection.
Disruption of the balance between cell proliferation and apoptosis can occur in chronic inflammation and tumorigenesis. In most neoplasms, proliferation is induced while apoptosis is inhibited. Previous animal studies have also reported promotion of cell proliferation and apoptosis in mixed reflux esophagitis[31], which is consistent with the results from this study. It is well known that H. pylori CagA- or VacA-positive strains can directly induce proliferation and apoptosis in human gastric cells[32-34]. CagA pathogenicity island-positive H. pylori induces apoptosis more rapidly[33]. In previous studies, VacA-positive H. pylori was able to induce apoptosis by a mitochondrial membrane permeability change[35]. The presence of CagA and VacA proteins is important for inducing apoptosis. H. pylori SS1, a virulent strain positive for both VacA and CagA, may induce the alteration of cell proliferation and apoptosis in the esophagus and initiate the development of EAC.
Cell cycle regulatory genes are known to be involved in the loss of control of cell proliferation and apoptosis and the development of EAC. Studies have revealed increased levels of Ki-67, cyclin D1, and c-Myc in BE and EAC[10,36,37]. In the present study, an increase in BE and EAC, in response to esophageal H. pylori colonization, further increased the expression of these genes compared to EGDA rats. Bax and Bcl-2 are required for the regulation of cell apoptosis[38,39]. Previous studies indicated that H. pylori induced apoptosis of gastric epithelium via a mitochondrial pathway. H. pylori VacA modulates the permeability of mitochondrial membrane in a transmembrane-potential dependent manner[40]. The imbalance of Bax/Bcl-2 expression, as indicated in this study, increased the apoptosis rate when H. pylori colonized the esophagus. In the current rat model with acid and bile reflux, the increased apoptotic rate might counteract the significant cell proliferation increase in the esophageal mucosa. Over-proliferation enhances the possibility of aberrant gene expression; therefore, the compensated cell death may be a protective mechanism against this over-proliferation. The alteration in the expression of genes involved in the regulation of cell proliferation and apoptosis and the subsequent imbalance between the two may play an important role in the development of BE and EAC.
In conclusion, H. pylori strains may colonize the esophageal mucosa, aggravate the inflammation of the lower esophagus, and induce intestinal metaplasia or even adenocarcinoma. Loss of balance between proliferation and apoptosis may be important in H. pylori-induced esophageal diseases.
The authors would like to thank Professor Ding-Fang Bu for excellent technical support.
The relationship between gastroesophageal reflux disease and Helicobacter pylori (H. pylori) is controversial. Previous studies have shown that H. pylori colonization in the esophagus increased the incidence of Barrett’s esophagus (BE) and esophageal adenocarcinoma (EAC). However, the relevant mechanism is not clear. The authors established a rat model of chronic acid and bile reflux with H. pylori infection and investigated the possible mechanisms in the process from inflammation to malignancy in response to H. pylori colonization in the esophagus.
Chronic gastric H. pylori infection in an animal model induces gastric adenocarcinoma. Studies have shown H. pylori colonizing the BE columnar epithelium. However, the outcomes of esophageal H. pylori colonization and the relevant mechanisms have not been determined.
The authors found that H. pylori colonization of the esophagus might play an aggressive role and initiate a pathogenic process from esophagitis to BE and EAC. This is the first study to investigate the effect esophageal H. pylori colonization has on cell proliferation and apoptosis in the esophagus.
Eradication of H. pylori infection in patients with gastroesophageal reflux disease (GERD) is a hot topic. This study indicates that esophageal H. pylori colonization aggravates esophagitis, and as a result, promotes the occurrence of BE and EAC via loss of balance between cell proliferation and apoptosis. Consequently, in order to prevent esophageal malignancy in patients with GERD, H. pylori should be eradicated in patients with concomitant esophageal colonization. These findings further our knowledge on potential ways of eradicating H. pylori infection.
