Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15358
Revised: May 31, 2014
Accepted: June 26, 2014
Published online: November 7, 2014
Processing time: 352 Days and 11.2 Hours
AIM: To determine the prevalence for hepatitis B virus (HBV) and HBV screening and vaccination practices for inflammatory bowel disease (IBD).
METHODS: This study is a retrospective, cross-sectional observational study. A retrospective chart review was performed in 500 patients who have been consecutively treated for IBD between September 2008 and January 2013 at the Rush University Medical Center Gastroenterology section. The patients were identified through the electronic medical record with the criteria that they attended the gastroenterology clinic, and that they had a diagnosis of IBD at the time of visit discharge. Once identified, each record was analyzed to determine whether the subject had been infected with HBV in the past, already been vaccinated against HBV, or advised to get vaccinated and followed through with the recommended vaccination.
RESULTS: About 254 out of 500 patients (51%) had HBV screening ordered. Among those ordered to have screening tests, 86% followed through with HBV serology. Gastroenterology physicians had significantly different screening ratios from each other (P < 0.001). There were no significant differences in the ratios of HBV screening when IBD specialists were compared to other gastroenterology physicians (0.505 ± 0.023 vs 0.536 ± 0.066, P = 0.66). Of those 220 patients screened, 51% of IBD patients were found not to be immune against HBV. Approximately 50% of gastroenterology physicians recommended HBV vaccinations to their patients in whom serology was negative for antibodies against HBV. IBD specialists recommended vaccinations to a higher percentage of their patients compared to other gastroenterology physicians (0.168 ± 0.019 vs 0.038 ± 0.026, P = 0.015). Present and/or past HBV infection was found in 3.6% of the patients who had serology checked. There was no statistically significant difference in the prevalence of hepatitis B surface antigen (HBsAg) between our study and that reported in previous studies done in Spain (4/220 vs 14/2076 respectively, P = 0.070); and in France (4/220 vs 3/315 respectively, P = 0.159). But, the prevalence of anti-HBcAb in this study was less than that reported in the study in Spain (7/220 vs 155/2076 respectively, P = 0.006); and was not significantly different from that reported in the study in France (7/220 vs 8/315 respectively, P = 0.313).
CONCLUSION: The prevalence of HBsAg in our IBD patients was not higher than previously reported European studies. Most IBD patients are not routinely screened or vaccinated against HBV at a tertiary referral center in the United States.
Core tip: With immunomodulatory drugs being used for treatment of inflammatory bowel disease (IBD) patients, it is important that IBD patients be protected against preventable infections, specifically hepatitis B. Compared to previous literature, the prevalence of hepatitis B virus (HBV) in IBD patients in a large tertiary gastroenterology practice in the United States was not higher than Europe. However, a significant portion of IBD patients were not routinely screened and vaccinated against HBV at a large tertiary gastroenterology practice. This corroborates the need for gastroenterologists and primary care physicians to be cognizant of treating IBD patients for preventable diseases in addition to managing IBD itself. Further education and use of electronic record prompters may be needed to increase prevention of HBV.
- Citation: Ben Musa R, Gampa A, Basu S, Keshavarzian A, Swanson G, Brown M, Abraham R, Bruninga K, Losurdo J, DeMeo M, Mobarhan S, Shapiro D, Mutlu E. Hepatitis B vaccination in patients with inflammatory bowel disease. World J Gastroenterol 2014; 20(41): 15358-15366
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15358.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15358
Inflammatory bowel disease (IBD) patients (and especially those on long-term immunosuppressive drugs) are at risk to contracting hepatitis B virus (HBV) infection, and should be considered candidates for vaccination against HBV[1]. Furthermore, immunosuppressive medications of all types may lead to reactivation of HBV replication in patients with chronic HBV infection[2-7]. HBV vaccine is effective and the mainstay control of HBV is by preventing infection and consequent acute and chronic liver disease[8].
Studies that document the prevalence of HBV in IBD patients have been reported in Europe[9-12] and show a decrease in the prevalence of viral hepatitis in the most recent reports, which has been attributed to the effective preventative measures taken, such as vaccination and serological screening[1]. Three studies document HBV vaccination rates in IBD patients in Europe, ranging from 12% to 49% of patients being vaccinated[9,10,12]. The evidence from these studies and others have led the European Crohn’s and Colitis Organization to recommend universal screening at the time of diagnosis for all IBD patients in their guideline statement[13].
No data exists from United states practices about HBV prevalence among IBD patients and little data exists for screening or vaccination practices[14,15]. Guidelines by the American Association for the Study of Liver Disease (AASLD) and Centers for Disease Control (CDC) recommend HBV screening and vaccination for any person seeking protection from hepatitis B, and also for those requiring immunosuppression (including IBD patients on steroids > 20 mg/d for 2 wk or more, high-dose purine analogs, and other immunosuppressive agents such as TNF inhibitors). According to these guidelines, HBV vaccination should ideally occur before the initiation of therapy with immunosuppressants, although the exact timing of screening is not specified[16-21]. Given the acute and unpredictable need for biologics and steroids in many IBD patients, many experts recommend screening and vaccination to begin at the time of diagnosis of IBD[22,23], especially considering that over 80% of patients will require steroids, 40% will require purine analogs and, 20% will require TNF inhibitors sometime in their lifetime[13]. Other professional society guidelines in the US recommend screening and vaccination for IBD patients considering TNF inhibitors[24,25].
