Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15335
Revised: March 20, 2014
Accepted: July 22, 2014
Published online: November 7, 2014
Processing time: 306 Days and 20.7 Hours
AIM: To investigate the effects and underlying mechanisms of resveratrol and genistein on contractile responses of rat gastrointestinal smooth muscle.
METHODS: Isolated strips of gastrointestinal smooth muscle from Spraque-Dawley rats were suspended in organ baths containing Kreb’s solution, and the contractility of smooth muscles was measured before and after incubation with resveratrol and genistein, and the related mechanisms were studied by co-incubation with various inhibitors.
RESULTS: Resveratrol and genistein dose-dependently decreased the resting tension, and also reduced the mean contractile amplitude of gastrointestinal smooth muscle. Estrogen receptor blockades (ICI 182780 and tamoxifen) failed to alter the inhibitory effects induced by resveratrol and genistein. However, their effects were attenuated by inhibitions of α-adrenergic receptor (phentolamine), nitric oxide synthase (levorotatory-NG-nitroarginine), ATP-sensitive potassium channels (glibenclamide), and cyclic adenosine monophosphate (SQ22536). In high K+/Ca2+-free Kreb’s solution containing 0.01 mmol/L egtazic acid, resveratrol and genistein reduced the contractile responses of CaCl2, and shifted its cumulative concentration-response curves rightward.
CONCLUSION: Resveratrol and genistein relax gastrointestinal smooth muscle viaα-adrenergic receptors, nitric oxide and cyclic adenosine monophosphate pathways, ATP-sensitive potassium channels, and inhibition of L-type Ca2+ channels.
Core tip: Female hormones can cause gastrointestinal motility disorder and may contribute to irritable bowel syndrome. As analogs of estrogen, the phytoestrogens resveratrol and genistein possess variable degrees of agonistic/antagonistic effects, but little is known about their effects on gastrointestinal motility. The present study demonstrates that, similar to estrogen, resveratrol and genistein attenuate gastrointestinal smooth muscle motility through various mechanisms. The results reflect the physiologic and pharmacologic actions of resveratrol and genistein, provide the pharmacologic guidance for the application of these compounds, and are also very valuable for medicine and nutrition.
- Citation: Zhang LX, Li HF, Wang LD, Jin S, Dou XC, Tian ZF, Ma Q. Resveratrol and genistein inhibition of rat isolated gastrointestinal contractions and related mechanisms. World J Gastroenterol 2014; 20(41): 15335-15342
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15335.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15335
Gastrointestinal motility is an important and complex physiologic activity within the digestive tract that is regulated by neural and endocrine hormones. A major contributing factor to gastrointestinal symptoms is dysfunction of gastric motility. Sex hormones, estrogens in particular, are known to cause gastrointestinal motility disorder and contribute to irritable bowel syndrome[1,2]. The phytoestrogens resveratrol and genistein are structurally and functionally similar to estrogens and possess a wide spectrum of physiologic and pharmacologic effects, such as estrogenic[3,4], anti-atherosclerosis[5], and anti-osteoporosis[6] effects, and relieve menopausal symptoms[7] and inhibit tyrosine kinases[8,9]. Recent data indicate that genistein and resveratrol can inhibit vasocontractile responses[10,11], and affect gallbladder[12] and gastrointestinal[13-17] smooth muscle contraction. Despite the increasing attention to the effects of phytoestrogens in smooth muscles, little is known about the mechanisms of these effects. The present study was designed to observe the effects of the phytoestrogens resveratrol and genistein on the contraction of isolated gastrointestinal muscle strips, and to study the underlying mechanisms.
The present work was conducted in accordance with the guidelines for the Care and Use of Laboratory Animals from the National Institutes of Health and our institution. Preliminary studies indicated that no differences existed between sexes with respect to either the contractile responsiveness to agonists or the sensitivities to genistein and resveratrol. Therefore, adult non-pregnant female Sprague-Dawley rats (220-280 g, provided by the Experimental Animal Center of Lanzhou University) were utilized in this study. After an overnight fast, animals stunned by striking their head, and whole stomach and duodenum were quickly removed and placed in Kreb’s solution containing (mmol/L): NaCl 120, KCl 5.9, NaH2PO4 1.2, MgCl2 1.2, NaHCO3 15.4, CaCl2 2.5, and glucose 11.5, buffered at pH 7.4. After removal of the mucosa by blunt dissection, muscle strips were prepared from the body and antrum of the stomach and duodenum by cutting parallel to the long axis of the tissue, and mounted horizontally in separate 5 mL tissue chambers containing 37 ± 0.5 °C Kreb’s solution, bubbled with 95% O2 and 5% CO2. The muscle preparations were allowed to equilibrate for 20-30 min with a resting tension of 1.0 g and the solution was changed every 20 min. The isometric contractions were measured with force transducers and recorded with the BL-420E+ experimental system of biologic function (TME, China) by microcomputer.