The macroscopic and microscopic scoring systems of esophageal injury are valid scores for quantifying the severity of esophageal injury based on the epithelial damage, vascular damage, and inflammatory extension.
In this study, the authors indicate that esophageal H. pylori colonization aggravates reflux esophagitis and increases the incidences of BE and EAC. Loss of balance between cell proliferation and apoptosis in the esophagus contributes to tumorigenesis caused by H. pylori infection. However, more studies are needed to further elucidate the mechanisms involved in the esophageal injury associated with H. pylori colonization.
P- Reviewer: Chai J, Shimi SM, Xia HHX S- Editor: Gou SX L- Editor: Webster JR E- Editor: Ma S
| 1. | Pohl H, Sirovich B, Welch HG. Esophageal adenocarcinoma incidence: are we reaching the peak? Cancer Epidemiol Biomarkers Prev. 2010;19:1468-1470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Neumann H, Mönkemüller K, Vieth M, Malfertheiner P. Chemoprevention of adenocarcinoma associated with Barrett’s esophagus: potential options. Dig Dis. 2009;27:18-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Falk GW. Barrett’s esophagus. Gastroenterology. 2002;122:1569-1591. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 253] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 4. | Ingravallo G, Dall’Olmo L, Segat D, Fassan M, Mescoli C, Dazzo E, Castoro C, Polimeno L, Rizzetto C, Baroni MD. CDX2 hox gene product in a rat model of esophageal cancer. J Exp Clin Cancer Res. 2009;28:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Almeida R, Silva E, Santos-Silva F, Silberg DG, Wang J, De Bolós C, David L. Expression of intestine-specific transcription factors, CDX1 and CDX2, in intestinal metaplasia and gastric carcinomas. J Pathol. 2003;199:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 212] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 6. | Yamamoto H, Bai YQ, Yuasa Y. Homeodomain protein CDX2 regulates goblet-specific MUC2 gene expression. Biochem Biophys Res Commun. 2003;300:813-818. [PubMed] |
| 7. | Henihan RD, Stuart RC, Nolan N, Gorey TF, Hennessy TP, O’Morain CA. Barrett’s esophagus and the presence of Helicobacter pylori. Am J Gastroenterol. 1998;93:542-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Contreras M, Salazar V, García-Amado MA, Reyes N, Aparcero M, Silva O, Castro D, Romero R, Gueneau P, Michelangeli F. High frequency of Helicobacter pylori in the esophageal mucosa of dyspeptic patients and its possible association with histopathological alterations. Int J Infect Dis. 2012;16:e364-e370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Liu FX, Wang WH, Wang J, Li J, Gao PP. Effect of Helicobacter pylori infection on Barrett’s esophagus and esophageal adenocarcinoma formation in a rat model of chronic gastroesophageal reflux. Helicobacter. 2011;16:66-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Kountouras J, Chatzopoulos D, Zavos C. Eradication of Helicobacter pylori might halt the progress to oesophageal adenocarcinoma in patients with gastro-oesophageal reflux disease and Barrett’s oesophagus. Med Hypotheses. 2007;68:1174-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 11. | Hayashi Y, Tsujii M, Wang J, Kondo J, Akasaka T, Jin Y, Li W, Nakamura T, Nishida T, Iijima H. CagA mediates epigenetic regulation to attenuate let-7 expression in Helicobacter pylori-related carcinogenesis. Gut. 2013;62:1536-1546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 101] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 12. | Lin YF, Wu MS, Chang CC, Lin SW, Lin JT, Sun YJ, Chen DS, Chow LP. Comparative immunoproteomics of identification and characterization of virulence factors from Helicobacter pylori related to gastric cancer. Mol Cell Proteomics. 2006;5:1484-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 13. | Bartchewsky W, Martini MR, Squassoni AC, Alvarez MC, Ladeira MS, Salvatore DM, Trevisan MA, Pedrazzoli J, Ribeiro ML. Effects of Helicobacter pylori infection on the expressions of Bax and Bcl-2 in patients with chronic gastritis and gastric cancer. Dig Dis Sci. 2010;55:111-116. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Targosz A, Brzozowski T, Pierzchalski P, Szczyrk U, Ptak-Belowska A, Konturek SJ, Pawlik W. Helicobacter pylori promotes apoptosis, activates cyclooxygenase (COX)-2 and inhibits heat shock protein HSP70 in gastric cancer epithelial cells. Inflamm Res. 2012;61:955-966. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 35] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 15. | Chen X, Yang Gy, Ding WY, Bondoc F, Curtis SK, Yang CS. An esophagogastroduodenal anastomosis model for esophageal adenocarcinogenesis in rats and enhancement by iron overload. Carcinogenesis. 1999;20:1801-1808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 96] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 16. | Lanas A, Royo Y, Ortego J, Molina M, Sáinz R. Experimental esophagitis induced by acid and pepsin in rabbits mimicking human reflux esophagitis. Gastroenterology. 1999;116:97-107. [PubMed] |
| 17. | Su Y, Chen X, Klein M, Fang M, Wang S, Yang CS, Goyal RK. Phenotype of columnar-lined esophagus in rats with esophagogastroduodenal anastomosis: similarity to human Barrett‘s esophagus. Lab Invest. 2004;84:753-765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 18. | Sampliner RE. Updated guidelines for the diagnosis, surveillance, and therapy of Barrett’s esophagus. Am J Gastroenterol. 2002;97:1888-1895. [PubMed] |
| 19. | Cronin J, McAdam E, Danikas A, Tselepis C, Griffiths P, Baxter J, Thomas L, Manson J, Jenkins G. Epidermal growth factor receptor (EGFR) is overexpressed in high-grade dysplasia and adenocarcinoma of the esophagus and may represent a biomarker of histological progression in Barrett’s esophagus (BE). Am J Gastroenterol. 2011;106:46-56. [PubMed] |
| 20. | Ferrero RL, Thiberge JM, Labigne A. Local immunoglobulin G antibodies in the stomach may contribute to immunity against Helicobacter infection in mice. Gastroenterology. 1997;113:185-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 57] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Loffeld RJ, Werdmuller BF, Kuster JG, Pérez-Pérez GI, Blaser MJ, Kuipers EJ. Colonization with cagA-positive Helicobacter pylori strains inversely associated with reflux esophagitis and Barrett’s esophagus. Digestion. 2000;62:95-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 83] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Fallone CA, Barkun AN, Friedman G, Mayrand S, Loo V, Beech R, Best L, Joseph L. Is Helicobacter pylori eradication associated with gastroesophageal reflux disease? Am J Gastroenterol. 2000;95:914-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Gronnier C, Bruyère E, Piessen G, Briez N, Bot J, Buob D, Leteurtre E, Van Seuningen I, Mariette C. Operatively induced chronic reflux in rats: a suitable model for studying esophageal carcinogenesis? Surgery. 2013;154:955-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Lee A, O’Rourke J, De Ungria MC, Robertson B, Daskalopoulos G, Dixon MF. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology. 1997;112:1386-1397. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 745] [Cited by in RCA: 756] [Article Influence: 27.0] [Reference Citation Analysis (0)] |
| 25. | Mallo GV, Rechreche H, Frigerio JM, Rocha D, Zweibaum A, Lacasa M, Jordan BR, Dusetti NJ, Dagorn JC, Iovanna JL. Molecular cloning, sequencing and expression of the mRNA encoding human Cdx1 and Cdx2 homeobox. Down-regulation of Cdx1 and Cdx2 mRNA expression during colorectal carcinogenesis. Int J Cancer. 1997;74:35-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 26. | Tatsuta T, Mukaisho K, Sugihara H, Miwa K, Tani T, Hattori T. Expression of Cdx2 in early GRCL of Barrett’s esophagus induced in rats by duodenal reflux. Dig Dis Sci. 2005;50:425-431. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Matsuda K, Yamauchi K, Matsumoto T, Sano K, Yamaoka Y, Ota H. Quantitative analysis of the effect of Helicobacter pylori on the expressions of SOX2, CDX2, MUC2, MUC5AC, MUC6, TFF1, TFF2, and TFF3 mRNAs in human gastric carcinoma cells. Scand J Gastroenterol. 2008;43:25-33. [PubMed] |
| 28. | Tatsugami M, Ito M, Tanaka S, Yoshihara M, Matsui H, Haruma K, Chayama K. Bile acid promotes intestinal metaplasia and gastric carcinogenesis. Cancer Epidemiol Biomarkers Prev. 2012;21:2101-2107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 29. | Forman D, Newell DG, Fullerton F, Yarnell JW, Stacey AR, Wald N, Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991;302:1302-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 941] [Cited by in RCA: 924] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 30. | Craanen ME, Dekker W, Blok P, Ferwerda J, Tytgat GN. Intestinal metaplasia and Helicobacter pylori: an endoscopic bioptic study of the gastric antrum. Gut. 1992;33:16-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 142] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 31. | Li Y, Wo JM, Su RR, Ray MB, Martin RC. Alterations in manganese superoxide dismutase expression in the progression from reflux esophagitis to esophageal adenocarcinoma. Ann Surg Oncol. 2007;14:2045-2055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 32. | Akazawa Y, Isomoto H, Matsushima K, Kanda T, Minami H, Yamaghchi N, Taura N, Shiozawa K, Ohnita K, Takeshima F. Endoplasmic reticulum stress contributes to Helicobacter pylori VacA-induced apoptosis. PLoS One. 2013;8:e82322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 59] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 33. | Minohara Y, Boyd DK, Hawkins HK, Ernst PB, Patel J, Crowe SE. The effect of the cag pathogenicity island on binding of Helicobacter pylori to gastric epithelial cells and the subsequent induction of apoptosis. Helicobacter. 2007;12:583-590. [PubMed] [DOI] [Full Text] |
| 34. | Himaya SW, Dewapriya P, Kim SK. EGFR tyrosine kinase inhibitory peptide attenuates Helicobacter pylori-mediated hyper-proliferation in AGS enteric epithelial cells. Toxicol Appl Pharmacol. 2013;269:205-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 35. | Willhite DC, Blanke SR. Helicobacter pylori vacuolating cytotoxin enters cells, localizes to the mitochondria, and induces mitochondrial membrane permeability changes correlated to toxin channel activity. Cell Microbiol. 2004;6:143-154. [PubMed] |
| 36. | van Dekken H, Hop WC, Tilanus HW, Haringsma J, van der Valk H, Wink JC, Vissers KJ. Immunohistochemical evaluation of a panel of tumor cell markers during malignant progression in Barrett esophagus. Am J Clin Pathol. 2008;130:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 37. | Rygiel AM, Milano F, Ten Kate FJ, Schaap A, Wang KK, Peppelenbosch MP, Bergman JJ, Krishnadath KK. Gains and amplifications of c-myc, EGFR, and 20.q13 loci in the no dysplasia-dysplasia-adenocarcinoma sequence of Barrett’s esophagus. Cancer Epidemiol Biomarkers Prev. 2008;17:1380-1385. [PubMed] |
| 38. | Tsujimoto Y. Role of Bcl-2 family proteins in apoptosis: apoptosomes or mitochondria? Genes Cells. 1998;3:697-707. [PubMed] |
| 39. | Goldberg AA, Titorenko VI, Beach A, Sanderson JT. Bile acids induce apoptosis selectively in androgen-dependent and -independent prostate cancer cells. PeerJ. 2013;1:e122. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 40. | Calvino-Fernández M, Benito-Martínez S, Parra-Cid T. Oxidative stress by Helicobacter pylori causes apoptosis through mitochondrial pathway in gastric epithelial cells. Apoptosis. 2008;13:1267-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |