Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15413
Revised: May 17, 2014
Accepted: June 12, 2014
Published online: November 7, 2014
Processing time: 231 Days and 2.7 Hours
AIM: To conduct an updated meta-analysis of prospective studies addressing the association between garlic consumption and colorectal cancer.
METHODS: Eligible cohort studies were identified by searching MEDLINE (PubMed) and screening the references of related articles published up to October 2013. Meta-analyses were conducted for colorectal cancer in relation to consumption of raw and cooked (RC) garlic and garlic supplements, separately. The summary relative risks (RR) with 95%CI were calculated using fixed-effects or random-effects model depending on the heterogeneity among studies.
RESULTS: A total of 5 prospective cohort studies were identified. In contrast to the previous meta-analysis, no significant associations were found between consumption of RC garlic (RR: 1.06; 95%CI: 0.95-1.19) or garlic supplements (RR: 1.12; 95%CI: 0.96-1.31) and risk of colorectal cancer. A non-significant protective effect of garlic supplement intake against colorectal cancer was observed in females (RR: 0.84; 95%CI: 0.64-1.11), but the opposite was the case in males (RR: 1.24; 95%CI: 0.96-1.59).
CONCLUSION: Consumption of RC garlic or garlic supplements is not significantly associated with reduced colorectal cancer risk.
Core tip: Garlic is consumed worldwide as a food additive and botanical supplement. The previous meta-analysis, mostly based on case-control studies, suggested that garlic consumption was associated with reduced colorectal cancer risk; however, our updated meta-analysis based on high-quality prospective studies showed no significant association between garlic consumption and risk of colorectal cancer. The recommendation of garlic consumption as a nutrition intervention against colorectal cancer should be cautious.
- Citation: Hu JY, Hu YW, Zhou JJ, Zhang MW, Li D, Zheng S. Consumption of garlic and risk of colorectal cancer: An updated meta-analysis of prospective studies. World J Gastroenterol 2014; 20(41): 15413-15422
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15413.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15413
Garlic, a member of the Allium genus, is served in many different ways worldwide. It has been frequently used as a dietary botanical supplement in the United States since the early 1990s[1-3]. The World Cancer Research Fund/American Institute for Cancer Research concluded that there was a possible association between garlic consumption and reduced risk of colorectal cancer mainly based on preclinical evidence and case-control studies, but rarely on cohort studies[4]. One meta-analysis of 4 case-control studies and 2 cohort studies published in 2000 showed at least a 30% reduction in colorectal cancer risk [relative risk (RR): 0.69; 95% confidence interval (CI): 0.55-0.89] due to a high intake of raw garlic and/or cooked garlic[5]. A similar inverse association was reported in another systematic review[6]. However, 3 cohort studies published in recent years reported no protective effect of garlic consumption against colorectal cancer[7-9]. In order to better understand the association between garlic consumption and risk of colorectal cancer, we conducted an updated meta-analysis of prospective cohort studies.
A systematic search of cohort studies addressing the association between garlic consumption and colorectal cancer was conducted in MEDLINE (PubMed) published up to October 25, 2013 by 2 investigators (Hu JY and Hu YW) independently. Other members of the Allium family were also included in the search in order to identify all relevant studies. The following key words (as free words and MeSH terms) were used: (allium, alliaceae; garlic; onion; leek; scallion; chive; food; or diet) and (colorectal, colon; or rectal) and (cancer, tumor; carcinoma; or neoplasm). The search was restricted to human studies published in English. Reference lists of retrieved articles were also examined to identify additional studies. Authors were contacted for additional information if needed.
Eligible studies met the following criteria: (1) prospective cohort studies; (2) evaluated the association between garlic consumption and risk of colorectal cancer; and (3) reported hazard ratio (HR) or RR with corresponding 95%CI, or data necessary to calculate them. In case of multiple studies from the same population, only the most recent study was included.
Data extraction was performed and cross-checked independently by 2 investigators (Zhou JJ and Zhang MW). Discrepancies were resolved through discussion with a third investigator (Li D). For each identified study, data were extracted on the first author’s last name, publication year, title of the study cohort, region/country of the study, follow-up period, sample size and number of identified cases, age range, dietary assessment, measures and types of garlic and consumption categories, crude and multivariate adjusted HRs or RRs with their 95%CI (the highest category of raw and cooked (RC) garlic consumption vs lowest category; exclusive garlic supplement users vs non-users) and covariate adjustment.
The quality of each included study was assessed on the basis of the 9-star Newcastle-Ottawa Scale (NOS)[10] by 2 reviewers (Hu JY and Hu YW) independently. A maximum of 9 stars would be assigned to each study and studies with a score no less than 7 were regarded as high quality[11].
RR was used as the risk estimate in this study. HR was regarded as RR directly because of the low absolute risk of colorectal cancer in humans[11,12]. If available, risk estimates adjusted for most variables were selected for the meta-analysis. For studies reporting risk estimates of colon cancer and rectal cancer separately, a fixed-effect model was used to pool the risk estimates and obtain an overall estimate for colorectal cancer[12-14]. In cases when risk estimates for RC garlic intake and garlic supplement intake were reported separately, overall risk estimates were obtained by pooling with a fixed-effect model[12,15]. The possible heterogeneity across included studies was assessed by the Cochrane Q test and I2 statistic[16,17]. P < 0.1 for the Q test or I2 > 50% was considered as significant heterogeneity[18]. A fixed-effects model was applied when no significant heterogeneity was detected; otherwise, a random-effects model was used. In order to evaluate the influence of each study on the pooled risk estimates, sensitivity analysis was conducted by sequentially excluding one study at a time. Subgroup analyses were performed by sex and site of cancer.
Publication bias was evaluated by Egger’s linear regression. A P-value less than 0.05 was regarded as significant. Stata 12.0 (StataCorp, College Station, TX, USA) was used for statistical analysis. A 2-sided P-value < 0.05 was considered statistically significant.
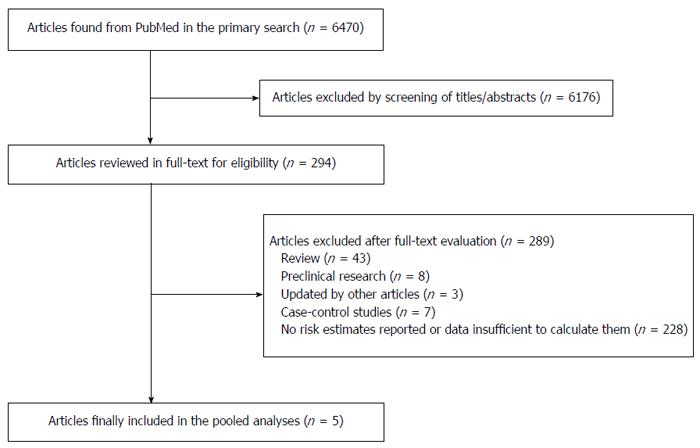
A total of 6470 potentially relevant articles were found in the primary search. Of these, 6176 articles were excluded by screening the titles or abstracts. The rest of the 294 articles were assessed in full-text. No eligible articles were identified by examining the references of reviews and retrieved articles. Three cohort studies[19-21], 2 of which were included in a previous systematic review and meta-analysis[19,20], were excluded because the information of the study cohorts was updated by other studies. Five prospective studies of 6 cohorts[7-9,22,23] (one study had 2 study populations) were identified and included in the meta-analysis after full-text assessment. Figure 1 shows the whole process of identifying relevant studies.
A total of 5 cohort studies published from 1996 through 2013 with 335923 subjects (4610 cases) were included in this meta-analysis[7-9,22,23]. Of these, a study conducted by Meng et al[7] was based on 2 cohorts, the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). The main characteristics of the 6 prospective cohorts are shown in Table 1. The follow-up period of the cohort studies ranged from 3.3 years to 24 years. Of the 5 studies, only one was conducted in the Netherlands with a relatively small sample size (3123)[22], and the other 4 were all large United States studies (sample size ranged from 35216 to 99700). The number of colorectal cancer cases identified in these studies varied from 241 to 1339[7].
| Ref. | Study | Country | Follow-up | Number of participants, age (yr) | Dietary | Study | Adjustment for confounders | |||
| Start | End | Male | Female | Total | assessment | quality | ||||
| Meng et al[7] (2013) | The Nurses’ Health Study (NHS) | United States | 1984 | June 2008 | NA | 76208 (1339 cases), 30-55 yr | 76208 (1339 cases) | Validated FFQ | 8 | Age, BMI, smoking, history of colorectal cancer, history of endoscopy screening, regular aspirin use, physical activity, postmenopausal hormone use, beef, pork and lamb as a main dish, consumption of processed meat, alcohol consumption, energy-adjusted total calcium intake, total folate, total vitamin D intake, total energy intake |
| Meng et al[7] (2013) | The Health Professionals Follow-up Study (HPFS) | United States | 1986 | Jan 2008 | 45592 (1029 cases), 40-75 yr | NA | 45592 (1029 cases) | Validated FFQ | 8 | Age, BMI, smoking, history of colorectal cancer, history of endoscopy screening, regular aspirin use, physical activity, postmenopausal hormone use, beef, pork and lamb as a main dish, consumption of processed meat, alcohol consumption, energy-adjusted total calcium intake, total folate, total vitamin D intake, total energy intake |
| McCullough et al[8] (2012) | The American Cancer Society Cancer Prevention Study (CPS) II Nutrition Cohort | United States | 1999 | June 2007 | 42824 (579 cases) | 56876 (551 cases) | 99700 (1130 cases) | Validated FFQ | 9 | Age, energy intake, gender, history of endoscopy, BMI, physical activity, smoking, alcohol consumption, NSAID use, PMH use, total calcium intake, fruits, vegetable and red/processed meat intake |
| Satia et al[9] (2009) | The VITamins And Lifestyle (VITAL) study | United States | Oct 2000-Dec 2002 | Dec 2006 | 36516 (220 cases), 50-76 yr | 39568 (208 cases), 50-76 yr | 76084 (428 cases) | Validated FFQ | 9 | Age, gender, education, physical activity, fruit and vegetable consumption, BMI, NSAID use, sigmoidoscopy, history of arthritis |
| Sellers et al[23] (1998) | The Iowa Women's Health Study | United States | 1986 | Dec 1995 | NA | 35216 (241 cases), 55-69 yr | 35216 (241 cases) | Validated FFQ | 9 | Age, total energy intake, history of colorectal polyps |
| Dorant et al[22] (1996) | The Netherlands Cohort Study | Netherlands | Sep 1986 | Dec 1989 | 1525 (243 cases), 55-69 yr | 1598 (200 cases), 55-69 yr | 3223 (443 cases) | Validated FFQ | 9 | Age, gender, family history of intestinal cancer, education, smoking, previous history of chronic intestinal disease or cholecystectomy, vitamin C and beta-carotene intake |
Quality scores of each study based on the NOS 9-star system are shown in Table 2. Both of the cohort studies conducted by Meng et al[7] were assigned a score of 8. The other 4 cohort studies all scored 9[8,9,22,23]. According to the predefined criteria of quality assessment, all studies included in this meta-analysis were of high quality. Three studies (4 cohorts) reported risk estimates on RC garlic consumption and the risk of colorectal cancer[7,8,23]. while 4 studies (5 cohorts) reported risk estimates on garlic supplement intake and risk of colorectal cancer[7-9,22].
| Subgroup | RC garlic intake | Garlic supplement intake | ||||||||
| No. of studies1 | Relative risk(95%CI) | Q-test | I2(%) | Egger’s test | No. of studies | Relative risk(95%CI) | Q-test | I2(%) | Egger’s test | |
| Colorectal cancer | 4 | 1.07 (0.95-1.19) | 0.66 | 0.0 | 0.50 | 5 | 1.12 (0.96-1.31) | 0.11 | 46.9 | 0.61 |
| Male | 2 | 1.18 (0.99-1.41) | 0.28 | 15.7 | NA | 3 | 1.24 (0.96-1.59) | 0.31 | 13.6 | 0.75 |
| Female | 3 | 1.04 (0.80-1.34) | 0.07 | 62.1 | 0.55 | 3 | 0.85 (0.64-1.11) | 0.39 | 0.0 | 0.95 |
| Colon cancer | 4 | 1.07 (0.94-1.21) | 0.63 | 0.0 | 0.23 | 3 | 1.01 (0.77-1.32) | 0.15 | 47.3 | 0.58 |
| Male | 2 | 1.18 (0.98-1.43) | 0.61 | 0.0 | NA | 2 | 1.30 (0.91-1.85) | 0.39 | 0.0 | NA |
| Female | 3 | 1.01 (0.86-1.19) | 0.10 | 56.5 | 0.49 | 2 | 0.73 (0.50-1.09) | 0.85 | 0.0 | NA |
| Rectal cancer | 3 | 1.02 (0.90-1.17) | 0.93 | 0.0 | 0.59 | 3 | 1.17 (0.74-1.83) | 0.41 | 0.0 | 0.31 |
| Male | 2 | 1.13 (0.86-1.49) | 0.26 | 22.5 | NA | 2 | 1.59 (0.94-2.69) | 0.85 | 0.0 | NA |
| Female | 2 | 0.97 (0.67-1.40) | 0.46 | 0.0 | NA | 2 | 0.69 (0.32-1.48) | 1.00 | 0.0 | NA |
| Proximal colon cancer | 3 | 1.06 (0.88-1.28) | 0.53 | 0.0 | 0.04 | 2 | 0.78 (0.51-1.21) | 0.77 | 0.0 | NA |
| Distal colon cancer | 3 | 1.12 (0.87-1.45) | 0.55 | 0.0 | 0.89 | 2 | 1.15 (0.43-3.12) | 0.04 | 75.4 | NA |
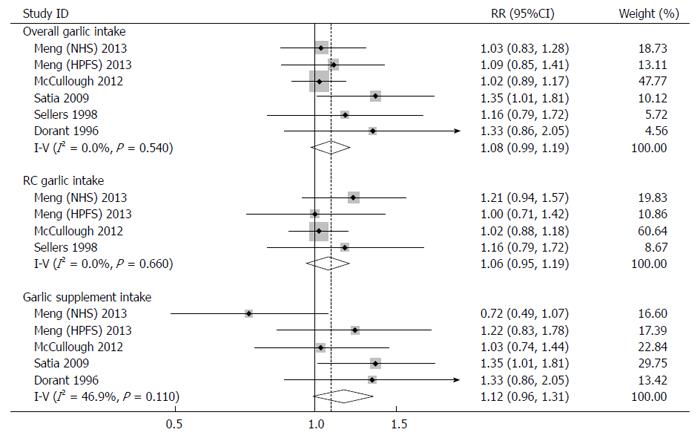
Three studies (4 cohorts) reported risk estimates on RC garlic intake and risk of colorectal cancer[7,8,23]. The meta-analysis using a fixed-effects model showed an elevated risk of colorectal cancer (RR: 1.07; 95%CI: 0.95-1.19), though this was not statistically significant (Figure 2). No heterogeneity (Q test: P = 0.66; I2 = 0.0%) or publication bias (Egger’s test: P = 0.50) was detected. Sensitivity analysis showed that RR ranged from 1.03 (95%CI: 0.91-1.17) by excluding the study by Meng et al[7] (NHS) to 1.14 (95%CI: 0.95-1.36) by excluding the study by McCullough et al[8]. No significant change in the pooled results was observed.
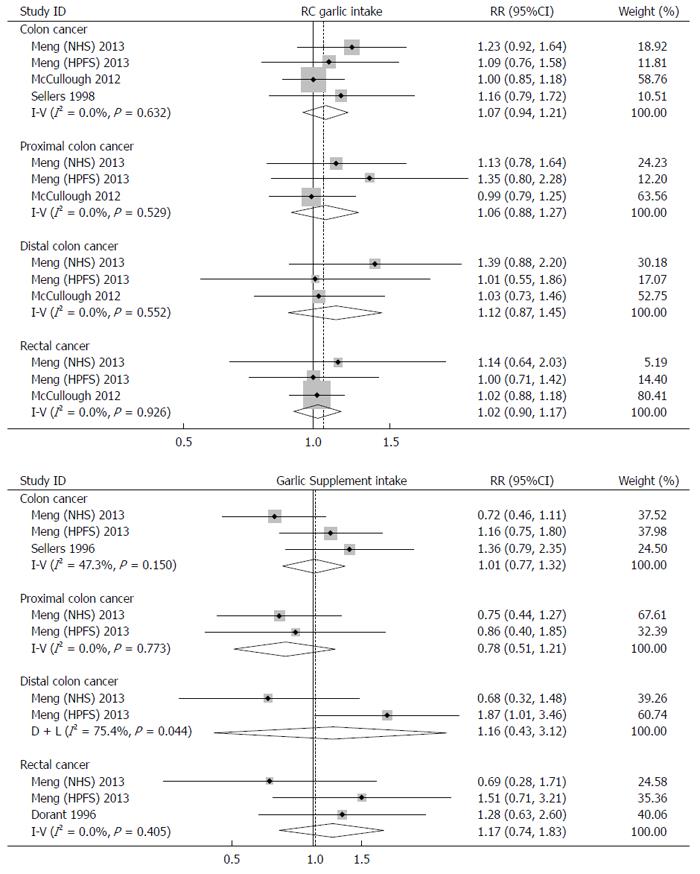
Subgroup analyses by sex and site of cancer were performed to further explore the potential source of heterogeneity (Figures 3 and 4). A fixed-effects model or random-effects model was applied for meta-analyses according to the heterogeneity across the included studies.
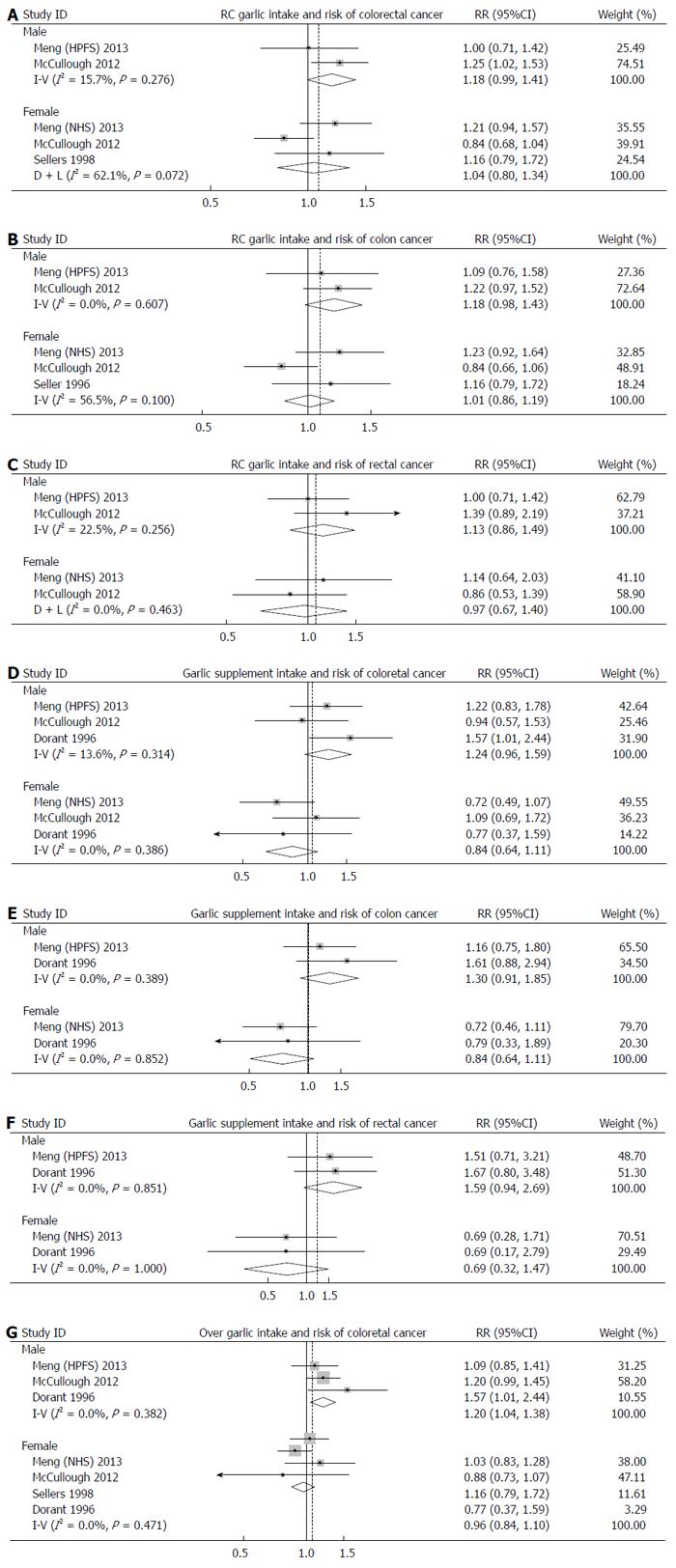
Interestingly, a borderline-significant positive effect of RC garlic consumption on risk of colorectal cancer was observed in males (RR: 1.18; 95%CI: 0.99-1.41), but not in females (RR: 1.04; 95%CI: 0.80-1.30). The pooled RR for colon cancer and rectal cancer were 1.07 (95%CI: 0.94-1.21) and 1.02 (95%CI: 0.90-1.17), respectively. When sex was taken into account, the pooled RR for colon cancer and rectal cancer were 1.18 (95%CI: 0.98-1.43) and 1.13 (95%CI: 0.86-1.49) in males, and 1.04 (95%CI: 0.80-1.35) and 0.97 (95%CI: 0.67-1.40) in females.
Four studies (5 cohorts) were conducted to investigate the association between garlic supplement intake and risk of colorectal cancer[7-9,22]. A positive association without statistical significance was observed in both the fixed-effects model (RR: 1.12; 95%CI: 0.96-1.31) and random-effects model (RR: 1.11; 95%CI: 0.89-1.38) (Figure 2). No evident heterogeneity (Q test: P = 0.11; I2 = 46.9%) or publication bias (Egger’s test: P = 0.61) was found. Sensitivity analysis showed that the pooled risk estimates ranged from 1.04 (95%CI: 0.86-1.25) by omitting the study by Satia et al[9] to 1.22 (95%CI: 1.03-1.46) by omitting the study by Meng et al[7] (NHS).
Similar to RC garlic consumption, subgroup analyses by sex and site of cancer were performed. The effects of garlic supplement intake on development of colorectal cancer was positive in males (RR: 1.24; 95%CI: 0.96-1.59), but negative in females (RR: 0.85; 95%CI: 0.64-1.11); however, these effects were not statistically significant. No significant associations were found between garlic supplement intake and development of colon cancer (RR: 1.01; 95%CI: 0.77-1.32) and rectal cancer (RR: 1.17; 95%CI: 0.83-1.32). Intake of garlic supplements showed a 30% increase in risk of colon cancer in males (RR: 1.30; 95%CI: 0.91-1.85), but a 27% reduction in females (RR: 0.73; 95%CI: 0.50-1.09). The results for rectal cancer were similar. The RRs of rectal cancer in males and females were 1.59 (95%CI: 0.94-2.69) and 0.69 (95%CI: 0.79-1.87), respectively.
The anticancer effect of garlic is mainly associated with its organosulfur compounds[24,25]. Garlic and its organosulfur compounds can: (1) inhibit cell-cycle progression, DNA adduct formation and angiogenesis; and (2) induce apoptosis and histone modification[6,26-29]. However, the association between garlic consumption and risk of colorectal cancer was inconsistent in epidemiological studies. According to a systematic review conducted in 2007, 4 (1 cohort study and 3 case-control studies) of 7 studies (3 cohort studies and 4 case-control studies) reported an inverse association. One meta-analysis of 4 case-control studies and 2 cohort studies in 2000 concluded that high intake of RC garlic may reduce the risk of colorectal cancer; however, several cohort studies published in recent years reported no protective effects of garlic consumption[7-9,23]. In addition, results of the cohort studies included in the previous meta-analysis were updated by recent studies[7,23]. Thus, an updated meta-analysis of currently available prospective cohort studies was performed in this study.
In this meta-analysis of 5 prospective studies (6 cohorts) including 335923 subjects (4610 cases), consumption of RC garlic was associated with a 7% increase in risk of colorectal cancer, though this was not statistically significant, while garlic supplement intake was associated with at least a 10% higher risk. Our results showed a different association between garlic intake and risk of colorectal cancer compared with a previous meta-analysis[5]. It is possibly because the studies summarized in the previous meta-analysis mostly were case-control studies, which are more susceptible to bias.
In subgroup analyses, our results suggested that RC garlic intake was associated with a slightly higher risk of colon cancer (7% elevated risk) compared with the risk of rectal cancer (2% elevated risk); however, for subjects taking garlic supplements, a 1% higher risk of colon cancer and a 17% higher risk of rectal cancer were observed. No associations were observed between RC garlic intake and risk of proximal and distal colon cancer. In subjects taking garlic supplementation, the point risk estimates for proximal and distal colon cancer were in opposite directions, though neither were statistically significant. The difference among risk estimates of different cancer sites is possibly because of their different predominance in the molecular genetic pathway[30]. Heterogeneity among different sites of colorectal cancer was also found in studies on other vegetable and fruit intake[31,32].
Subgroup analyses by sex revealed that RC garlic intake was associated with elevated risks of both colon and rectal cancer in males, though these were not statistically significant, while a null association was found in females. The associations between garlic supplement intake and risks of colon and rectal cancer were in opposite directions. A protective effect with borderline significance was observed in females consuming garlic supplements, but in males, the results were similar to RC garlic intake. Previous studies suggested that women might be affected differently by dietary factors, which may be explained, at least partially, by the interactions of sex hormones and nutrition[33,34]. Another possible reason for the opposite association between RC garlic consumption and colorectal cancer risk in males and females is that men are not as adept at estimating garlic consumption as women (i.e., meals are usually prepared by women)[8].
The major strength of this meta-analysis was the collection of prospective studies with large sample sizes to assess how garlic intake is associated with development of colorectal cancer. Firstly, the large sample size (a total of 335923 subjects and 4610 cases) provided sufficient power to reveal the true relationship between garlic intake and risk of colorectal cancer. Secondly, the prospective design of the included studies could help to minimize the influence of recall and selection bias[11]. Meanwhile, all the studies met the criteria of high quality based on 9-star NOS. High quality could reduce the influence caused by poor study design.
There are several limitations in this meta-analysis. Firstly, the observational nature of the data made it subject to unmeasured or residual confounders, though known confounding factors were adjusted in most of the included studies. Secondly, the definition and unit of RC garlic consumption of each category were not standardized among included studies. More specifically, the exact consumption level of garlic in lowest and highest categories was different across studies. Thirdly, a limited dataset was included in some of the subgroup analyses. Finally, only studies published in English were included in this meta-analysis. Thus, potential publication bias should be considered, though no significant publication bias was detected by Egger’s test.
In conclusion, compared with previous studies, the results of this meta-analysis suggested that garlic intake is not associated with risk of colorectal cancer when analyzed as RC garlic or garlic supplements. Furthermore, in females, garlic supplement intake showed a protective effect against colorectal cancer; however, in males, both RC garlic intake and garlic supplement intake were associated with elevated risk of colorectal cancer, although none of the associations were statistically significant. Further studies are warranted to confirm the results and clarify the underlying mechanism. The sex-specific effects of dietary factors in development of colorectal cancer are worthy of investigation.
Whether garlic consumption is associated with reduced risk of colorectal cancer requires further evaluation. The previous meta-analysis suggesting a negative association was mainly based on case-control studies, which are more prone to bias compared with cohort studies. Furthermore, 3 cohort studies on this topic have been published recently which were not included in the previous meta-analysis. The aim of this study was to evaluate the association between garlic consumption and colorectal cancer risk.
An updated meta-analysis of high-quality prospective studies was conducted to more accurately evaluate the association between garlic and garlic supplement consumption and risk of colorectal cancer.
In this meta-analysis, only prospective studies were included, and each study was regarded as high quality on the basis of the 9-star Newcastle-Ottawa Scale. The results indicated that garlic or garlic supplements were not associated with reduced risk of colorectal cancer. Additionally, a non-significant protective effect of garlic supplement against colorectal cancer was found in females, but the opposite was the case in males.
Dietary intervention provides an attractive way for colorectal cancer prevention. Garlic, as a member of the Allium genus, was believed and suggested to be protective against a series of cancers, including colorectal cancer. It was recommended by the World Cancer Research Fund/American Institute for Cancer Research as one of the anti-cancer foods in 2007, based on epidemiological studies; however, this updated meta-analysis showed no protective effects of garlic consumption against colorectal cancer. More high quality studies with large sample sizes and well-balanced confounders are needed to draw a precise conclusion on this subject. Meanwhile, the recommendation of garlic consumption as an approach for colorectal cancer prevention should be cautious.
This meta-analysis is well conducted and the paper is brief and easy to read. The topic of the meta-analysis is well defined and clinically relevant because we need to know the environmental, particularly dietary, risk factors for the development of colorectal cancer. The number of subjects involved is over 300000.
P- Reviewer: Karatapanis S, Ponzetti A S- Editor: Qi Y L- Editor: Cant MR E- Editor: Wang CH
| 1. | Kelly JP, Kaufman DW, Kelley K, Rosenberg L, Anderson TE, Mitchell AA. Recent trends in use of herbal and other natural products. Arch Intern Med. 2005;165:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 174] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 2. | Millen AE, Dodd KW, Subar AF. Use of vitamin, mineral, nonvitamin, and nonmineral supplements in the United States: The 1987, 1992, and 2000 National Health Interview Survey results. J Am Diet Assoc. 2004;104:942-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 161] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 3. | Wold RS, Lopez ST, Yau CL, Butler LM, Pareo-Tubbeh SL, Waters DL, Garry PJ, Baumgartner RN. Increasing trends in elderly persons’ use of nonvitamin, nonmineral dietary supplements and concurrent use of medications. J Am Diet Assoc. 2005;105:54-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 4. | World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Washington, DC: AICR 2007; . |
| 5. | Fleischauer AT, Poole C, Arab L. Garlic consumption and cancer prevention: meta-analyses of colorectal and stomach cancers. Am J Clin Nutr. 2000;72:1047-1052. [PubMed] |
| 6. | Ngo SN, Williams DB, Cobiac L, Head RJ. Does garlic reduce risk of colorectal cancer? A systematic review. J Nutr. 2007;137:2264-2269. [PubMed] |
| 7. | Meng S, Zhang X, Giovannucci EL, Ma J, Fuchs CS, Cho E. No association between garlic intake and risk of colorectal cancer. Cancer Epidemiol. 2013;37:152-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 8. | McCullough ML, Jacobs EJ, Shah R, Campbell PT, Gapstur SM. Garlic consumption and colorectal cancer risk in the CPS-II Nutrition Cohort. Cancer Causes Control. 2012;23:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Satia JA, Littman A, Slatore CG, Galanko JA, White E. Associations of herbal and specialty supplements with lung and colorectal cancer risk in the VITamins and Lifestyle study. Cancer Epidemiol Biomarkers Prev. 2009;18:1419-1428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 76] [Cited by in RCA: 69] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 10. | Wells BS, O’Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. |
| 11. | Zheng JS, Hu XJ, Zhao YM, Yang J, Li D. Intake of fish and marine n-3 polyunsaturated fatty acids and risk of breast cancer: meta-analysis of data from 21 independent prospective cohort studies. BMJ. 2013;346:f3706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 239] [Article Influence: 19.9] [Reference Citation Analysis (0)] |
| 12. | Wu QJ, Yang Y, Vogtmann E, Wang J, Han LH, Li HL, Xiang YB. Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann Oncol. 2013;24:1079-1087. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 164] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 13. | Aune D, Chan DS, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 830] [Cited by in RCA: 721] [Article Influence: 51.5] [Reference Citation Analysis (0)] |
| 14. | Aune D, Lau R, Chan DS, Vieira R, Greenwood DC, Kampman E, Norat T. Nonlinear reduction in risk for colorectal cancer by fruit and vegetable intake based on meta-analysis of prospective studies. Gastroenterology. 2011;141:106-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 191] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 15. | Zhou Y, Zhuang W, Hu W, Liu GJ, Wu TX, Wu XT. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology. 2011;141:80-89. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 112] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539-1558. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21630] [Cited by in RCA: 25797] [Article Influence: 1121.6] [Reference Citation Analysis (0)] |
| 17. | Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39087] [Cited by in RCA: 46521] [Article Influence: 2114.6] [Reference Citation Analysis (3)] |
| 18. | Jin M, Cai S, Guo J, Zhu Y, Li M, Yu Y, Zhang S, Chen K. Alcohol drinking and all cancer mortality: a meta-analysis. Ann Oncol. 2013;24:807-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 80] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Ascherio A, Willett WC. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994;54:2390-2397. [PubMed] |
| 20. | Steinmetz KA, Kushi LH, Bostick RM, Folsom AR, Potter JD. Vegetables, fruit, and colon cancer in the Iowa Women’s Health Study. Am J Epidemiol. 1994;139:1-15. [PubMed] |
| 21. | Lin J, Zhang SM, Wu K, Willett WC, Fuchs CS, Giovannucci E. Flavonoid intake and colorectal cancer risk in men and women. Am J Epidemiol. 2006;164:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 71] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 22. | Dorant E, van den Brandt PA, Goldbohm RA. A prospective cohort study on the relationship between onion and leek consumption, garlic supplement use and the risk of colorectal carcinoma in The Netherlands. Carcinogenesis. 1996;17:477-484. [PubMed] |
| 23. | Sellers TA, Bazyk AE, Bostick RM, Kushi LH, Olson JE, Anderson KE, Lazovich D, Folsom AR. Diet and risk of colon cancer in a large prospective study of older women: an analysis stratified on family history (Iowa, United States). Cancer Causes Control. 1998;9:357-367. [PubMed] |
| 24. | Knowles LM, Milner JA. Possible mechanism by which allyl sulfides suppress neoplastic cell proliferation. J Nutr. 2001;131:1061S-1066S. [PubMed] |
| 26. | Matsuura N, Miyamae Y, Yamane K, Nagao Y, Hamada Y, Kawaguchi N, Katsuki T, Hirata K, Sumi S, Ishikawa H. Aged garlic extract inhibits angiogenesis and proliferation of colorectal carcinoma cells. J Nutr. 2006;136:842S-846S. [PubMed] |
| 27. | Powolny AA, Singh SV. Multitargeted prevention and therapy of cancer by diallyl trisulfide and related Allium vegetable-derived organosulfur compounds. Cancer Lett. 2008;269:305-314. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 28. | Nian H, Delage B, Ho E, Dashwood RH. Modulation of histone deacetylase activity by dietary isothiocyanates and allyl sulfides: studies with sulforaphane and garlic organosulfur compounds. Environ Mol Mutagen. 2009;50:213-221. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 29. | Druesne-Pecollo N, Latino-Martel P. Modulation of histone acetylation by garlic sulfur compounds. Anticancer Agents Med Chem. 2011;11:254-259. [PubMed] |
| 30. | Li FY, Lai MD. Colorectal cancer, one entity or three. J Zhejiang Univ Sci B. 2009;10:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 31. | Terry P, Giovannucci E, Michels KB, Bergkvist L, Hansen H, Holmberg L, Wolk A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst. 2001;93:525-533. [PubMed] |
| 32. | Voorrips LE, Goldbohm RA, van Poppel G, Sturmans F, Hermus RJ, van den Brandt PA. Vegetable and fruit consumption and risks of colon and rectal cancer in a prospective cohort study: The Netherlands Cohort Study on Diet and Cancer. Am J Epidemiol. 2000;152:1081-1092. [PubMed] |
| 33. | Jacobs ET, Thompson PA, Martínez ME. Diet, gender, and colorectal neoplasia. J Clin Gastroenterol. 2007;41:731-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 34. | DeCosse JJ, Ngoi SS, Jacobson JS, Cennerazzo WJ. Gender and colorectal cancer. Eur J Cancer Prev. 1993;2:105-115. [PubMed] |