To observe the direct effects of resveratrol and genistein on the basal contractile activities of gastrointestinal, different concentrations (0.01, 0.05, 0.10, 0.50, 1.00, 5.00, 10.00, and 50.00 μmol/L) of resveratrol and genistein or the same dose of solvent (control) was added in progressively increasing cumulative concentrations every 2 min. In order to investigate the mechanism of action, tissues were pre-incubated for 2 min with inhibitors of the estrogen receptors (1 μmol/L tamoxifen and 10 μmol/L ICI 182780), or 10 μmol/L of inhibitors of α-adrenergic receptor (phentolamine), nitric oxide (NO) synthase (levorotatory-NG-nitroarginine), cyclic adenosine monophosphate (cAMP) (SQ22536), and the ATP-sensitive potassium channel (KATP) (glibenclamide) before administration of different cumulative concentrations of genistein or resveratrol.
To determine the effects of resveratrol and genistein on contractile responses to CaCl2, gastric smooth muscle strips were first equilibrated with calcium-free Krebs solution for 30 min. KCl (final concentration 80 mmol/L) was added to the tissue chambers and tension was measured after 10 min. Afterward, a calcium-contraction curve experiment (CaCl2 final concentration: 10 μmol/L to 0.1mol/L) was performed according to a reported method[10]. After washing with calcium-free solution several times and recovering resting tension, the muscle strips were incubated with KCl (80 mmol/L) and resveratrol or genistein (final concentrations 1.0 or 50.0 μmol/L) for 5 min, then the calcium-contraction experiment was repeated.
Resveratrol, genistein, tamoxifen, SQ22536, glibenclamide, and levorotatory-NG-nitroarginine were purchased from Sigma-Aldrich (St. Louis, MO, USA). ICI 182780 was purchased from Tocris Bioscience (Bristol, UK). All drugs were dissolved in dimethyl sulphoxide so that the final concentration of dimethyl sulphoxide was never > 0.1%, which had no effect on basal contraction.
All results are expressed as mean ± standard error or as percentage change [(effect size - baseline contractile response)/baseline contractile response × 100]. In experiments involving concentration-response curves, the results are expressed as percentage of control maximal contractile responses induced by 0.1 mol/L CaCl2. The maximum contractile response (Emax) and the half-maximum effective concentration value (EC50) were determined for each curve by using a non-linear least square fitting procedure of individual experimental data and presented as pD2 (pD2 = -logEC50). Concentration-response curves were performed by using the Prism statistical software (Graph Pad Software, La Jolla, CA, USA) according to a sigmoid regression equation: Y = bottom + (top - bottom)/[1 + 10(logEC50-X) × Hill slope)], where X is the logarithm of the drug concentration and Y is the response. The statistical significance in concentration-response curves of the two groups was determined by two-way analysis of variance, and the others were evaluated by the Student’s t-test for paired and unpaired observations. P values less than 0.05 were considered statistically significant.
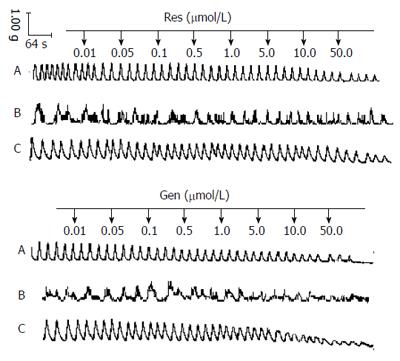
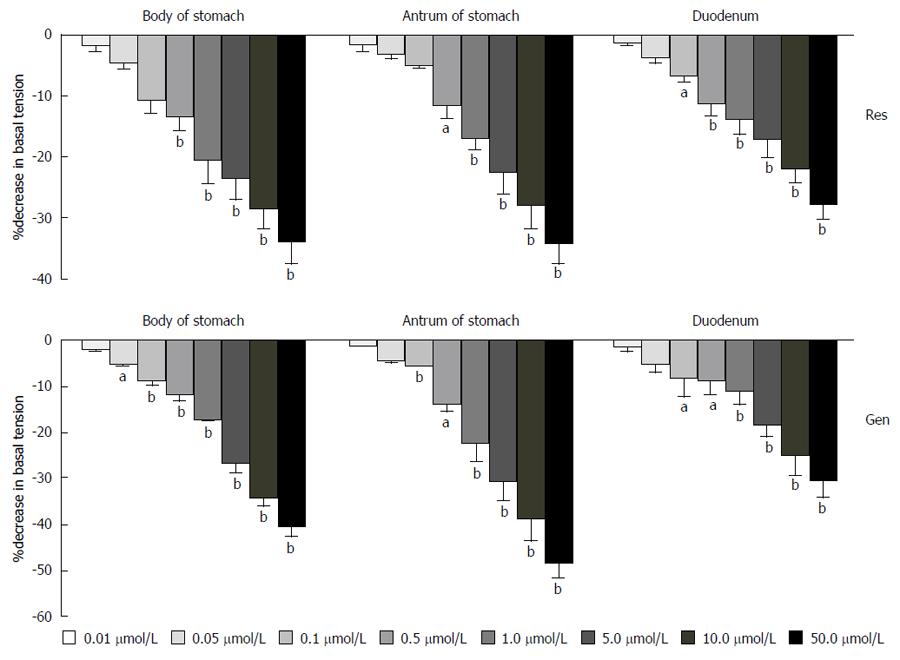
Representative tracings show the response to cumulatively applied resveratrol and genistein in body of stomach, duodenum and antrum of stomach smooth muscle strips (Figure 1). Resveratrol and genistein dose-dependently decreased the resting tension (n = 10; r = 0.99; P < 0.01) (Figure 2), the mean contractile amplitude (n = 10; r = 0.98; P < 0.01) and the contraction frequencies (data not shown).
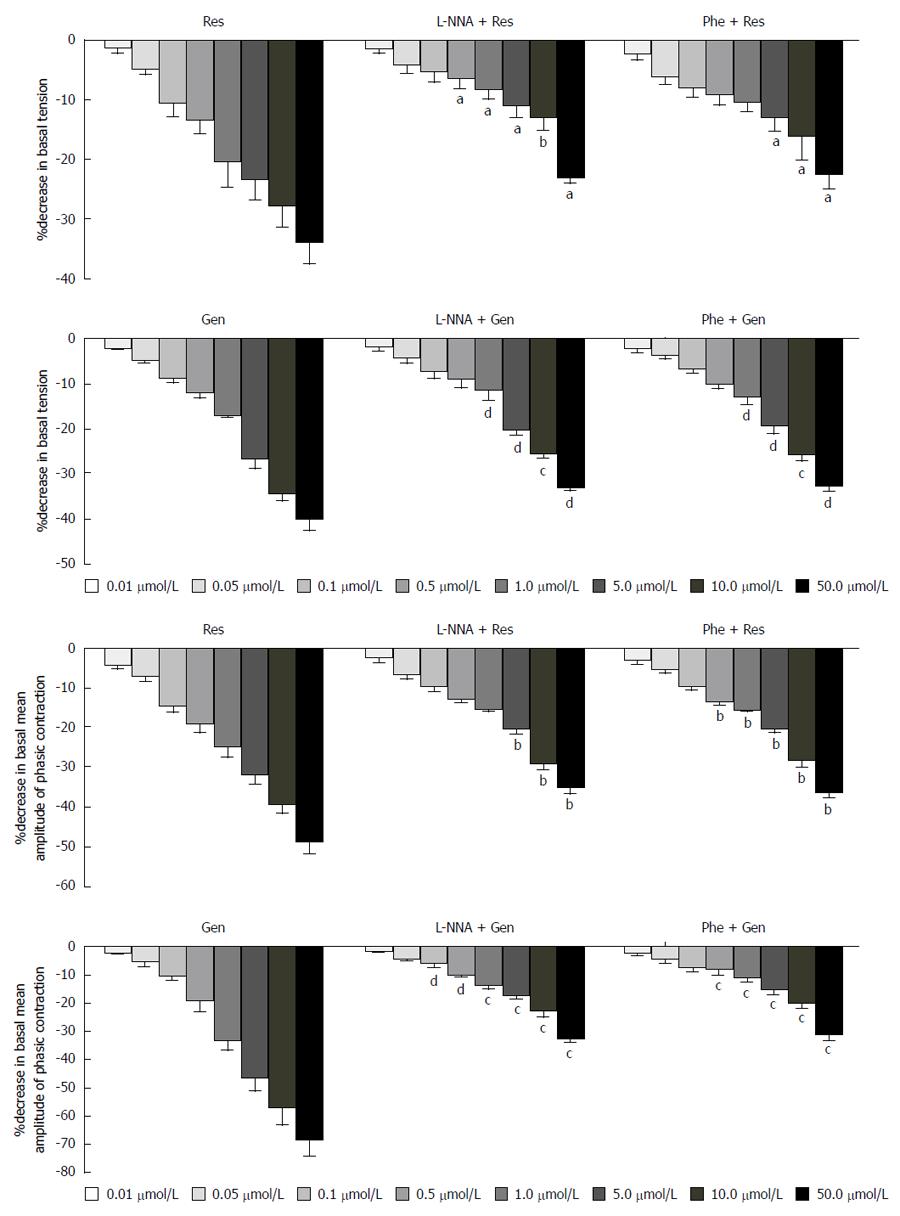
Incubation of gastric smooth muscle strips with 10 μmol/L phentolamine or levorotatory-NG-nitroarginine attenuated the inhibitory effects induced by resveratrol and genistein (Figure 3). Phentolamine and levorotatory-NG-nitroarginine alone had no obvious effect on basal activity.
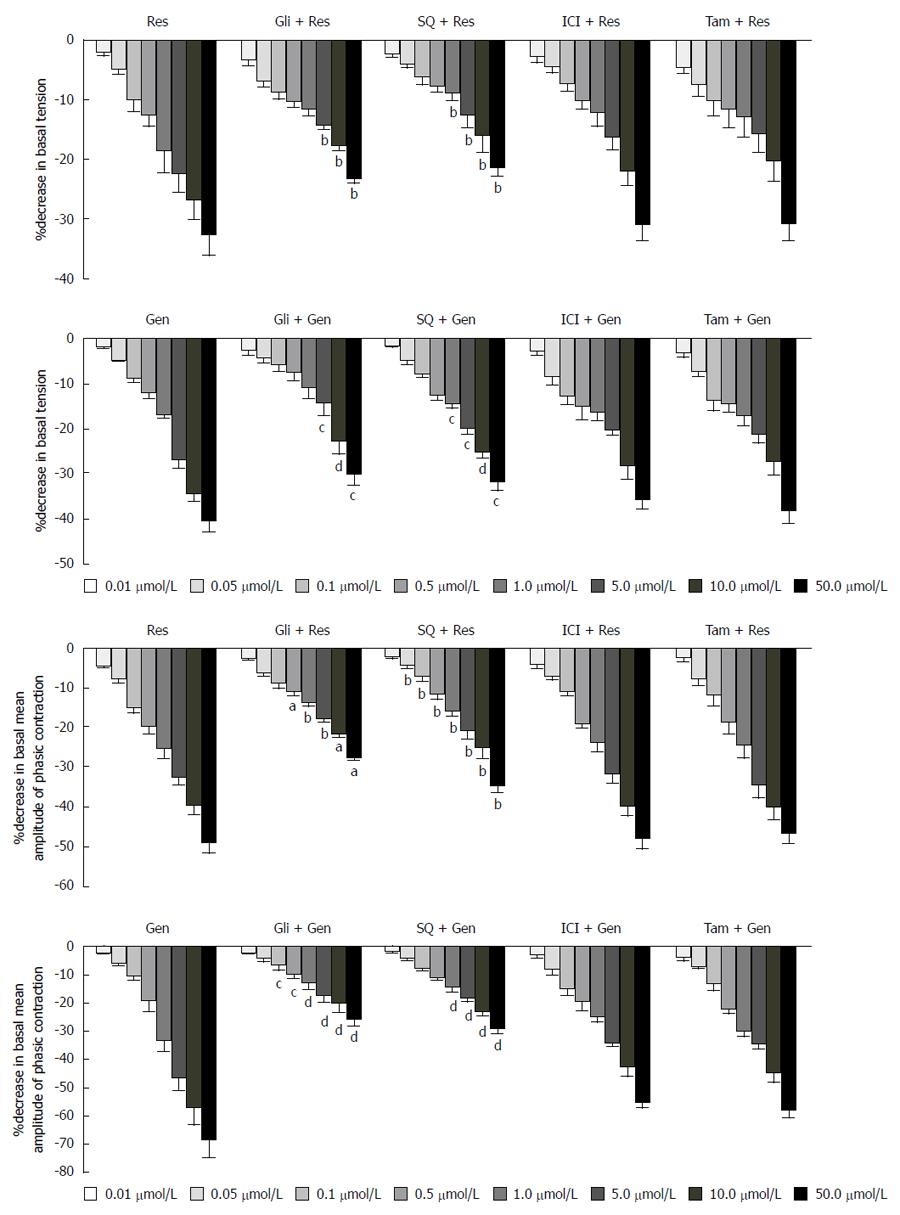
Incubation of gastric smooth muscle strips with 10 μmol/L glibenclamide or SQ22536 attenuated the inhibitory effects induced by resveratrol and genistein (Figure 4). However, ICI 182780 (10 μmol/L) and tamoxifen (1 μmol/L) did not alter the effects of resveratrol or genistein. ICI 182780, tamoxifen, glibenclamide and SQ22536 alone had no obvious effect on basal activity.
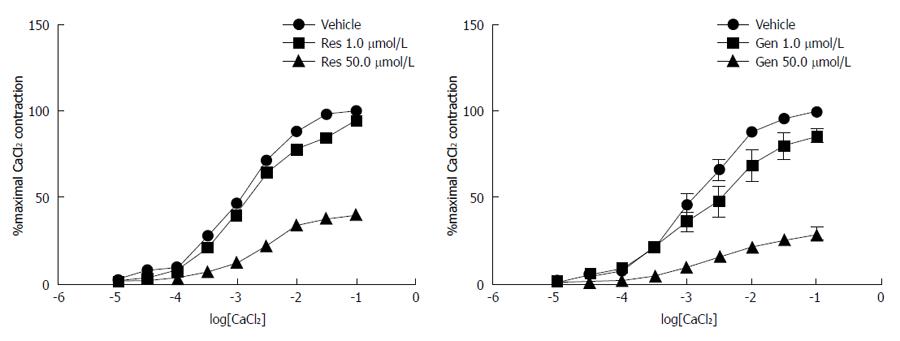
As summarized in Figure 5, incubation with 50.0 μmol/L resveratrol inhibited contractile responses of the body of the stomach to CaCl2 (pD2: 2.90 ± 0.03 vs 2.56 ± 0.07, Emax: 100.50 ± 1.22 vs 41.62 ± 1.32; Ps < 0.05). Similar effects were seen with 50.0 μmol/L genistein (pD2: 2.85 ± 0.06 vs 2.46 ± 0.22, Emax: 102.00 ± 3.36 vs 31.57 ± 4.40; Ps < 0.05). However, 1.0 μmol/L concentrations of resveratrol and genistein had no significant effect on calcium-dependent contraction.
There are structural similarities between the steroidal nucleus of 17 β-estradiol and the rigid ring structure of the phytoestrogen genistein, both of which can easily enter the cell and affect gene expression due to their lipid-solubility and relatively small molecular weight[10,11]. The structural similarity between resveratrol and the synthetic estrogen diethylstilbestrol prompted us to investigate whether resveratrol might exhibit estrogenic activity in gastrointestinal motility[1,2]. Studies in vitro have shown that ICI 182780, a nonselective estrogen receptor (ER) antagonist, blocks resveratrol-induced signaling pathway activation in endothelial cells[18]. In addition, resveratrol has been shown to inhibit vascular smooth muscle cell proliferation via an ER-dependent mechanism[19]. As smooth muscle cells express functional ERs, it is possible that the effects of resveratrol and genistein may be attributed to their interaction with these receptors. However, the results of the present study demonstrate that this is unlikely, as the inhibitory effects of these phytoestrogens were not affected by the ER antagonists ICI 182780 and tamoxifen, which can block the classical ERα and the novel ERβ[20]. These results suggest that the acute inhibitory effects caused by resveratrol and genistein are not mediated by the classical estrogen receptors.
The gastrointestinal tract has its own local nervous system, known as enteric nervous system, which contains adrenergic and cholinergic neurons. Norepinephrine is an important neurotransmitter in both the central and the peripheral components of nervous system. There are two major classes of adrenergic receptors for norepinephrine, α and β, which are G protein-coupled receptors that signal through cAMP pathways to regulate sarcoplasmic reticulum Ca2+ mobilization and myofilament Ca2+ sensitivity. Although the α-adrenoceptors mediate excitatory functions in rat liver, spleen and cerebral cortex, and hamster smooth muscle cell lines, they have inhibitory effects in the gastrointestinal tract[21]. To analyze the contribution of α-adrenoceptors and the cAMP pathway in the phytoestrogen-induced relaxing effects in rat isolated gastric smooth muscle, we used phentolamine, one of the α-adrenoceptors blockers[22], and SQ22536, an inhibitor of cAMP synthesis. Our data indicate that these compounds partially blocked the resveratrol and genistein-induced relaxations, indicating that the mechanism involves α-adrenergic receptors and the intracellular cAMP-signaling pathway.
NO is present in the majority of mesenteric neurons, including in the human gastric fundus, and is released to produce proximal gastric relaxation[23]. In addition, relaxation responses to graded mechanical distention are mostly ascribed to passive viscoelastic properties, with a slight NO-mediated neurogenic component[24]. The regulatory mechanisms are not well understood, and differ depending on stimulation[24,25]. NO initiates proximal gastric accommodation in the human stomach. Our data show that resveratrol and genistein-induced relaxation was partially blocked by inhibition of NO with levorotatory-NG-nitroarginine, indicating that NO is partially responsible for these effects in rat corpus longitudinal smooth muscle.
Reduced ATP and increased ADP concentrations activate KATP channels. Thus, it is thought that these channels provide a link between cell metabolism and membrane excitability. Furthermore, KATP channels are activated by a number of neuropeptides and neurotransmitters. Experiments in vivo show that glibenclamide interferes with the excitatory effects of active KATP channels in smooth muscle[26]. Therefore, we used this compound to analyze the contribution of KATP channels to the genistein or resveratrol-induced relaxation of rat isolated gastric smooth muscle. Although glibenclamide is one of the most selective blockers of KATP channels, it can block other types of K+ channels when used in high concentrations (> 30 μmol/L)[27]. As glibenclamide inhibited the effects of resveratrol and genistein, our data indicate that KATP channels are involved in resveratrol- and genistein-induced relaxation of gastric smooth muscle. This data is in agreement with a report by Wang et al[28] showing that resveratrol induced negative chronoscopic and isotropic actions via KATP and large conductance Ca2+ channels in the isolated guinea pig atrium. In contrast, Gojkovic-Bukarica et al[27] reported that glibenclamide does not antagonize the antinociceptive and vasorelaxant effects of resveratrol.
Two sources of calcium contribute to the initiation of smooth muscle contraction: release from the sarcoplasmic reticulum, and influx through calcium channels[29,30] that are activated membrane depolarization from high extracellular K+ concentrations[27]. In our experiments, CaCl2 increased the contractile response of gastric smooth muscle in high K+ depolarization medium in a concentration-dependent manner. Resveratrol and genistein inhibited these contractile responses and shifted the CaCl2 concentration-response curves rightward. Thus, resveratrol and genistein may exert their effects, in part, by inhibiting Ca2+ influx through voltage-dependent calcium channels. The calcium-antagonistic properties contribute to gastrointestinal smooth muscle relaxation in a manner similar to the effect of estrogen[1,10-12].
Resveratrol and genistein are also tyrosine kinase inhibitors[12,14]. Smooth muscle cells exhibit high tyrosine kinase activity, which is involved in the signaling associated with receptor-mediated contractions. Tyrosine phosphorylation is accompanied by capacitive Ca2+ entry evoked by thapsigargin or thrombin in platelets, suggesting a regulatory role for tyrosine kinase[14]. Data show that genistein can suppress the responses to Ca2+ reintroduction, such as sustained contraction, in rat ileal smooth muscles, suggesting that genistein inhibits capacitive Ca2+ entry rather than tyrosine kinase[14], which has supported our speculation of Ca2+-antagonistic properties of genistein. Evidences also demonstrate that the activity of apamin-sensitive K+ channels is facilitated by tyrosine kinase in the rat duodenal smooth muscle[13], and genistein can abolish the spontaneous rhythmic myogenic contractions of the gastric fundus and decrease the amplitude of the spontaneous contractions of rat colon longitudinal smooth muscle in a concentration-dependent manner, however it does not affect the inhibition of the colon circular muscle[15-17]. These findings are consistent with our study, and suggest that the direct inhibitory effects induced by resveratrol and genistein are mediated via diverse mechanisms and pathways. Whether the inhibition of tyrosine kinase is involved in the inhibitory effects of resveratrol and genistein still needs further investigation.
In summary, the phytoestrogens resveratrol and genistein can directly inhibit the contractile activity of isolated gastrointestinal smooth muscle. These effects are due to activation of α-adrenergic receptors, the NO and cAMP pathways, and KATP channels, but are not related to the activation of estrogen receptors.
We gratefully acknowledge the contributions of Associate Professor Xiao-Hong Duan (School of Foreign Language and Literatures, Lanzhou University) for polishing English grammatical errors.
The phytoestrogens resveratrol and genistein are structurally and functionally similar to estrogens and possess a wide spectrum of estrogenic physiologic and pharmacologic effects. These compounds can decrease contractile responses of vascular and gallbladder smooth muscles via a Ca2+ antagonistic property which is similar to estradiol, however little is known about the effect of resveratrol and genistein on gastrointestinal smooth muscle motility.
Gastrointestinal disorders can be influenced by ovary function, and it is noted that the prevalence of visceral pain disorders such as irritable bowel syndrome, gastroesophageal reflux disease, gallbladder and biliary tract diseases are significantly higher in women. Furthermore, symptoms such as nausea, vomiting, abdominal pain, distension, satiety, bloating, diarrhea, and constipation frequently appear in pregnancy, suggesting that sex hormones may play a significant role in the physiology and pathology of the gastrointestinal tract, including regulation of motor and sensory function. Phytoestrogens such as resveratrol and genistein have estrogen agonistic/antagonistic effects, and are proposed to affect gastrointestinal motility similarly to estrogen.
The aim of the present study was to observe direct effects of resveratrol and genistein on isolated gastrointestinal smooth muscle motility, and to elucidate the underlying mechanisms.
The results reflect the pharmacologic actions of resveratrol and genistein on gastrointestinal motility, provide pharmacologic guidance for the application of these compound, and are also very valuable for nutritional study of vegetables, fruits and plants that contain these phytoestrogens.
This is a nice paper looking at the effects of the phytoestrogens genistein and resveratrol on the inhibition of gastrointestinal motility. The data support the conclusions of the authors. Overall the manuscript is well written.
P- Reviewer: Alican I, Plaza MA, Srinivasan S S- Editor: Ma YJ L- Editor: AmEditor E- Editor: Wang CH
| 1. | Mulak A, Taché Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol. 2014;20:2433-2448. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 155] [Cited by in RCA: 178] [Article Influence: 16.2] [Reference Citation Analysis (2)] |
| 2. | Everson GT. Gastrointestinal motility in pregnancy. Gastroenterol Clin North Am. 1992;21:751-776. [PubMed] |
| 3. | Valeri A, Fiorenzani P, Rossi R, Aloisi AM, Valoti M, Pessina F. The soy phytoestrogens genistein and daidzein as neuroprotective agents against anoxia-glucopenia and reperfusion damage in rat urinary bladder. Pharmacol Res. 2012;66:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Rajah TT, Peine KJ, Du N, Serret CA, Drews NR. Physiological concentrations of genistein and 17β-estradiol inhibit MDA-MB-231 breast cancer cell growth by increasing BAX/BCL-2 and reducing pERK1/2. Anticancer Res. 2012;32:1181-1191. [PubMed] |
| 5. | Xu HS, Dai SL, Sun RY. [Cardiovascular effects of phytoestrogens]. Zhongguo Yixue Kexueyuan Xuebao. 2005;27:258-261. [PubMed] |
| 6. | Ji G, Yang Q, Hao J, Guo L, Chen X, Hu J, Leng L, Jiang Z. Anti-inflammatory effect of genistein on non-alcoholic steatohepatitis rats induced by high fat diet and its potential mechanisms. Int Immunopharmacol. 2011;11:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 90] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 7. | Eden JA. Phytoestrogens for menopausal symptoms: a review. Maturitas. 2012;72:157-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Zhang YH, Wu W, Sun HY, Deng XL, Cheng LC, Li X, Tse HF, Lau CP, Li GR. Modulation of human cardiac transient outward potassium current by EGFR tyrosine kinase and Src-family kinases. Cardiovasc Res. 2012;93:424-433. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Zhu H, Cheng H, Ren Y, Liu ZG, Zhang YF, De Luo B. Synergistic inhibitory effects by the combination of gefitinib and genistein on NSCLC with acquired drug-resistance in vitro and in vivo. Mol Biol Rep. 2012;39:4971-4979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 10. | Li HF, Tian ZF, Qiu XQ, Wu JX, Zhang P, Jia ZJ. A study of mechanisms involved in vasodilatation induced by resveratrol in isolated porcine coronary artery. Physiol Res. 2006;55:365-372. [PubMed] |
| 11. | Li HF, Wang LD, Qu SY. Phytoestrogen genistein decreases contractile response of aortic artery in vitro and arterial blood pressure in vivo. Acta Pharmacol Sin. 2004;25:313-318. [PubMed] |
| 12. | Wang LD, Qiu XQ, Tian ZF, Zhang YF, Li HF. Inhibitory effects of genistein and resveratrol on guinea pig gallbladder contractility in vitro. World J Gastroenterol. 2008;14:4955-4960. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 13. | Kawabata A, Kuroda R, Kuroki N, Nishikawa H, Kawai K, Araki H. Characterization of the protease-activated receptor-1-mediated contraction and relaxation in the rat duodenal smooth muscle. Life Sci. 2000;67:2521-2530. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Ohta T, Yasuda W, Hasegawa A, Ito S, Nakazato Y. Effects of inhibitors for tyrosine kinase and non-selective cation channel on capacitative Ca(2+) entry in rat ileal smooth muscle. Eur J Pharmacol. 2000;387:211-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 15. | Mulè F, Baffi MC, Falzone M, Cerra MC. Signal transduction pathways involved in the mechanical responses to protease-activated receptors in rat colon. J Pharmacol Exp Ther. 2002;303:1265-1272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Mustafa S, Oriowo M. Cooling-induced contraction of the rat gastric fundus: mediation via transient receptor potential (TRP) cation channel TRPM8 receptor and Rho-kinase activation. Clin Exp Pharmacol Physiol. 2005;32:832-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Takeuchi T, Kishi M, Hirayama N, Yamaji M, Ishii T, Nishio H, Hata F, Takewaki T. Tyrosine kinase involvement in apamin-sensitive inhibitory responses of rat distal colon. J Physiol. 1999;514:177-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Klinge CM, Blankenship KA, Risinger KE, Bhatnagar S, Noisin EL, Sumanasekera WK, Zhao L, Brey DM, Keynton RS. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J Biol Chem. 2005;280:7460-7468. [PubMed] |
| 19. | Khandelwal AR, Hebert VY, Dugas TR. Essential role of ER-alpha-dependent NO production in resveratrol-mediated inhibition of restenosis. Am J Physiol Heart Circ Physiol. 2010;299:H1451-H1458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Molero L, García-Durán M, Diaz-Recasens J, Rico L, Casado S, López-Farré A. Expression of estrogen receptor subtypes and neuronal nitric oxide synthase in neutrophils from women and men: regulation by estrogen. Cardiovasc Res. 2002;56:43-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 92] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 21. | Civantos Calzada B, Aleixandre de Artiñano A. Alpha-adrenoceptor subtypes. Pharmacol Res. 2001;44:195-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 202] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 22. | Petkov GV, Nelson MT. Differential regulation of Ca2+-activated K+ channels by beta-adrenoceptors in guinea pig urinary bladder smooth muscle. Am J Physiol Cell Physiol. 2005;288:C1255-C1263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Kim YC, Choi W, Yun HY, Sung R, Yoo RY, Park SM, Yun SJ, Kim MJ, Song YJ, Xu WX. Nitric Oxide-mediated Relaxation by High K in Human Gastric Longitudinal Smooth Muscle. Korean J Physiol Pharmacol. 2011;15:405-413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Tsuda Y, Nakahara T, Mori A, Sakamoto K, Ishii K. Resveratrol prevents bradykinin-induced contraction of rat urinary bladders by decreasing prostaglandin production and calcium influx. Eur J Pharmacol. 2011;666:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Dixit D, Zarate N, Liu LW, Boreham DR, Huizinga JD. Interstitial cells of Cajal and adaptive relaxation in the mouse stomach. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1129-G1136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 26. | Teramoto N. Physiological roles of ATP-sensitive K+ channels in smooth muscle. J Physiol. 2006;572:617-624. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 86] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 27. | Gojkovic-Bukarica L, Novakovic A, Kanjuh V, Bumbasirevic M, Lesic A, Heinle H. A role of ion channels in the endothelium-independent relaxation of rat mesenteric artery induced by resveratrol. J Pharmacol Sci. 2008;108:124-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 28. | Wang GY, Song CM, Zhang LN, Li Q, Yue H, Feng JK, Wang N. [Roles of potassium channel in effects of resveratrol on isolated myocardial contractility and heart rate research in guinea pig]. Zhongguo Zhongyao Zazhi. 2007;32:1317-1319. [PubMed] |
| 29. | Luo YL, Wang YL, Li NL, Zheng TZ, Zhang L, She YL, Hu SM. Actions of genistein on contractile response of smooth muscle isolated from guinea pig gallbladder. Hepatobiliary Pancreat Dis Int. 2009;8:614-619. [PubMed] |
| 30. | Ji G, Feldman M, Doran R, Zipfel W, Kotlikoff MI. Ca2+ -induced Ca2+ release through localized Ca2+ uncaging in smooth muscle. J Gen Physiol. 2006;127:225-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |