Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15387
Revised: March 25, 2014
Accepted: June 26, 2014
Published online: November 7, 2014
Processing time: 295 Days and 8 Hours
AIM: To investigate the impact of spleen operation (SO) on interferon-α (IFN-α)-based antiviral treatment in patients with hepatitis C virus (HCV)-related cirrhosis.
METHODS: Studies were systematically identified by searching electronic databases including MEDLINE, Cochrane Library, Elsevier, and Embase up to September 30, 2013, and relevant clinical studies were reviewed. Sustained virological response (SVR) rate and adherence to therapy were taken as the endpoints of interest.
RESULTS: A total of 603 patients from 16 studies were included in the systematic review. Of 372 patients who underwent SO followed by antiviral treatment, the total SVR rate was 39.5%. SVR was associated with HCV genotypes 2/3 (OR = 10.84; 95%CI: 5.47-21.47; P < 0.00001). IFN-α dose needed to be reduced in 29.4%, and IFN-α-based therapy was discontinued in 11.5% of patients. Analysis of controlled studies showed that SVRs were achieved in 34.1% of patients with SO and 31.1% of patients without SO. SO had no effect on the SVR rate in cirrhotic patients with genotype 1 HCV infection (OR = 1.28; 95%CI: 0.51-3.22; P = 0.60), but improved the SVR rate in patients with genotypes 2/3 infection, though the difference was not significant (OR = 0.36; 95%CI: 0.13-1.02; P = 0.05).
CONCLUSION: SO combined with IFN-α-based antiviral therapy may be suitable in cirrhotic patients with genotypes 2/3 HCV infection, but not in those with genotype 1 infection.
Core tip: Hematologic abnormalities caused by hypersplenism and interferon-α (IFN-α) severely affect IFN-α-based therapy in cirrhotic patients with hepatitis C virus (HCV) infection. Splenectomy and partial splenic embolization followed by IFN-α-based therapy have been increasingly performed to address cytopenias, including thrombocytopenia, in patients with HCV-related cirrhosis with hypersplenism. However, the therapeutic effect and long-term safety of such treatments remain controversial. We performed a systematic review and demonstrated that spleen operation can improve hematologic parameters before and during IFN-α-based antiviral therapy in cirrhotic patients infected with HCV. However, this treatment is more suitable for patients with genotypes 2/3 HCV infection than for those with genotype 1.
- Citation: Feng B, Zhang W, Luo BF, Song GJ, Wang J, Jin Q, Qin H, Wei L. Effect of spleen operation on antiviral treatment in hepatitis C virus-related cirrhotic patients. World J Gastroenterol 2014; 20(41): 15387-15397
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15387.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15387
The World Health Organization estimated that about 170 million persons worldwide might be infected with hepatitis C virus (HCV)[1]. Over a 25-30-year period, 5%-25% of patients with chronic HCV infection will develop cirrhosis[2,3]. Patients with HCV-related cirrhosis develop hepatic decompensation at a rate of 30% over 10 years, and hepatocellular carcinoma (HCC) at an estimated incidence of 3% per annum in North America, Europe, and Australia, and 8% in Asia and Africa[4,5]. HCV eradication is the only therapeutic intervention able to delay or halt disease progression and improve the quality of life of infected patients. In patients with compensated cirrhosis, sustained virological response (SVR) is associated with a decreased incidence of end-stage liver diseases, including decompensated cirrhosis and HCC[6].
In the past two decades, interferon-α (IFN-α)-based administration has been increasingly applied for chronic HCV infection. Moreover, a combination of pegylated IFN (PegIFN)α and ribavirin (RBV) has become the standard treatment, resulting in a SVR in 65%-82% of patients with genotypes 2 and 3 HCV, and 40%-54% of patients with genotype 1[7]. However, cirrhosis has long been considered to be one of the strongest negative predictors of antiviral treatment outcomes[8]. A landmark, prospective, randomized trial reported that the highest SVR rate (32%) was obtained in patients treated with PegIFN-α 180 μg/wk, compared with those treated with conventional IFN-α-2a 3 MU three times weekly or PegIFN-α 90 μg/wk, with no significant difference in the tolerance of the regimens among cirrhotic patients[9]. Helbling et al[10] reported an SVR rate of 32% for HCV genotypes 1/4 and 58% for genotypes 2/3 in patients with advanced fibrosis/cirrhosis receiving PegIFN-α-2a 180 μg/wk and RBV 600-1200 mg/d.
Why do cirrhotic patients achieve such a low SVR? Cirrhotic patients are often men, of older age, and with high alcohol consumption, and commonly experience comorbidities such as obesity and diabetes mellitus, which negatively influence the safety and efficacy of IFN-α-based treatment[11]. Importantly, hematologic abnormalities caused by hypersplenism and IFN-α severely affect adherence to antiviral therapy, and it can be difficult to maintain the IFN-α dose in cirrhotic patients complicated by hypersplenism. Thrombocytopenia and neutropenia associated with hypersplenism can develop and/or be exacerbated following IFN-α therapy, possibly necessitating its discontinuation. In addition, the initiation of IFN-α therapy may be difficult in some of these patients[12]. Feng et al[13] found that 66.7% of cirrhotic patients who discontinued antiviral therapy did so because of significant neutropenia and thrombocytopenia.
As a novel anti-HCV strategy, direct-acting antiviral agents (DAAs) have received increasing attention in recent years[14]. Some new, IFN-free administration protocols have also been used in clinical trials and achieved good outcomes[15]. However, their relatively short period in clinical practice, less experience in cirrhotic patients, and higher costs mean that it will be difficult to administer antiviral treatment with DAAs in HCV-related cirrhotic patients worldwide, especially in developing countries, in the near future. It is therefore important to increase the rate of SVR to IFN-α-based therapy in patients with cirrhosis.
Splenectomy and partial splenic embolization (PSE) (collectively referred to as spleen operation, SO) followed by IFN-α-based therapy have been performed to address cytopenias, including thrombocytopenia, in patients with HCV-related cirrhosis with hypersplenism[16]. However, the therapeutic effect and long-term safety of such treatments remain controversial. We therefore conducted a systematic review to clarify the impact of SO on antiviral treatment in patients with HCV-related cirrhosis.
We searched the PubMed, Cochrane Library, Elsevier (ScienceDirect OnLine), and Embase databases using electronic and manual strategies for publications reporting the effects of splenectomy or PSE on anti-HCV treatment. Eligible trials were identified up to September 30, 2013, by electronic search. Manual search was used to follow references in identified trials. The terms “hepatitis C” or “HCV” and “interferon” and “splenectomy” or “splenic embolization” were used as search terms.
The inclusion and exclusion criteria were determined by all authors. Studies were considered for inclusion if they met the following criteria: (1) English or Chinese language; (2) participants with HCV-related cirrhosis; (3) splenectomy or PSE before IFN-α-based antiviral treatment; and (4) published in peer-reviewed journals as full papers. Studies were excluded if they involved: (1) unconfirmed HCV infection; (2) case reports or reviews; (3) in vitro or animal studies; (4) participants co-infected with other viruses; and (5) liver transplantation. In the event of duplicate reports of the same study subjects, we included the most recent one.
Data were extracted independently by two investigators (FB and ZW). Inconsistent data were discussed with the other authors when necessary. Consensus was achieved for all data inclusion before analysis. The following data were extracted: first author, publication year, number of patients, study design, hematologic parameters pre-/postoperation, and protocols, outcomes and adverse effects of therapy. The primary outcome of interest was SVR rate. Additional data were obtained through direct communication with the investigators when necessary.
Meta-analysis was performed using Review Manager 5.2 (The Cochrane Collaboration, Oxford, United Kingdom). Dichotomous variable data are presented as odds ratio (OR) with 95%CI. χ2 test was used to assess the heterogeneity. An I2 value < 25% was regarded as no heterogeneity. A fixed-effects model was used when no significant heterogeneity existed among the studies analyzed; otherwise a random-effects model was used. The Mantel-Haenszel method was used to pool the effects. A P value < 0.05 was considered to be significant.
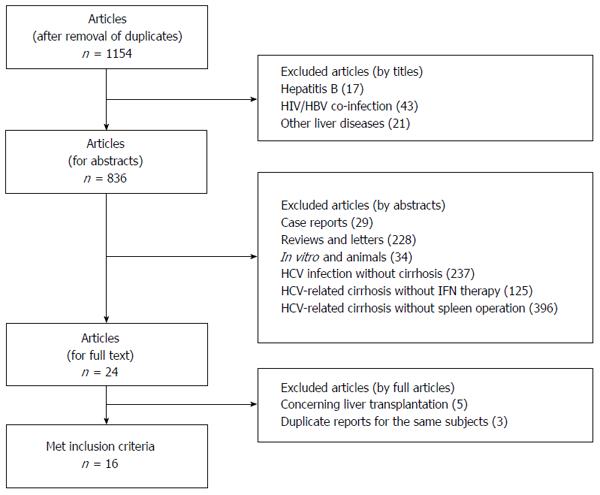
Electronic and manual searches identified a total of 1154 citations. After review of the titles and abstracts, 1130 citations were excluded according to the eligibility and ineligibility criteria. The remaining 24 studies were selected for full article review. After careful examination of the full texts, eight studies were excluded because of liver transplantation or duplicate reports for the same subjects. Sixteen studies were thus included in this systematic review (Figure 1)[11,17-31]. There was full concordance between the two independent reviewers with respect to the final inclusion and exclusion of the studies reviewed.
As shown in Table 1, among the 16 studies included in this review, 10 were from Japan, three from China, two from the United States and one from Spain. Seven were controlled, clinical studies, and nine were uncontrolled, retrospective, cohort studies. Among a total of 603 patients who received IFN-α and RBV treatment, 372 underwent prior SO. There were 11 and 4 studies where patients underwent splenectomy or PSE, respectively, and splenectomy and PSE were compared in one study.
| Ref. | Number of patients, n (total/SO + IFN1) | Country | Study design | Spleen operation | Antiviral agents | Doses of IFN and RBV | Therapy duration |
| Aizawa et al[17], 2013 | 90/30 | Japan | Retrospective, controlled | Splenectomy (2 PSE) | PegIFN-α-2a or PegIFN-α-2b + RBV | PegIFN-α-2a: 180 ug/wk; PegIFN-α-2b: 1.0-1.5 μg/kg per week; RBV: 600-1000 mg/d | GT 1: at least 48 wk; |
| GT 2/3: at least 24 wk | |||||||
| Akahoshi et al[18], 2012 | 100/97 | Japan | Retrospective, cohort | Splenectomy | PegIFN-α-2b + RBV | PegIFN-α-2b: 1.5 μg/kg per week; RBV: 600-1000 mg/d | GT 1: 48 wk; |
| GT 2/3: 24 wk | |||||||
| Hayashi et al[11], 2006 | 6/6 | United States | Retrospective, cohort | Splenectomy | PegIFN-α-2b + RBV | PegIFN-α-2b: 1.5 μg/kg per week; RBV: 600-1000 mg/d | GT 1: 48 wk; |
| GT 2/3: 24 wk | |||||||
| Ikezawa et al[19], 2010 | 10/10 | Japan | Retrospective, cohort | Splenectomy | PegIFN-α-2a or PegIFN-α-2b + RBV | PegIFN-α-2a: 180 ug/wk; PegIFN-α-2b: 1.5 μg/kg per week; RBV: 600-1000 mg/d | GT 1: 48 wk; |
| GT 2/3: 24 wk | |||||||
| Ji et al[20], 2013 | 13/13 | China | Prospective, cohort | Splenectomy | PegIFN-α-2a (2) or PegIFN-α-2b (3) or IFN-α-2b (8) + RBV | PegIFN-α-2a: 180 ug/wk; PegIFN-α-2b: 1.5 μg/kg per week; IFN-α-2b: 2.1-3.0 MU, tiw; RBV: 800-1000 mg/d | GT 1: 48 wk; |
| GT 2/3: 24 wk | |||||||
| Kedia et al[21], 2012 | 6/6 | Japan | Retrospective, cohort | Splenectomy | PegIFN (2) or IFN (4) + RBV | NA | NA |
| Kercher et al[22], 2004 | 11/11 | United States | Prospective, cohort | Splenectomy | PegIFN + RBV | NA | NA |
| Liang et al[23], 2008 | 9/9 | China | Retrospective, cohort | Splenectomy | PegIFN-α-2a (6) or IFN-α-2b (3) + RBV | PegIFN-α-2a: 180 ug/wk; IFN-α-2b: 5MU tiw; RBV: 900-1200 mg/d | GT 1: 48 wk; |
| GT 2/3: 24 wk | |||||||
| Morihara et al[24], 2009 | 48/16 | Japan | Retrospective, controlled | Splenectomy | PegIFN-α-2b (2) or IFN-α (14) + RBV | PegIFN-α-2b: 50 μg/wk; IFN-α: 3.0-6.0 MU, tiw; RBV: 400 mg/d | 1.4 yr (range: 0.2-12.4 yr) |
| Motomura et al[25], 2012 | 127/37 | Japan | Retrospective, controlled | Splenectomy | PegIFN + RBV | NA | NA |
| Shigekawa et al[26], 2011 | 29/292 | Japan | Retrospective, cohort | Splenectomy | PegIFN-α-2a or PegIFN-α-2b + RBV | PegIFN-α-2a: 90 μg/wk; or PegIFN-α-2b: 0.75 μg/kg per week; RBV: 800 mg/d | GT 1: 48 wk; |
| GT 2/3: 24 wk | |||||||
| Xie et al[27], 2012 | 49/49 | China | Retrospective, controlled | Splenectomy PSE | PegIFN-α-2a + RBV | PegIFN-α-2a: 135 or 180 μg/wk; RBV: 800-1200 mg/d | NA |
| Foruny et al[28], 2005 | 8/8 | Spain | Retrospective, cohort | PSE | PegIFN-α-2a (2) or PegIFN-α-2b (6) + RBV | PegIFN-α-2a: 180 ug/wk; PegIFN-α-2b: NA; RBV: 8.8-18.18 mg/kg per day | 24-48 wk |
| Miyake et al[29], 2008 | 20/10 | Japan | Case controlled | PSE | PegIFN-α-2b (5) or IFN-α-2b (5) + RBV | PegIFN-α-2b: 1.2 μg/kg per week; IFN-α-2b: 6MU tiw; RBV: 400 - 800 mg/d | 24-48 wk |
| Tahara et al[30], 2011 | 53/30 | Japan | Case controlled | PSE | PegIFN-α-2a (2) or PegIFN-α-2b (2) + RBV | NA | 24-48 wk |
| Takahara et al[31], 2011 | 24/11 | Japan | Case controlled | PSE | PegIFN-α-2b + RBV | PegIFN-α-2b: 1.2 μg/kg per week; | GT 1: 48 wk; |
| RBV: 400-800 mg/d | GT 2/3: 24 wk | ||||||
| Summary | 603/372 |
PegIFN-α (2a or 2b) and conventional IFN-α were used in all participants. PegIFN-α combined with RBV was administered in 90.9% (338/372) of patients with SO plus IFN-α-based antiviral treatment. The majority of patients with HCV genotypes 1 and 2/3 were treated for 48 and 24 wk, respectively. Based on patient request and early virological response, a minority of patients increased the duration of antiviral therapy[17,24]. The initial dosages of IFN-α and RBV are shown in Table 1. The dosages were adjusted if necessary, based on the adverse effects.
The demographic characteristics of the subjects who underwent SO plus IFN-α-based antiviral treatment are shown in Table 2. Their mean ages ranged from 45.4 ± 11.1 to 63.3 ± 6.6 years old. The total proportion of males was 50.0% (range: 20.0%-81.3%). Information on HCV genotype, which included genotypes 1, 2 and 3, was provided in 14 studies, and HCV genotype 1 accounted for 75.0% of subjects (237/316). Six studies described baseline viral load, and 16.1% (31/193) of patients in these studies had low viral load (< 105 IU/mL). Child-Pugh scores were provided in 10 studies, and the total numbers of patients in these studies with classes A, B and C were 160, 72 and 2, respectively.
| Ref. | Age | Male/female | HCV genotype, n (1/2 and 3) | HCV RNA(low/high) | Child-Pugh, n (A/B/C) | Pre-spleen operation | Post-spleen operation | ||||
| Leukocyte(×109/L) | Hemoglobin(g/L) | Platelet count(×109/L) | Leukocyte(×109/L) | Hemoglobin(g/L) | Platelet count (×109/L) | ||||||
| Aizawa et al[17], 2013 | 61.0 ± 7.1 | 16/14 | 25/5 | 0/30 | NA | 3.41 ± 1.27 | 125 ± 22 | 60 ± 14 | 5.13 ± 1.34 | 122 ± 19 | 167 ± 51 |
| Akahoshi et al[18], 2012 | 58.1 ± 7.0 | 54/46 | 80/20 | 12/88 | 61/39/0 | 3.19 ± 1.14 | 128 ± 15 | 56 ± 21 | 5.17 ± 1.34 | 125 ± 14 | 222 ± 98 |
| Hayashi et al[11], 2006 | 45.4 ± 11.1 | 4/3 | 4/21 | NA | 5/2/0 | 2.20 ± 1.70 | NA | 32.4 ± 12.3 | NA | NA | 224.4 ± 74.9 |
| Ikezawa et al[19], 2010 | 62.5 (51-69) | 4/6 | 9/1 | 8/2 | 9/1/0 | 3.84 ± 0.36 | NA | 64.2 ± 6.9 | 5.53 ± 0.64 | NA | 209 ± 40.6 |
| Ji et al[20], 2013 | 51.8 ± 8.0 | 4/9 | 9/4 | 11/2 | 9/4/0 | 2.10 ± 0.49 | NA | 48.2 ± 15.9 | 5.70 ± 1.42 | NA | 186.0 ± 70.6 |
| Kedia et al[21], 2012 | NA | NA | 1/5 | NA | NA | NA | NA | NA | NA | NA | NA |
| Kercher et al[22], 2004 | 45.4 (27-55) | 7/4 | NA | NA | 11/0/0 | NA | NA | 55.4 ± 20.8 | NA | NA | 441.1 ± 164.8 |
| Liang et al[23], 2008 | 47.6 ± 13.9 | 6/3 | 4/5 | NA | 6/3/0 | 2.10 ± 0.70 | 121 ± 16 | 36 ± 12 | 5.10 ± 1.20 | 131 ± 14 | 190 ± 65 |
| Morihara et al[24], 2009 | 52 (36-60) | 13/3 | 11/5 | NA | 7/1/2008 | 3.20 (1.80-5.60) | NA | 44 (27-78) | 5.20 (3.70-9.00) | NA | 110 (79-275) |
| Motomura et al[25], 2012 | 58 ± 1 | 19/18 | 32/5 | NA | NA | NA | 125 ± 3 | 59 ± 2 | NA | NA | 168.7 ± NA |
| Shigekawa et al[26], 2011 | 63.3 ± 6.6 | 12/23 | 26/9 | NA | 10/1/2024 | 3.05 ± 1.05 | NA | 48 ± 15 | 4.52 ± 0.98 | NA | 155 ± 55 |
| Xie et al[27], 2012 | 45.6 (32-65) | 22/27 | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Foruny et al[28], 2005 | NA | 5/3 | 7/1 | NA | 2/6/0 | 2.11 (1.30-0.59) | 129 (101-149) | 46.2 (21-70.3) | 5.57 (3.51-7.05) | 141 (124-152) | 180.5 (113-214) |
| Miyake et al[29], 2008 | 61 (42-70) | 2/8 | 6/4 | 0/10 | NA | 3.59 (2.62-6.33) | 130 (112-151) | 75 (44-115) | 5.70 (2.48-8.34) | 122 (111-138) | 161 (113-293) |
| Tahara et al[30], 20112 | 57.5 (36-71) | 12/13 | 13/12 | 0/30 | 25/0/0 | 3.55 (1.50-7.20) | 134 (99-160) | 70 (32-101) | 5.40 (2.60-8.10) | 126 (101-160) | 174 (90-338) |
| Takahara et al[31], 2011 | 62 (44-72) | 5/6 | 10/1 | NA | NA | 4.71 (2.95-6.33) | 127 (112-151) | 93 (61-117) | 7.20 (2.84-11.8) | 122 (111-138) | 191 (113-302) |
| Summary | 185/186 | 237/79 | 31/162 | 160/72/2 | |||||||
As shown in Table 2, all patients had thrombocytopenia and/or leukopenia at baseline. Platelet and leukocyte counts were significantly elevated after SO compared with those at baseline. The platelet count increased 2.05-7.96-fold and the leukocyte count 1.44-2.71-fold compared with pre-operation levels. There was no significant difference in hemoglobin levels between before and after SO.
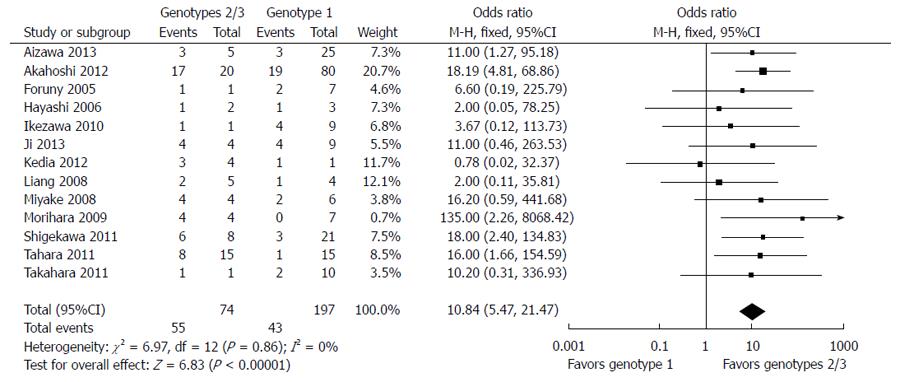
Among the 372 subjects who underwent SO followed by anti-HCV therapy, 11 were receiving treatment when the paper was written and 147 achieved SVR (39.5%). Excluding the patients still receiving treatment, the total SVR rate was 40.7%, and the SVR rates in patients infected with genotype 1 and genotype 2/3 were 29.7% (73/246) and 74.3% (55/74), respectively (Table 3). There was no evidence of substantial between-study heterogeneity (I2 = 0%; P > 0.1) and a fixed-effects model was therefore used to pool the results. Meta-analysis demonstrated that SVR was associated with genotypes 2/3 in cirrhotic patients (OR = 10.84; 95%CI: 5.47-21.47; P < 0.00001) (Figure 2).
| Ref. | SVR rate | SVR rate (GT) | IFN dosage reduction | Discontinuation of therapy | Reasons of discontinuation therapy | HCC |
| Aizawa et al[17], 2013 | 6/30 | GT 1: 3/25 | 12/30 | 41/1202 | HCC occurrence 10; No VR 10; Fatigue 5; Thrombocytopenia 3; Fundal hemorrhage 2; Interstitial pneumonia 2; Anemia, auditory disturbance, pruritus, depression, presyncope, liver function flare, infection and death of accident 1,respectively | 112 |
| GT 2/3: 3/5 | ||||||
| Akahoshi et al[18], 2012 | 36/971 | GT 1: 19/80 | NA | 8/97 | Depression 3; Neutropenia 2; Appearance of HCC 2; Pneumococcal meningitis 1 | 2 |
| GT 2/3: 17/20 | ||||||
| Hayashi et al[11], 2006 | 2/6 | GT 1: 1/3 | 0/6 | 0/6 | NO | NA |
| GT 2/3: 1/2 | ||||||
| Ikezawa et al[19], 2010 | 5/10 | GT 1: 4/9 | 2/10 | 1/10 | Angina 1 | NA |
| GT 2/3: 1/1 | ||||||
| Ji et al[20], 2013 | 8/13 | GT 1: 4/9 | 1/13 | 0/13 | NO | NA |
| GT 2/3: 4/4 | ||||||
| Kedia et al[21], 2012 | 4/6 | GT 1: 1/1 | 0/6 | 0/6 | NO | NA |
| GT 2/3: 3/4 | ||||||
| Kercher et al[22], 2004 | 3/11 | NA | 8/11 | 0/11 | NO | NA |
| Liang et al[23], 2008 | 3/9 | GT 1: 1/4 | 0/9 | 2/9 | Neutropenia 1; Anemia 1 | NA |
| GT 2/3: 2/5 | ||||||
| Morihara et al[24], 2009 | 4/16 | GT 1: 0/7 | 0/16 | 3/16 | Severe thrombocytopenia 1; NSAID-induced liver injury 1; peripheral neuropathy 1 | 4 |
| GT 2/3: 4/4 | ||||||
| Motomura et al[25], 2012 | 16/37 | NA | NA | NA | NA | NA |
| Shigekawa et al[26], 2011 | 9/29 | GT 1: 3/21 | NA | 11/29 | HCC 9; Neutropenia 1; Peritonitis 1 | 9 |
| GT 2/3: 6/8 | ||||||
| Xie et al[27], 2012 | 30/49 | GT 1: 30/49 | NA | 1/49 | Hypothyroidism 1 | NA |
| Foruny et al[28], 2005 | 3/8 | GT 1: 2/7 | 0/8 | 2/8 | Intolerance (with no evidence of hematological toxicity) 1; Neutropenia 1 | NA |
| GT 2/3: 1/1 | ||||||
| Miyake et al[29], 2008 | 6/10 | GT 1: 2/6 | 3/10 | 2/10 | Hepatic coma 1; Plural effusion 1 | NA |
| GT 2/3: 4/4 | ||||||
| Tahara et al[30], 2011 | 9/30 | GT 1: 1/15 | 16/30 | 4/30 | Neutropenia2; Severe fatigue 2 | 4 |
| GT 2/3: 8/15 | ||||||
| Takahara et al[31], 2011 | 3/11 | GT 1: 2/10 | 5/11 | 1/11 | Hepatic coma 1 | NA |
| GT 2/3: 1/1 | ||||||
| Summary | 147/3723 | GT 1: 73/246 | 47/160 | 35/3054 | 19/1604 | |
| GT 2/3: 55/74 |
Only two studies showed virological response in relation to different baseline viral loads, and there were no data on the relationship between Child-Pugh score and antiviral outcomes.
Eleven of the included studies provided data about IFN-α dose reduction during antiviral treatment. Of those who underwent SO plus antiviral treatment, 29.4% (47/160) needed a reduction of IFN-α dose, partly because of thrombocytopenia recurrence, while 11.5% (35/305) discontinued IFN-α-based therapy for other reasons, including HCC occurrence, neutropenia, severe fatigue, severe thrombocytopenia, and depression (Table 3).
Excluding the study by Aizawa et al[17], in which HCC patients were not classified on the basis of whether or not they underwent SO, 11.9% (19/160) of patients who underwent SO plus IFN developed HCC during follow-up. In a comparative study, HCC occurred in 17.4% (8/46) of patients with SO and 50.0% (16/32) of patients without SO (Table 4).
| Ref. | SVR | SVR for GT-1 | SVR for GT-2/3 | IFN dosage reduction | Discontinuation | HCC | ||||||
| SO | Non-SO | SO | Non-SO | SO | Non-SO | SO | Non-SO | SO | Non-SO | SO | Non-SO | |
| Aizawa et al[17], 2013 | 6/30 | 12/60 | 3/25 | 8/50 | 3/5 | 4/10 | NA | NA | NA | NA | NA | NA |
| Morihara et al[24], 2009 | 4/111 | 7/32 | 0/7 | 1/18 | 4/4 | 6/14 | 0/16 | 8/32 | 3/16 | 8/32 | 4/16 | 16/32 |
| Motomura et al[25], 2012 | 16/37 | 38/90 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Miyake et al[29], 2008 | 6/10 | 3/10 | 2/6 | 0/6 | 4/4 | 3/4 | 3/10 | 5/10 | 2/10 | 1/10 | NA | NA |
| Tahara et al[30], 2011 | 9/30 | 5/23 | 1/15 | 1/14 | 8/15 | 4/9 | 16/30 | 10/23 | 4/30 | 2/23 | 4/30 | NA |
| Takahara et al[31], 2011 | 3/11 | 6/13 | 2/10 | 4/8 | 1/1 | 2/5 | 5/11 | 11/13 | 1/11 | 1/13 | NA | NA |
| Summary | 44/129 | 71/228 | 8/63 | 14/96 | 20/29 | 19/42 | 24/67 | 34/78 | 10/67 | 12/78 | 8/46 | 16/32 |
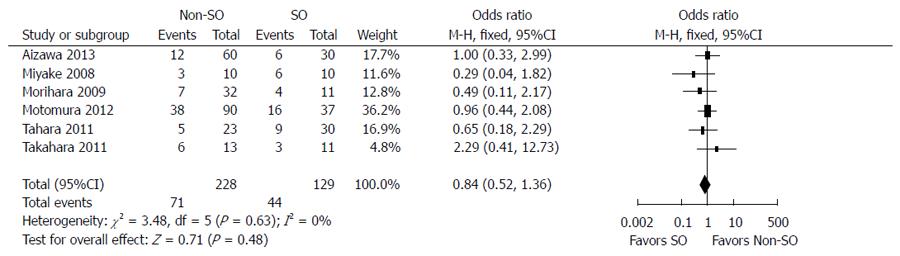
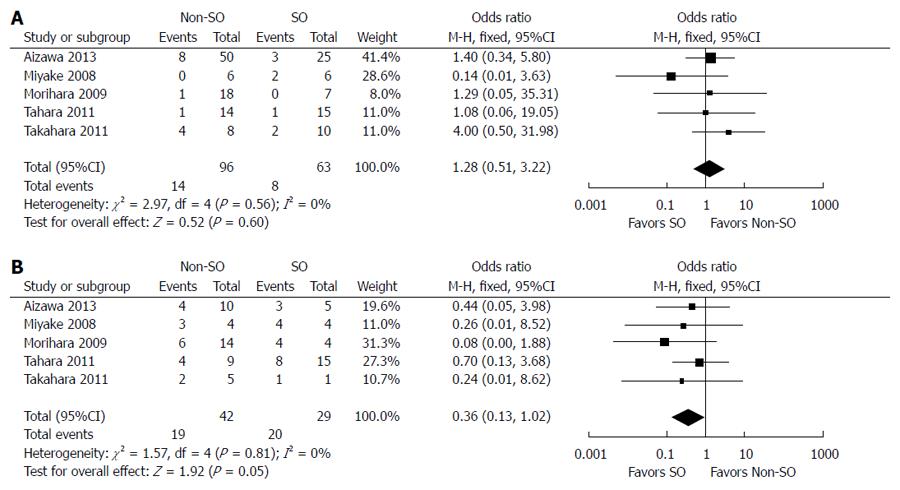
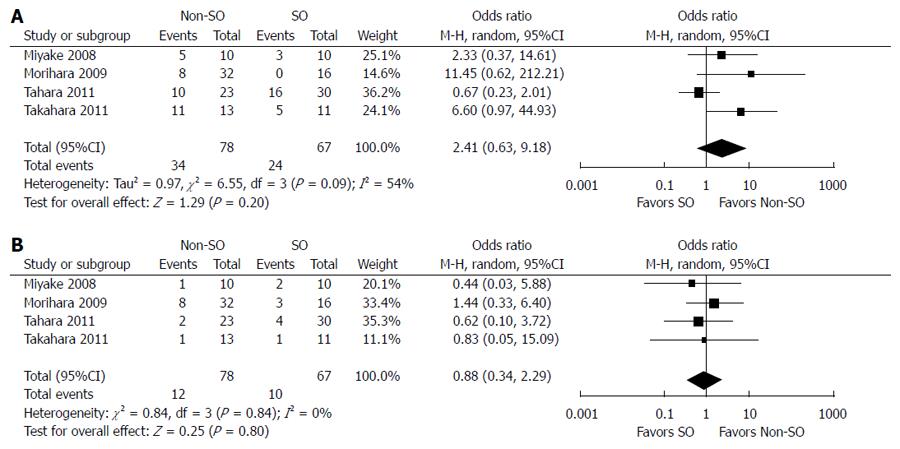
The outcomes of and adherence to antiviral therapy in patients with and without SO from six controlled studies are compared in Table 4. SVR was achieved in 34.1% (44/129) of patients with SO and 31.1% (71/228) of patients without SO. The fixed-effects model demonstrated no significant difference between the two groups (OR = 0.84; 95%CI: 0.52-1.36; P = 0.48) (Figure 3). Stratified analysis according to HCV genotype showed that SO did not increase the SVR rate in cirrhotic patients with genotype 1 HCV infection (8/63 vs 14/96; OR = 1.28; 95%CI: 0.51-3.22; P = 0.60) but did improve the virological response in individuals infected with genotypes 2/3 (OR = 0.36; 95%CI: 0.13-1.02; P = 0.05) (Figure 4). The random-effects model showed no difference between patients with and without SO in terms of the incidence of IFN dosage reduction (24/67 vs 34/78; OR = 2.41; 95%CI: 0.63-9.18; P = 0.20). There was no significant difference in withdrawal of IFN-α-based therapy between patients with SO plus IFN and those with IFN-α-based treatment alone (10/67 vs 12/78; OR = 0.88; 95%CI: 0.34-2.29; P = 0.80) (Figure 5).
Thrombocytopenia and neutropenia are common manifestations of liver cirrhosis and have been reported to occur in up to 76% of cirrhotic patients[32]. Although the exact mechanisms are not fully understood, portal hypertension associated with cirrhosis generally diverts the flow of blood from the portal system towards the spleen, resulting in spleen congestion and hypersplenism, with increased destruction of platelets and neutrophils. As the current standard of care, IFN-based treatment exacerbates thrombocytopenia and neutropenia as a result of IFN-induced bone marrow suppression, as well as the direct inhibitory effect of IFN on megakaryocytes, necessitating dose reductions or discontinuation of antiviral treatment in up to 25% of patients[22].
Splenectomy and PSE are therefore used to elevate platelet and neutrophil levels and improve the adherence to IFN-based therapy in HCV-related cirrhotic patients with hypersplenism[17,18]. They were recommended in Japan for cirrhotic patients with thrombocytopenia before initiation of IFN-α treatment in 2008[33]. In the current review, the platelet count increased by a mean of 2.80-fold and leukocyte count by a mean of 1.83-fold compared with pre-operation levels. It was also reported that platelet counts could remain high during IFN and RBV therapy, which could enable PEG-IFN-α/RBV therapy to be performed safely, with a higher SVR[18].
In this systematic review, 39.5% (147/372) of cirrhotic patients who underwent SO achieved SVR, giving a total SVR rate of 40.7% (147/361), excluding those patients who were still receiving treatment. Additionally, patients infected with genotype 1 HCV achieved an SVR rate of 29.7% (73/246), which was similar to the reported approximately 30% of cirrhotic patients infected with HCV genotypes 1/4 who obtained SVR even without SO[9,10]. Based on the data from six controlled studies, SVRs were achieved in 34.1% (44/129) of patients with SO and 31.1% (71/228) of patients without SO. Splenectomy and PSE did not significantly increase the SVR in cirrhotic patients with genotype 1 HCV infection, but did increase the SVR in individuals with genotypes 2/3 infection, as supported by stratified analysis of controlled studies[10].
Adherence is another important factor affecting SVR in cirrhotic patients. Higher SVR rates were reported in patients with genotype 1 infection who received more than 80% of their PegIFN-α and RBV dosage for more than 80% of the expected duration[34]. Hematologic parameters remained at relatively high levels during IFN-based treatment in most patients who underwent SO. Antiviral therapy was only withdrawn in 2.6% (11/425) of cases because of thrombocytopenia and neutropenia, which was far lower than that reported in some studies[13]. Overall, dose reduction of IFN occurred in 29.4% (47/160) of cirrhotic patients with SO, while 11.5% (35/305) of individuals discontinued therapy. A recent national survey conducted by Ikeda et al[16] showed that, among all patients who received pretreatment (splenectomy or PSE), discontinuation of IFN occurred in 22% of cases of splenectomy, 28% of cases of PSE and 33% of those without pretreatment. Stratified analysis of controlled studies showed that SO before antiviral therapy could decrease the incidence of IFN dose reduction (though the difference was not significant), but had little effect on discontinuation of therapy.
SVR is known to decrease the incidence of HCC in chronic hepatitis C (CHC) patients with compensated cirrhosis[6]. However, the spleen is thought to confer antitumor immunity, and SO, especially splenectomy, leads to reduction and dysfunction of T cells and decreased specific humoral immunity, which may increase the risk of carcinogenesis[27]. We also analyzed the effect of combination therapy on hepatocarcinogenesis in cirrhotic patients with splenomegaly. In the current study, 11.9% of patients with SO plus IFN-α developed HCC during follow-up. A comparative study showed that splenectomy and SPE could suppress the occurrence of HCC in a small population sample, which may have been partly the result of the antiviral and antitumor activities of IFN-α.
The orally active thrombopoietin receptor agonist eltrombopag was recently used to treat thrombocytopenia resulting from IFN-α in CHC patients with cirrhosis[35]. However, its long-term efficacy in patients with hypersplenism remains unclear, because the enlarged spleen can immediately destroy the increased platelets. PegIFN λ plus RBV may be used to treat these patients with hematologic abnormalities because of slightly decreased blood cells[36]. However, DAAs, with fewer adverse effects (especially IFN-free) and short duration, have greatly altered the therapeutic principles for CHC, and may be the most promising strategy for treating these cirrhotic patients[37].
There were some limitations to our study. Most included studies were retrospective, uncontrolled and observational studies and there was a lack of randomized controlled trials. Furthermore, the small number of study subjects made it difficult to perform subgroup analysis on the basis of factors such as baseline Child-Pugh score, viral load, and comorbidities. Small case-controlled studies could only provide information on the possible impact of SO on the outcomes of and adherence to antiviral treatment, but it is difficult to draw firm conclusions from them. We therefore compared the results for SVR and adherence to therapy with those reported in the literature. Information from small case-controlled studies and short follow-up periods was insufficient to clarify the impact of SO on HCC occurrence. However, despite these limitations, several conclusions can be drawn from careful consideration of the available data.
In conclusion, splenectomy and PSE can elevate platelet and leukocyte levels and improve the tolerance to IFN-α-based antiviral therapy in cirrhotic patients with HCV infection. Stratified analysis showed that SO does not improve the outcomes of antiviral therapy in cirrhotic patients with genotype 1 HCV infection, but may be indicated in patients with genotypes 2/3 infection receiving IFN-α therapy, who are more likely to achieve an SVR by dose maintenance. We suggest that the strategy of SO combined with IFN-α in cirrhotic patients with HCV infection may be replaced by new, IFN-free therapies, especially DAAs, in the future.
As we well knew, the sustained virological response (SVR) rate is very low in cirrhotic patients with hepatitis C virus (HCV) infection. One of important reasons is that hematologic abnormalities caused by hypersplenism and interferon-α (IFN-α) severely affect IFN-α-based therapy. Splenectomy and partial splenic embolization followed by IFN-α-based therapy have been increasingly performed to address cytopenias, including thrombocytopenia, in patients with HCV-related cirrhosis with hypersplenism. However, the therapeutic effect and long-term safety of such treatments remain controversial.
To investigate the efficacy and safety of spleen operation (SO) on IFN-α-based antiviral treatment in patients with HCV-related cirrhosis.
The number of studies on this topic was small, and therefore, the authors aimed to summarize all of these findings which they could research and proposed the position of spleen operation in HCV-related cirrhosis by a systematic review.
The results demonstrated that SO combined with IFN-α-based antiviral therapy may be suitable in cirrhotic patients with genotypes 2/3 HCV infection, but not in those with genotype 1 infection. And so, spleen operation should be reassessed and performed with careful considerations for those cirrhotic patients with genotype 1 infection in clinical practice.
The manuscript is an interesting systematic review on the impact of spleen operation on antiviral therapy in HCV-related cirrhotic patients. It deals with a difficult subject that is not well documented. The results demonstrated that spleen operation combined with IFN-α-based antiviral therapy may be suitable to HCV cirrhotic patients with genotypes 2/3 other than those with genotype 1.
P- Reviewer: Georgopoulou U, Matsumori A, Trifan A S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Wang CH
| 1. | Te HS, Jensen DM. Epidemiology of hepatitis B and C viruses: a global overview. Clin Liver Dis. 2010;14:1-21, vii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 304] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 2. | Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35-S46. [PubMed] |
| 3. | Liang TJ, Rehermann B, Seeff LB, Hoofnagle JH. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann Intern Med. 2000;132:296-305. [PubMed] |
| 4. | Fattovich G, Giustina G, Degos F, Tremolada F, Diodati G, Almasio P, Nevens F, Solinas A, Mura D, Brouwer JT. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology. 1997;112:463-472. [PubMed] |
| 5. | Thomas DL, Seeff LB. Natural history of hepatitis C. Clin Liver Dis. 2005;9:383-398, vi. [PubMed] |
| 6. | Ogawa E, Furusyo N, Kajiwara E, Takahashi K, Nomura H, Maruyama T, Tanabe Y, Satoh T, Nakamuta M, Kotoh K. Efficacy of pegylated interferon alpha-2b and ribavirin treatment on the risk of hepatocellular carcinoma in patients with chronic hepatitis C: a prospective, multicenter study. J Hepatol. 2013;58:495-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 7. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: management of hepatitis C virus infection. J Hepatol. 2011;55:245-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 889] [Cited by in RCA: 919] [Article Influence: 65.6] [Reference Citation Analysis (0)] |
| 8. | Vezali E, Aghemo A, Colombo M. A review of the treatment of chronic hepatitis C virus infection in cirrhosis. Clin Ther. 2010;32:2117-2138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 78] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 9. | Heathcote EJ, Shiffman ML, Cooksley WG, Dusheiko GM, Lee SS, Balart L, Reindollar R, Reddy RK, Wright TL, Lin A. Peginterferon alfa-2a in patients with chronic hepatitis C and cirrhosis. N Engl J Med. 2000;343:1673-1680. [PubMed] |
| 10. | Helbling B, Jochum W, Stamenic I, Knöpfli M, Cerny A, Borovicka J, Gonvers JJ, Wilhelmi M, Dinges S, Müllhaupt B. HCV-related advanced fibrosis/cirrhosis: randomized controlled trial of pegylated interferon alpha-2a and ribavirin. J Viral Hepat. 2006;13:762-769. [PubMed] |
| 11. | Hayashi PH, Mehia C, Joachim Reimers H, Solomon HS, Bacon BR. Splenectomy for thrombocytopenia in patients with hepatitis C cirrhosis. J Clin Gastroenterol. 2006;40:740-744. [PubMed] |
| 12. | Giannini EG, Marenco S, Fazio V, Pieri G, Savarino V, Picciotto A. Peripheral blood cytopaenia limiting initiation of treatment in chronic hepatitis C patients otherwise eligible for antiviral therapy. Liver Int. 2012;32:1113-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 13. | Feng B, Li XB, Fang JL, Ma H, Rao HY, Song GJ, Wu N, Wang H, Wei L. Factors affecting the efficacy of antiviral treatment on patients with HCV-related cirrhosis. Infect Dis Info. 2012;25:104-106. |
| 14. | Ghany MG, Nelson DR, Strader DB, Thomas DL, Seeff LB. An update on treatment of genotype 1 chronic hepatitis C virus infection: 2011 practice guideline by the American Association for the Study of Liver Diseases. Hepatology. 2011;54:1433-1444. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 803] [Cited by in RCA: 844] [Article Influence: 60.3] [Reference Citation Analysis (0)] |
| 15. | Conteduca V, Sansonno D, Russi S, Pavone F, Dammacco F. Therapy of chronic hepatitis C virus infection in the era of direct-acting and host-targeting antiviral agents. J Infect. 2014;68:1-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 16. | Ikeda N, Imanishi H, Aizawa N, Tanaka H, Iwata Y, Enomoto H, Saito M, Iijima H, Iimuro Y, Fujimoto J. Nationwide survey in Japan regarding splenectomy/partial splenic embolization for interferon treatment targeting hepatitis C virus-related chronic liver disease in patients with low platelet count. Hepatol Res. 2014;44:829-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Aizawa N, Enomoto H, Takashima T, Sakai Y, Iwata K, Ikeda N, Tanaka H, Iwata Y, Saito M, Imanishi H. Thrombocytopenia in pegylated interferon and ribavirin combination therapy for chronic hepatitis C. J Gastroenterol. 2014;49:1253-1263. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 18. | Akahoshi T, Tomikawa M, Kawanaka H, Furusyo N, Kinjo N, Tsutsumi N, Nagao Y, Hayashi J, Hashizume M, Maehara Y. Laparoscopic splenectomy with interferon therapy in 100 hepatitis-C-virus-cirrhotic patients with hypersplenism and thrombocytopenia. J Gastroenterol Hepatol. 2012;27:286-290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | Ikezawa K, Naito M, Yumiba T, Iwahashi K, Onishi Y, Kita H, Nishio A, Kanno T, Matsuura T, Ono A. Splenectomy and antiviral treatment for thrombocytopenic patients with chronic hepatitis C virus infection. J Viral Hepat. 2010;17:488-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Ji F, Zhang S, Huang N, Deng H, Li Z. Splenectomy prior to antiviral therapy in patients with hepatitis C virus related decompensated cirrhosis. Braz J Infect Dis. 2013;17:601-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Kedia S, Goyal R, Mangla V, Kumar A, S S, Das P, Pal S, Sahni P, Acharya SK. Splenectomy in cirrhosis with hypersplenism: improvement in cytopenias, Child’s status and institution of specific treatment for hepatitis C with success. Ann Hepatol. 2012;11:921-929. [PubMed] |
| 22. | Kercher KW, Carbonell AM, Heniford BT, Matthews BD, Cunningham DM, Reindollar RW. Laparoscopic splenectomy reverses thrombocytopenia in patients with hepatitis C cirrhosis and portal hypertension. J Gastrointest Surg. 2004;8:120-126. [PubMed] |
| 23. | Liang M, Li JY, Zhao YH, Kang P, Gao J, Li SC. [The therapeutic effects and safety of antiviral therapy in hepatitis C patients with cirrhosis after splenectomy]. Zhonghua Ganzangbing Zazhi. 2008;16:789-790. [PubMed] |
| 24. | Morihara D, Kobayashi M, Ikeda K, Kawamura Y, Saneto H, Yatuji H, Hosaka T, Sezaki H, Akuta N, Suzuki Y. Effectiveness of combination therapy of splenectomy and long-term interferon in patients with hepatitis C virus-related cirrhosis and thrombocytopenia. Hepatol Res. 2009;39:439-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 25. | Motomura T, Shirabe K, Furusyo N, Yoshizumi T, Ikegami T, Soejima Y, Akahoshi T, Tomikawa M, Fukuhara T, Hayashi J. Effect of laparoscopic splenectomy in patients with Hepatitis C and cirrhosis carrying IL28B minor genotype. BMC Gastroenterol. 2012;12:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Shigekawa Y, Uchiyama K, Takifuji K, Ueno M, Hama T, Hayami S, Tamai H, Ichinose M, Yamaue H. A laparoscopic splenectomy allows the induction of antiviral therapy for patients with cirrhosis associated with hepatitis C virus. Am Surg. 2011;77:174-179. [PubMed] |
| 27. | Xie YM, Li B, Ma L, Pan L, Wei X, Peng XJ, Hao CQ, Zhang Y, Bai XF, Kang WZ. [Peg-IFNa-2a/RBV antiviral efficacy in cirrhotic hepatitis C patients after splenectomy or partial splenic embolization]. Zhonghua Gan Zang Bing Za Zhi. 2012;20:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 28. | Foruny JR, Blázquez J, Moreno A, Bárcena R, Gil-Grande L, Quereda C, Pérez-Elías MJ, Moreno J, Sánchez J, Muriel A. Safe use of pegylated interferon/ribavirin in hepatitis C virus cirrhotic patients with hypersplenism after partial splenic embolization. Eur J Gastroenterol Hepatol. 2005;17:1157-1164. [PubMed] |
| 29. | Miyake Y, Ando M, Kaji E, Toyokawa T, Nakatsu M, Hirohata M. Partial splenic embolization prior to combination therapy of interferon and ribavirin in chronic hepatitis C patients with thrombocytopenia. Hepatol Res. 2008;38:980-986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 30. | Tahara H, Takagi H, Sato K, Shimada Y, Tojima H, Hirokawa T, Ohyama T, Horiuchi K, Naganuma A, Arai H. A retrospective cohort study of partial splenic embolization for antiviral therapy in chronic hepatitis C with thrombocytopenia. J Gastroenterol. 2011;46:1010-1019. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 31. | Takahara M, Miyake Y, Miyatake H, Imagawa A, Nakatsu M, Ando M, Hirohata M, Yamamoto K. Partial splenic embolization facilitates the adherence to peginterferon in chronic hepatitis C with thrombocytopenia. Intern Med. 2011;50:2731-2736. [PubMed] |
| 32. | Giannini EG. Review article: thrombocytopenia in chronic liver disease and pharmacologic treatment options. Aliment Pharmacol Ther. 2006;23:1055-1065. [PubMed] |
| 33. | Kumada H, Okanoue T, Onji M, Moriwaki H, Izumi N, Tanaka E, Chayama K, Sakisaka S, Takehara T, Oketani M, Suzuki F, Toyota J, Nomura H, Yoshioka K, Seike M, Yotsuyanagi H, Ueno Y; Study Group for the Standardization of Treatment of Viral Hepatitis Including Cirrhosis, Ministry of Health, Labour and Welfare of Japan. Guidelines for the treatment of chronic hepatitis and cirrhosis due to hepatitis C virus infection for the fiscal year 2008 in Japan. Hepatol Res. 2010;40:8-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 89] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 34. | McHutchison JG, Manns M, Patel K, Poynard T, Lindsay KL, Trepo C, Dienstag J, Lee WM, Mak C, Garaud JJ, Albrecht JK; International Hepatitis Interventional Therapy Group. Adherence to combination therapy enhances sustained response in genotype-1-infected patients with chronic hepatitis C. Gastroenterology. 2002;123:1061-1069. [PubMed] |
| 35. | McHutchison JG, Dusheiko G, Shiffman ML, Rodriguez-Torres M, Sigal S, Bourliere M, Berg T, Gordon SC, Campbell FM, Theodore D, Blackman N, Jenkins J, Afdhal NH; TPL102357 Study Group. Eltrombopag for thrombocytopenia in patients with cirrhosis associated with hepatitis C. N Engl J Med. 2007;357:2227-2236. [PubMed] |
| 36. | Muir AJ, Shiffman ML, Zaman A, Yoffe B, de la Torre A, Flamm S, Gordon SC, Marotta P, Vierling JM, Lopez-Talavera JC. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology. 2010;52:822-832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 192] [Cited by in RCA: 186] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 37. | Chayama K, Takahashi S, Toyota J, Karino Y, Ikeda K, Ishikawa H, Watanabe H, McPhee F, Hughes E, Kumada H. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology. 2012;55:742-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 284] [Cited by in RCA: 303] [Article Influence: 23.3] [Reference Citation Analysis (0)] |