The aims of this study are to determine the prevalence of HBV infection among IBD patients, to determine HBV screening and vaccination ratios and to determine whether a significant majority of IBD patients are being vaccinated against HBV, in a tertiary gastroenterology practice in the US.
This study is a retrospective and cross-sectional observational study. A retrospective chart review of 500 patients who have been consecutively treated for IBD at the Rush University Medical Center Gastroenterology Section was performed. The patients were identified through the EPIC electronic medical record scheduling module between September 13, 2010 and January 31, 2013 by looking up every provider’s schedule and checking the gastroenterology notes. Subjects were included in the study if a specific diagnosis of IBD was written into the clinic notes by a board certified gastroenterologist in our practice. In general it is our practice that a diagnosis of IBD is made on the basis of classic IBD symptoms, and classic radiology or endoscopy findings. All IBD cases in our practice additionally are required to have endoscopic or surgical histopathology data, and two expert board certified gastrointestinal pathologists review all of the biopsies. Every part of the electronic record was examined to look for any evidence of hepatitis B screening or vaccination including the electronic record’s immunization recording module; the scanned in documents section to look for results outside of the Rush system provided to Rush physicians; the results section for test results at our institution; and all gastroenterology clinic visit notes.
The variables gathered from the charts included demographic information: age, gender, race; diagnosis (CD, UC, indeterminate colitis); disease duration; past history of medical illnesses; medications; treating physician; and immunosuppressive therapy use. Additional data gathered on HBV were whether the gastroenterologist ordered an HBV serology at the time of the visit, whether the patients followed through with the HBV serology orders, their history of HBV infections in past, presence of a chronic carrier state for HBV, whether or not IBD patients were vaccinated for HBV in the past, and the age at which they reported to have been vaccinated. Records were also reviewed for a mean follow-up of 9 mo (1-48 mo) to determine whether the patients who had negative serology for HBV antibody were recommended to be vaccinated and whether vaccination recommendations were followed through. Also, records were reviewed for a mean follow-up of 6 mo (1-34 mo) to determine whether those patients who had HBV serology ordered actually had blood drawn and checked for HBV serology. Records were also reviewed for a mean follow up of 13 mo (1-46 mo) to determine whether those patients who did not have HBV serology ordered at their initial visit were recommended to be screened.
The lab tests recorded in retrospective review were hepatitis B surface antigen (HBsAg), antibodies to HBsAg (anti-HBs Ab), antibodies to hepatitis B core protein (anti-HBc Ab), hepatitis B e antigen (HBeAg) and antibodies to hepatitis B e antigen (HBeAg Ab), and HBV DNA titers. HBV screening at our own institution was performed in the clinical laboratories using an immunoassay technique within the Architect® System; Abbott; Abbott pale, Illinois, United States. There is no standard hepatitis B panel for screening patients at our institution, and all the tests are ordered individually. All other test results reviewed at our institution but were performed elsewhere, were done at a clinically certified outpatient or hospital laboratory.
In this study, prior infection, carrier status and prior vaccination were defined, according to the CDC criteria[26]: Prior HBV infection was defined by loss of HBsAg, presence of anti-HBc and anti-HBs. Carrier state was defined by the presence of low level of HBsAg in the blood for longer than 6 mo even if all symptoms have disappeared. Prior vaccination was defined as presence of anti-HBs without anti-HBc.
Quantitative variables were summarized and reported as the mean ± SD. Binary variables were compared with references using the Binomial test and Statistical comparisons for categorical variables were performed using the χ2 test and Fisher exact test. All statistical analyses were performed using PASW (formerly called SPSS) version 19.0 (IBM corporation). All P values are two-sided and P values less than 0.05 were reported as statistical significant.
Of the total 500 patients with IBD, 206 (41.2%) patients had UC and 292 (58.4%) had CD and 2 (0.4%) patients had indeterminate colitis (IC). 305 (61%) patients were female, and 195 (39%) patients were male, with a mean age of 42.5 ± 16.5 years. The mean IBD disease duration was 134 ± 127.6 mo (Table 1). 60% of patients were on immunosuppressive medications. 85 (17%) patients were on thiopurines alone; 101 (20.2%) patients were on biologics; 23 (4.6%) patients were on both thiopurines and biologics; and 92 (18.4%) patients were on other immunosuppressants including prednisone.
| Characteristics | CD (n =292) | UC (n = 206) | IC (n = 2) |
| Age (yr, mean ± SD) | 41.94 ± 16.3 | 43.2 ± 16.6 | 46.5 ± 37.5 |
| Sex (Male/Female) | 110/182 | 85/121 | 0/2 |
| Race | |||
| African American | 66 (22%) | 37 (18%) | 0 (0%) |
| Asian | 3 (1%) | 2 (1%) | 0 (0%) |
| Native American | 2 (0.7%) | 1 (0.5%) | 0 (0%) |
| White | 200 (68%) | 148 (72%) | 2 (100%) |
| Ethnicity | |||
| Hispanic | 14 (4.8%) | 16 (8%) | 0 (0%) |
| Not hispanic | 278 (95%) | 190 (92%) | 2 (100%) |
| Immunosuppressants | 187 (64%) | 109 (53%) | 2 (100%) |
| IBD disease duration | |||
| (mo, mean ± SD) | 154 ± 138 | 107 ± 106 | 4 ± 4 |
Of the total 500 IBD patients, 254 (51%) had a HBV screening ordered. Of these 254 patients who had HBV serology ordered, 220 patients (86%) had blood drawn and checked for HBV serology.
Of these 220 patients who had HBV serology checked, 220 patients (100%) had their blood drawn and checked for HBsAg, of which 4 patients were positive (1.8%). 114 patients (51%) had their blood drawn and checked for anti-HBcAb, of which 7 patients were positive (6%). 193 patients (87%) had their blood drawn and checked for the anti-HBs Ab. Of those patients, who had the anti-HBs Ab checked, 73 patients (38%) had reactive titers greater than 12 mlU/mL, suggesting that they were either previously vaccinated or infected with hepatitis B and became immune; while 120 patients (62%) had nonreactive titers less than 8 mlU/mL. There were no significant differences in the proportion of follow-through with HBV screening between the male (0.523 ± 0.035) and female patients (0.498 ± 0.028), (P = 0.590), and between IBD patients who were less than 50 years of age (0.507 ± 0.027) and more than 50 years of age (0.51 ± 0.039), (P = 0.965). Furthermore, there was no significant difference in the proportions of HBV screening in IBD patients who are on and who are not on immunosuppressive medications (0.534 ± 0.028 vs 0.47 ± 0.035, respectively, P = 0.165).
Present and/or past HBV infection was found in 3.6% of the 220 IBD patients who had any serology checked. Prevalence of the different viral markers in the CD, UC and IC patients is shown in Table 2.
| Disease Type | HBsAg | Anti-HBc Ab |
| Crohn’s disease (n = 143) | 4 (2.8) | 6 (4.2) |
| Ulcerative colitis (n = 76) | 0 (0) | 1 (1.3) |
| Indeterminate colitis (n = 1) | 0 (0) | 0 (0) |
| Total (n = 220) | 4 (1.8) | 7 (3.2) |
The characteristics of the HBV positive patients are shown in Table 3. Anti-HBc Ab was present in 7 (3.2%) of the 220 patients who had any screening test done. Anti-HBc Ab was present in 7 (6.1%) of the 114 patients who had the test drawn. Among the 7 HBV positive patients, only 4 (1.8%) of the 220 IBD patients had positive HBsAg (patient 1, 2, 3, and 4), and viral loads above 10000 copies/mL were observed in 2 patients (patients 1, and 4). Three patients had only anti-HBc Ab (patients 5, 6, and 7). Anti-HBsAb was associated with anti-HBc Ab in 3 patients (patient 5, 6, and 7).
| Patients | Age (yr) | Sex | Ethnicity | IBD | Immunosupression | Serology | Viral Load (copies/mL) | HBV states |
| 1 | 54 | Male | White | CD | Adalimumab | HBsAg+ | 135039 | Chronic infection |
| Azathioprine | Anti-HBs Ab- | |||||||
| HBeAg+ | ||||||||
| Anti-HBe Ab- | ||||||||
| Anti-HBc Ab+ | ||||||||
| 2 | 56 | Female | African american | CD | - | HBsAg+ | 244 | Chronic infection |
| Anti-HBs Ab- | ||||||||
| Anti-HBc Ab+ | ||||||||
| HBeAg- | ||||||||
| 3 | 56 | Female | White | CD | - | HBsAg+ | 121 | Chronic infection |
| Anti-HBs Ab- | ||||||||
| Anti-HBc Ab+ | ||||||||
| HBeAg- | ||||||||
| 4 | 61 | Male | African american | CD | - | HBsAg+ | 1000000 | Chronic infection |
| Anti-HBs Ab- | ||||||||
| HBeAg+ | ||||||||
| Anti-HBe Ab- | ||||||||
| Anti-HBc Ab+ | ||||||||
| 5 | 54 | Female | African american | CD | Adalimumab | HBsAg- | < 10 | Past infection, |
| Anti-HBs Ab+ | Naturally immune | |||||||
| Anti-HBc Ab+ | ||||||||
| 6 | 62 | Female | African american | UC | - | HBsAg- | - | Past infection, |
| Anti-HBs Ab+ | Naturally immune | |||||||
| Anti-HBc Ab+ | ||||||||
| 7 | 64 | Female | African american | CD | Prednisolone | HBsAg- | - | Past infection, |
| Anti-HBs Ab+ | Naturally immune | |||||||
| Anti-HBc Ab+ |
Of the total 193 patients who had their serology checked for anti-HBsAb, 73 patients had detectable antibody titers. Of the other 120 patients who had negative titers, 69 (57%) patients were recommended to get vaccination. Of the 65 (94%) out of 69 patients who were recommended to get the vaccine at the gastroenterology practice, 20 (31%) got their vaccine. The 4 (6%) patients were recommended to get their vaccination through their primary care physician office, of which 2 (50%) patients got their vaccine. Total of 95 patients (49%) were vaccinated against HBV, indicating that greater than 50% of the IBD patients were not vaccinated against HBV. There were no statistical significant differences in the proportions of follow-through with vaccination recommendations between the male (0.45 ± 0.012) and female patients (0.48 ± 0.008, P = 0.821); and between IBD patients less than 50 years of age (0.46 ± 0.009) and more than 50 years of age (0.5 ± 0.011, P = 0.790). There was no statistical difference in the proportion of HBV vaccination in IBD patients who are on and who are not on immunosuppressive medications (0.205 ± 0.023 vs 0.144 ± 0.024, P = 0.081). Also, there was no significant difference in the proportions of patients having HBV vaccine recommended to them between IBD patients who are on and who are not on immunosuppressive medications (0.158 ± 0.023 vs 0.144 ± 0.026, P = 0.689).
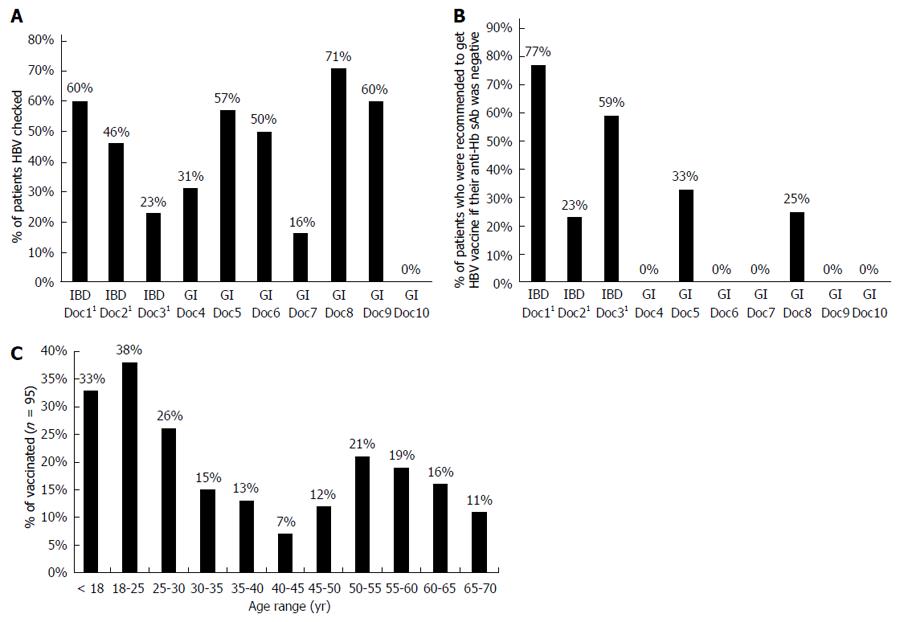
We compared the differences in the ratio of HBV vaccine administrated to IBD patients who are greater than 50 years old vs IBD patients who are under 25 years old. More patients with IBD younger than 25 years of age had been vaccinated against HBV, compared to patients greater than 50 years of age (0.9 ± 0.009 vs 0.393 ± 0.004, P = 0.003), which may relate to the changes in the last 20 years that have included HBV vaccine as part of childhood immunizations following availability of the recombinant vaccines[27] (Figure 1C).
Screening for HBV markers was carried out by most of gastroenterology physicians in our tertiary medical clinic. Physicians had significantly different screening rates from each other (P < 0.001, Figure 1A). Doctors 1, 2, and 3 were IBD specialists. There was no statistically significant difference in the proportion of patients screened for HBV when IBD specialists were compared to the other gastroenterology physicians (0.505 ± 0.023 vs 0.536 ± 0.066, P = 0.66). The 5/10 (50%) of the gastroenterology physicians recommended HBV vaccination to at least one IBD patient with seronegative antibodies against HBV (Figure 1B). IBD specialists, had a higher proportion of patients recommended to get vaccinated with seronegative antibodies compared to the other gastroenterology physicians (doctors 4-10, 0.168 ± 0.019 vs 0.038 ± 0.026, P = 0.015).
The average prevalence of chronic HBV reported in studies in Spain and France, were extracted from the published studies and these were compared to the HBV data of this study’s IBD patients in the United States[9,10].
There was no significant difference in the prevalence of HBsAg among IBD patients between our study and that reported in the study in Spain[9] (4/220 vs 14/2076, P = 0.070), and that reported in the study in France[10] (4/220 vs 3/315, P = 0.159) Table 4. But, the prevalence of anti-HBcAb in this study was significantly less than that reported in the study in Spain[9] (7/220 vs 155/2076, P = 0.006), and the prevalence was not significantly different from that reported in the study in France[10] (7/220 vs 8/315, P = 0.313).
| Loras 2009 (study 1) | Chevaux 2010 (study 2) | Our IBD study | Study 1 vs our study(P value) | Study 2 vs our study(P value) | |
| Chronic HBV (HBsAg) | 0.7% | 0.95% | 1.8% | 0.070 | 0.159 |
| Exposure to HBV (Anti-HBc Ab) | 7.5% | 2.50% | 3.2% | 0.006 | 0.313 |
Of the 18 patients who had their HBV vaccination at the gastroenterology office, only 4 patients underwent serological testing for anti-HBsAb after receiving the full 3 doses of the vaccine to confirm immunity during the course of this study. All 4 of those patients were on immunosuppressive medications at the time of vaccination. Of these 4 patients, 3 patients (75%) had detectable anti-HBsAb titer more than 12 mIU/mL after testing, implying development of immunity to HBV.
IBD patients (especially those on immunosuppressive therapy) are at risk for the development of HBV[28], The risk of HBV infection is increased in IBD patients due to immunosuppressive medications, other risk factors related to infection such as prior blood transfusions, surgery and endoscopy during the course of IBD[1,29,30]. Despite an increased risk for this infection, many IBD patients are not being vaccinated appropriately[31]. In this study, we found that less than half of our IBD patients received the HBV vaccine. More patients with IBD younger than 25 years of age had been vaccinated against HBV, compared to patients greater than 50 years of age, and this perhaps could be the result of recommendations for routine use of vaccines in children and adolescents upto age 18 since the early 1990s in the United States, as stated by the Advisory Committee on Immunization Practices (ACIP)[27]. On the other hand, for adults, HBV vaccination is required if there is a particular risk factor such as in the case of healthcare workers, and military recruits[32], persons who use iv drugs, men who have sex with men, persons who are not in a monogamous relationship, international travelers, persons who are seeking care for a sexually transmitted disease, long term care facilities for the developmentally disabled, patients with HIV or ESRD, etc. Interestingly, the CDC also recommends vaccination of any person who is seeking protection from HBV[33].
Additionally, gastroenterologists failed to order HBV serology in a significant majority of IBD patients. This suggests that all gastroenterologists or physicians placing the orders may not be adequately educated and do not routinely recommend HBV screening and vaccination for their IBD patients on or off immunosuppressive agents. This is in contrast to a French survey study, in which HBV serology was carried out by 91% of the gastroenterologists, and about 46% of practitioners recommended HBV vaccination for seronegative patients[34]. Furthermore, the ordered screening tests varied in patients highlighting the need for a standardized HBV screening panel, at a teaching institution like ours, where some orders are being placed by trainees.
In this study the prevalence of chronic HBV infection by HBsAg was not higher than those reported in France and Spain, however our study is not powered to detect single digit percentage point differences in disease prevalence. Two recent surveys of gastroenterologists highlighted the lack of physician awareness for HBV vaccination[31,35]. Consequently, it is important to address the need for educating physicians on the guidelines for HBV screening and the importance of vaccinating IBD patients[36].
HBV can be reactivated in chronic carriers during immunosuppressive therapy[37]. A recent paper has reported a patient with CD who developed subfulminant hepatitis due to reactivation of a previously unknown carrier state during treatment with an immunosuppressive agent[4]. Esteve et al[3] have observed two CD patients with severe reactivation of chronic HBV during treatment with TNF inhibitors, resulting in one death. Those studies illustrate that all IBD patients would be at risk and should be screened by checking HBV serological markers prior to therapy with immunosuppressants on a routine basis, for this preventable disease[3,4,36]. In this study we found that there is no difference in the proportion of patients screened or vaccinated between IBD patients who are and who are not on immunosuppressive therapy.
Furthermore, the response rate to HBV vaccine can potentially be significantly lower among patients who are being treated with immunosuppressants, so the vaccination should preferably be administered at least 14 d prior to immunosuppressive therapy, and the response to the vaccine should be assessed 1 mo after the last vaccination dose[38]. Among previous studies, it has been reported that, only 3 (33%) out of 9 vaccinated IBD patients had detectable anti-HBs Abs[14] in one study. Another study reported that the HBV vaccination non-response rate was found to be 24% among patients with IBD and 0% in the control group[38]. Our findings are parallel to that of the later study and show that 3 (75%) out of 4 vaccinated IBD patients at our practice, had detectable anti-HBs Abs, and all these patients were on immunosuppressive therapy at the time of vaccination. Another study has reported that the response to the HBV vaccine was normal in IBD patients with immunosuppressive medications and that the vaccination can effectively be given after immunomodulator therapy has been initiated[39]. Thus, it is recommended to vaccinate an IBD patient even if they are already on treatment to prevent any future infection.
The strength of our study comes from a large group of stable patients that are treated with IBD in our practice with follow up appointments; and availability of data from several IBD and general gastroenterology physicians who treat IBD; and our urban location with several other university centers, reflecting real time practice patterns at a tertiary referral center. The limitation of this study was that it was retrospective; hence the study is limited by the existing data that has been recorded in patient records. In fact, there was no information on the vaccination status for some IBD patients. This suggests that documentation of HBV status by gastroenterologists is clearly suboptimal for IBD patients. Therefore, we believe some gastroenterologists failed to ask about past HBV infection or vaccination and did not order HBV screening. In other cases, it is possible that HBV vaccination status was asked about, but never recorded into the chart especially, if the patient’s care has also included other physicians outside of the tertiary health care system in which we conducted the study. The results may not indicate screening practices elsewhere outside of this tertiary center and further studies are needed before this can be attributed to all IBD patients in the US. In this tertiary practice, some physicians may be more familiar with vaccination guidelines and use them. On the other hand, many patients may also be more complex and sick. This can mean that vaccination may not be as important to them and also may not be their physician’s top priority and concern during a particular clinic visit with limited time allocated to follow-up patients in the United States. All of these factors may have some effect on the data. However, the data reflects real time practice patterns and is expected to be still of value.
In conclusion, physicians managing IBD patients should be aware of the need for screening and vaccination to prevent HBV infection and the European and AASLD and CDC guidelines for HBV screening and vaccination. This can be achieved through education of gastroenterology health care providers as well as IBD patients of the importance HBV screening and vaccination. Use of the electronic medical records to prompt vaccination is another way HBV screening and vaccination can be achieved and presented to patients with IBD. Further prospective studies on HBV prevalence, screening and vaccination rates in IBD patients in the United States are needed.
A significant majority of inflammatory bowel disease (IBD) patients are treated by immunosuppressive agents (including steroids and biologics) and are at risk for hepatitis B infection or reactivation. Most of the studies that document the prevalence of HBV in IBD patients have been reported in Europe. There is little information on HBV infection prevalence, screening and vaccination rates among IBD patients in the United States.
Some guidelines and expert opinion suggest that all IBD patients should be screened and vaccinated for HBV ideally at the time of diagnosis. However, there is little information on whether this is being performed by gastroenterologists who treat IBD in the United States. Survey studies suggest many gastroenterologists do not screen or vaccinate, but real time practice information has not been reported before in the United States.
In 1986, the development of the recombinant HBV vaccine revolutionized the immunization practices for people with and without IBD alike. Children began to be vaccinated and now it is standard of care to vaccinate everyone before the age of 18. However a majority of older adults have not been vaccinated for HBV, likely due to the fact that the vaccine was not available before 1986. IBD patients compose a special group of individuals who require frequent healthcare contact and undergo many medical interventions such as blood transfusions as well as surgeries. Considering the fact that IBD patients also frequently require immunosuppressive medications that depress the ability to fight off any infections, they represent a high risk group to acquire HBV or to have it reactivate (if they have been previously exposed and generated immunity against HBV). This study presents actual practice information from a tertiary care practice in the United States about the prevalence of HBV, the screening and vaccination practices of gastroenterologists. The study suggests that there is high practice variability and considerable room for improvement in HBV prevention among IBD patients.
This study shows that the prevalence of IBD patients with HBV positivity in a large tertiary gastroenterology practice in the United States is similar to what has been previously reported in European studies. This study also shows that a significant majority of IBD patients are not actually screened and vaccinated for HBV despite having been diagnosed with IBD. These findings highlight the need to increase awareness of gastroenterologists regarding HBV screening and vaccination recommendations for IBD patients, and the need to standardize and automate screening and vaccination practices, ideally through practice initiated quality improvement projects. Such projects could prevent HBV infection and related morbidity and mortality among IBD patients in the United States.
Inflammatory bowel disease, IBD, consists of two categories: Crohn’s Disease (CD) and Ulcerative Colitis (UC) which have different characteristics. To name a few, CD can lead to lesions anywhere in the GI tract from the mouth to the anus, and these lesions can skip portions of the tract and spare the rectum. On the other hand, UC is a continuous disease and always involves the rectum. Both diseases are similar in that there is inflammation of the mucosa of the gastrointestinal the tract. In fact, sometimes signs and symptoms are so similar that it can become difficult for a concrete diagnosis for one of these disorders. In this case, once chronic inflammation is confirmed, the term Indeterminate Colitis (IC) is given until additional features for either CD or UC become more apparent over time in a particular patient.
Ascertainment of hepatitis B infection and immunity with subsequent treatment or vaccination has been recommended in several guidelines. However, the strength of evidence on which these recommendations are based is relatively weak. There is therefore critical need for observational studies that can highlight the magnitude of risk and/or the variability of practice in following guidelines. The authors have conducted an extensive and topical review of the practice pattern at their institution and highlighted the need for greater vigilance in screening within the IBD population. This is good study about physicians managing IBD patients and the guidelines for HBV screening and vaccination. This is an interesting paper that deserves to be published in WJG. However, additional studies have to be performed in order to extend these data to all of the United States.
P- Reviewer: Feuerstein JD, Kisiel JB, Velin D, Yang CH S- Editor: Qi Y L- Editor: A E- Editor: Ma S
| 1. | Hou JK, Velayos F, Terrault N, Mahadevan U. Viral hepatitis and inflammatory bowel disease. Inflamm Bowel Dis. 2010;16:925-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 53] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 2. | Xunrong L, Yan AW, Liang R, Lau GK. Hepatitis B virus (HBV) reactivation after cytotoxic or immunosuppressive therapy--pathogenesis and management. Rev Med Virol. 2001;11:287-299. [PubMed] |
| 3. | Esteve M, Saro C, González-Huix F, Suarez F, Forné M, Viver JM. Chronic hepatitis B reactivation following infliximab therapy in Crohn’s disease patients: need for primary prophylaxis. Gut. 2004;53:1363-1365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 331] [Cited by in RCA: 363] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 4. | Millonig G, Kern M, Ludwiczek O, Nachbaur K, Vogel W. Subfulminant hepatitis B after infliximab in Crohn’s disease: need for HBV-screening? World J Gastroenterol. 2006;12:974-976. [PubMed] |
| 5. | Madonia S, Orlando A, Scimeca D, Olivo M, Rossi F, Cottone M. Occult hepatitis B and infliximab-induced HBV reactivation. Inflamm Bowel Dis. 2007;13:508-509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 106] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 6. | Esteve M, Loras C, González-Huix F. Lamivudine resistance and exacerbation of hepatitis B in infliximab-treated Crohn’s disease patient. Inflamm Bowel Dis. 2007;13:1450-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 7. | Loras C, Gisbert JP, Mínguez M, Merino O, Bujanda L, Saro C, Domenech E, Barrio J, Andreu M, Ordás I. Liver dysfunction related to hepatitis B and C in patients with inflammatory bowel disease treated with immunosuppressive therapy. Gut. 2010;59:1340-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 156] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 8. | Nguyen CT, Tran TT. Hepatitis vaccination and prophylaxis. Clin Liver Dis. 2009;13:317-329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 9. | Loras C, Saro C, Gonzalez-Huix F, Mínguez M, Merino O, Gisbert JP, Barrio J, Bernal A, Gutiérrez A, Piqueras M. Prevalence and factors related to hepatitis B and C in inflammatory bowel disease patients in Spain: a nationwide, multicenter study. Am J Gastroenterol. 2009;104:57-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 99] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 10. | Chevaux JB, Nani A, Oussalah A, Venard V, Bensenane M, Belle A, Gueant JL, Bigard MA, Bronowicki JP, Peyrin-Biroulet L. Prevalence of hepatitis B and C and risk factors for nonvaccination in inflammatory bowel disease patients in Northeast France. Inflamm Bowel Dis. 2010;16:916-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 11. | Katsanos KH, Tsianos VE, Zois CD, Zioga H, Vagias I, Zervou E, Christodoulou DK, Tsianos EV. Inflammatory bowel disease and hepatitis B and C in Western Balkans: a referral centre study and review of the literature. J Crohns Colitis. 2010;4:450-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 12. | Papa A, Felice C, Marzo M, Andrisani G, Armuzzi A, Covino M, Mocci G, Pugliese D, De Vitis I, Gasbarrini A. Prevalence and natural history of hepatitis B and C infections in a large population of IBD patients treated with anti-tumor necrosis factor-α agents. J Crohns Colitis. 2013;7:113-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 62] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 13. | Rahier JF, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, Cottone M, de Ridder L, Doherty G, Ehehalt R. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014;8:443-468. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 694] [Cited by in RCA: 746] [Article Influence: 67.8] [Reference Citation Analysis (0)] |
| 14. | Melmed GY, Ippoliti AF, Papadakis KA, Tran TT, Birt JL, Lee SK, Frenck RW, Targan SR, Vasiliauskas EA. Patients with inflammatory bowel disease are at risk for vaccine-preventable illnesses. Am J Gastroenterol. 2006;101:1834-1840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 235] [Cited by in RCA: 245] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 15. | Wasan SK, Calderwood AH, Long MD, Kappelman MD, Sandler RS, Farraye FA. Immunization rates and vaccine beliefs among patients with inflammatory bowel disease: an opportunity for improvement. Inflamm Bowel Dis. 2014;20:246-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 87] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 16. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50:661-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2125] [Cited by in RCA: 2171] [Article Influence: 135.7] [Reference Citation Analysis (0)] |
| 17. | Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology (Baltimore, Md). 2009;50:1-36. |
| 18. | National Center for Immunization and Respiratory Diseases. General recommendations on immunization --- recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2011;60:1-64. [PubMed] |
| 19. | Centers for Disease Control and Prevention (CDC). Recommended adult immunization schedule--United States, 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1-4. [PubMed] |
| 20. | Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, Neitzel SM, Ward JW. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep. 2008;57:1-20. [PubMed] |
| 21. | Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, Rodewald LE, Douglas JM, Janssen RS, Ward JW. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR Recomm Rep. 2006;55:1-33; quiz CE1-4. [PubMed] |
| 22. | Morisco F, Castiglione F, Rispo A, Stroffolini T, Vitale R, Sansone S, Granata R, Orlando A, Marmo R, Riegler G. Hepatitis B virus infection and immunosuppressive therapy in patients with inflammatory bowel disease. Dig Liver Dis. 2011;43 Suppl 1:S40-S48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 25] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | López-Serrano P, Pérez-Calle JL, Sánchez-Tembleque MD. Hepatitis B and inflammatory bowel disease: role of antiviral prophylaxis. World J Gastroenterol. 2013;19:1342-1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 23] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 24. | Sands BE, Cuffari C, Katz J, Kugathasan S, Onken J, Vitek C, Orenstein W. Guidelines for immunizations in patients with inflammatory bowel disease. Inflamm Bowel Dis. 2004;10:677-692. [PubMed] |
| 25. | Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. Am J Gastroenterol. 2010;105:501-523; quiz 524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 899] [Cited by in RCA: 942] [Article Influence: 62.8] [Reference Citation Analysis (0)] |
| 26. | Interpretation of Hepatitis B Serologic Test Results. Centers for Disease Control and Prevention. Available from: http: //www.cdc.gov/hepatitis/hbv/pdfs/serologicchartv8.pdf. |
| 27. | Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices (ACIP) recommended immunization schedules for persons aged 0 through 18 years and adults aged 19 years and older--United States, 2013. MMWR Surveill Summ. 2013;62 Suppl 1:1. [PubMed] |
| 28. | Dezfoli S, Melmed GY. Vaccination issues in patients with inflammatory bowel disease receiving immunosuppression. Gastroenterol Hepatol (N Y). 2012;8:504-512. [PubMed] |
| 29. | Tolentino YF, Fogaca HS, Zaltman C, Ximenes LL, Coelho HS. Hepatitis B virus prevalence and transmission risk factors in inflammatory bowel disease patients at Clementino Fraga Filho university hospital. World J Gastroenterol. 2008;14:3201-3206. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 34] [Cited by in RCA: 33] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 30. | Yakut M, Kabaçam G, Üstün Y, Cetinkaya H, Soykan I. Hepatitis B and C virus prevalence and related factors in patients with inflammatory bowel diseases. Clin Res Hepatol Gastroenterol. 2011;35:857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 31. | Wasan SK, Coukos JA, Farraye FA. Vaccinating the inflammatory bowel disease patient: deficiencies in gastroenterologists knowledge. Inflamm Bowel Dis. 2011;17:2536-2540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 116] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 32. | Army DoT. Vaccination of New Recruits Aginst Hepatitis B Virus. 2002. Available from: http://www.vaccines.mil/documents/371hbvaccination.pdf. |
| 33. | Centers for Disease Control and Prevention. Recommended adult immunization schedule -- United States, 2014. J Midwifery Womens Health. 2014;59:205-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 34. | Poupardin C, Nahon S, Pariente A, Cadranel JF, Renou C. Hepatitis B reactivation in patients with inflammatory bowel disease: A prospective survey on screening and prevention practices at general hospitals in France. Inflamm Bowel Dis. 2011;17:669-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 35. | Yeung JH, Goodman KJ, Fedorak RN. Inadequate knowledge of immunization guidelines: a missed opportunity for preventing infection in immunocompromised IBD patients. Inflamm Bowel Dis. 2012;18:34-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 36. | Gordon JA, Jakhete N, Borum ML. Improving care for inflammatory bowel disease patients infected with hepatitis B virus. Inflamm Bowel Dis. 2013;19:E53-E54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 37. | Melmed GY. Vaccination strategies for patients with inflammatory bowel disease on immunomodulators and biologics. Inflamm Bowel Dis. 2009;15:1410-1416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 79] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 38. | Altunöz ME, Senateş E, Yeşil A, Calhan T, Ovünç AO. Patients with inflammatory bowel disease have a lower response rate to HBV vaccination compared to controls. Dig Dis Sci. 2012;57:1039-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Dotan I, Werner L, Vigodman S, Agarwal S, Pfeffer J, Horowitz N, Malter L, Abreu M, Ullman T, Guzner-Gur H. Normal response to vaccines in inflammatory bowel disease patients treated with thiopurines. Inflamm Bowel Dis. 2012;18:261-268. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |