Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15319
Revised: May 28, 2014
Accepted: July 11, 2014
Published online: November 7, 2014
Processing time: 226 Days and 22.9 Hours
AIM: To investigate the effect of diazoxide administration on liver ischemia/reperfusion injury.
METHODS: Wistar male rats underwent partial liver ischemia performed by clamping the pedicle from the medium and left anterior lateral segments for 1 h under mechanical ventilation. They were divided into 3 groups: Control Group, rats submitted to liver manipulation, Saline Group, rats received saline, and Diazoxide Group, rats received intravenous injection diazoxide (3.5 mg/kg) 15 min before liver reperfusion. 4 h and 24 h after reperfusion, blood was collected for determination of aspartate transaminase (AST), alanine transaminase (ALT), tumor necrosis factor (TNF-α), interleukin-6 (IL-6), interleukin-10 (IL-10), nitrite/nitrate, creatinine and tumor growth factor-β1 (TGF-β1). Liver tissues were assembled for mitochondrial oxidation and phosphorylation, malondialdehyde (MDA) content, and histologic analysis. Pulmonary vascular permeability and myeloperoxidase (MPO) were also determined.
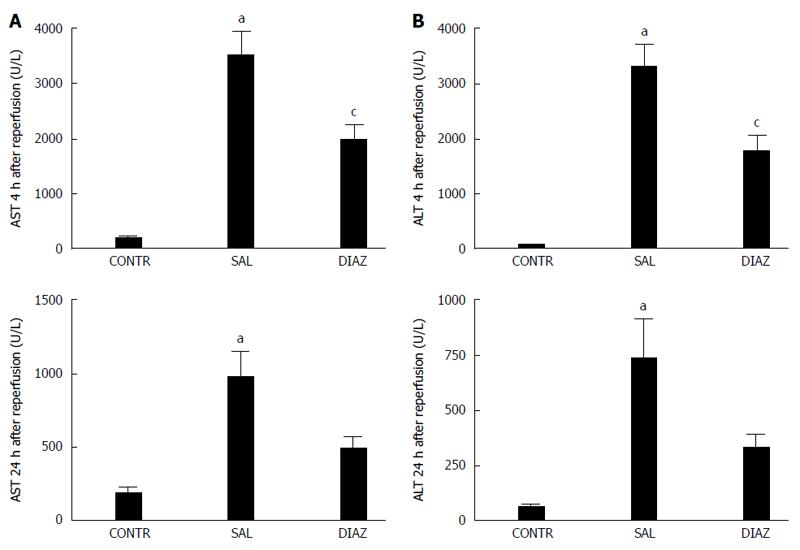
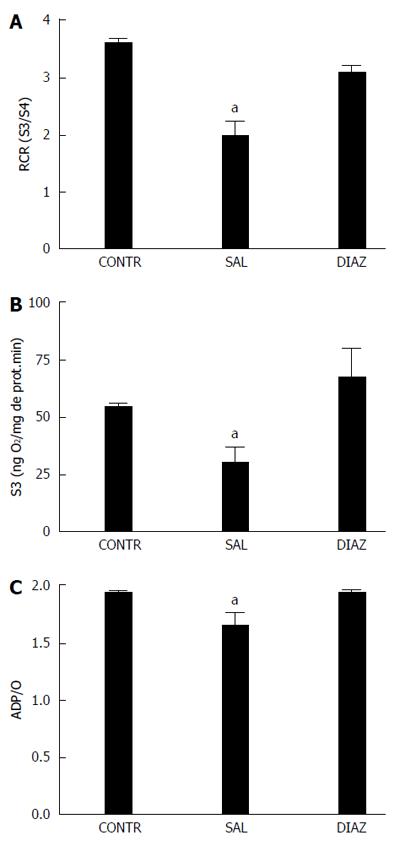
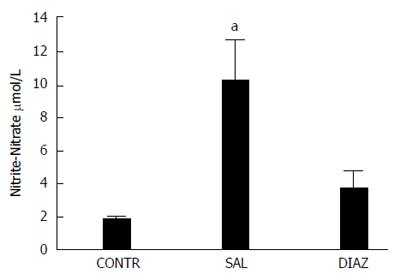
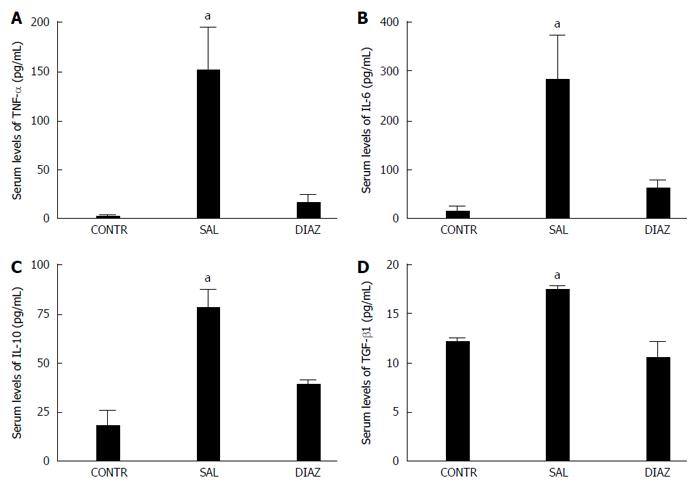
RESULTS: Four hours after reperfusion the diazoxide group presented with significant reduction of AST (2009 ± 257 U/L vs 3523 ± 424 U/L, P = 0.005); ALT (1794 ± 295 U/L vs 3316 ± 413 U/L, P = 0.005); TNF-α (17 ± 9 pg/mL vs 152 ± 43 pg/mL, P = 0.013; IL-6 (62 ± 18 pg/mL vs 281 ± 92 pg/mL); IL-10 (40 ± 9 pg/mL vs 78 ± 10 pg/mL P = 0.03), and nitrite/nitrate (3.8 ± 0.9 μmol/L vs 10.2 ± 2.4 μmol/L, P = 0.025) when compared to the saline group. A significant reduction in liver mitochondrial dysfunction was observed in the diazoxide group compared to the saline group (P < 0.05). No differences in liver MDA content, serum creatinine, pulmonary vascular permeability and MPO activity were observed between groups. Twenty four hours after reperfusion the diazoxide group showed a reduction of AST (495 ± 78 U/L vs 978 ± 192 U/L, P = 0.032); ALT (335 ± 59 U/L vs 742 ± 182 U/L, P = 0.048), and TGF-β1 (11 ± 1 ng/mL vs 17 ± 0.5 ng/mL, P = 0.004) serum levels when compared to the saline group. The control group did not present alterations when compared to the diazoxide and saline groups.
CONCLUSION: Diazoxide maintains liver mitochondrial function, increases liver tolerance to ischemia/reperfusion injury, and reduces the systemic inflammatory response. These effects require further evaluation for using in a clinical setting.
Core tip: Diazoxide is a selective mitoKATP channel opener and has a protective effect against organ ischemia/reperfusion (I/R) injury. This report shows that diazoxide maintains liver mitochondrial function, increases liver tolerance to I/R injury, and reduces the systemic inflammatory response. Since diazoxide has also a hypotensive effect its administration may also reduce bleeding in liver surgery during hepatic parenchyma transection. These effects require further evaluation for use in a clinical setting.
- Citation: Nogueira MA, Coelho AMM, Sampietre SN, Patzina RA, Pinheiro da Silva F, D'Albuquerque LAC, Machado MCC. Beneficial effects of adenosine triphosphate-sensitive K+ channel opener on liver ischemia/reperfusion injury. World J Gastroenterol 2014; 20(41): 15319-15326
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15319.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15319
The physiological function of mitochondrial ATP-sensitive potassium channel (mitoKATP) is to permit K+ transport into the mitochondrial matrix and therefore maintain its volume[1]. This mitochondrial channel has been identified in many tissues, such as heart[2], brain[3], skeletal muscle[4] and liver[5].
This mitoKATP channel has been related to the preconditioning protection of the ischemic heart[6]. It was also observed that the preconditioning-like effect of morphine in the intact heart can be the result of mitoKATP channel activation[7].
Diazoxide is a selective mitoKATP channel opener and could have a protective effect against organ ischemia/reperfusion (I/R) injury. In fact a protective effect has been demonstrated in the heart[8]. This protective effect is related to mitoKATP opening since other drugs with this action also reduce the heart I/R injury[9].
The protective effect of diazoxide in I/R injury has been reported in several organs, such as brain[10] and spinal cord[11] in which mitoKATP has been identified. Since mitoKATP channels are present in liver mitochondria[12] it is conceivable that diazoxide may have a protective effect on liver I/R injury. In the present study, we evaluated the effect of diazoxide on local and systemic effects of liver I/R injury.
Seventy adult male Wistar rats weighing 230-270 g housed in individual cages in a 12 h dark-light controlled environment were used for the experimental protocol. Rats had free access to standard rat chow and water. The experimental protocol was approved by the Ethics Committee for Animal Research from the Medical School of São Paulo University and received humanized care according to the criteria outlined in the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences and National Research Council. National Academic Press, Washington, DC, 1996.)
The rats were randomly submitted to the following experimental protocols: Control Group (n = 18), rats underwent laparotomy and liver manipulation; Saline Group (n = 26), rats received intravenous injection (iv) saline 15 min before liver ischemia; Diazoxide Group (n = 26), rats received iv diazoxide (Sigma Chemical CO, St. Louis, MO, USA) (3.5 mg/kg) 15 min before liver ischemia. Saline and Diazoxide groups received the same iv volume in mL/kg.
The animals were anesthetized with intra-peritoneal ketamine (Cristalia, São Paulo, Brazil) (30 mg/kg) and xylazine (Bayer, São Paulo, Brazil) (30 mg/kg) and submitted to orotracheal intubation, and ventilated with a tidal volume of 0.08 mL/g body weight, at a respiratory rate of 60/min, and FiO2 of 0.21 (Small Animal Ventilator model 683, Harvard Apparatus, Holliston, MA, USA). During the surgical procedure, body temperature was monitored using a rectal digital thermometer (YSI Precision 4000A Thermometer, USA), being maintained at 37 °C. Median laparotomy was performed and the hepatic pedicles of the median and left anterolateral segments were isolated, exposed and clamped with a non-traumatic microvascular bulldog clamp during 1 h to induce ischemia to 70% of the total liver volume. In this model, intestinal congestion is avoided, allowing the possibility to study the effects of isolated liver ischemia. The incision was closed, and after a 60-min ischemic period, the abdomen was reopened allowing clamp removal and liver reperfusion[13,14].
After liver reperfusion, rats were re-anesthetized for blood sampling through cardiac puncture and killed by exsanguination. At 4 h and 24 h after reperfusion, blood was collected for determination of aspartate aminotransferase (AST), alanine aminotransferase (ALT), tumor necrosis factor (TNF-α), interleukin-6 (IL-6), interleukin-10 (IL-10), tumor growth factor-β1 (TGF-β1), nitrite/nitrate and creatinine. Hepatic tissues were assembled for evaluation of mitochondrial oxidation and phosphorylation, malondialdehyde (MDA) content, and histologic analysis. The liver tissue for post-ischemic analysis was obtained from the median and left anterolateral segments previously submitted to I/R injury. Lungs were perfused via the trachea with 30-50 mL of 0.9% NaCl at 10 mL/min, using a syringe pump (model 975) from Harvard Apparatus, and fragments were harvested and divided for analysis of microvascular permeability and myeloperoxidase (MPO) activity. No mortality was observed in this model of partial liver ischemia.
Serum AST and ALT levels were measured to assess the extent of hepatocellular injury. The enzyme activities were assayed by using the optimized ultraviolet method (COBAS MIRA) from Roche (Roche Diagnostics, Rotkrenz, Switzerland). Results are expressed as units per liter (U/L).
Liver mitochondria were prepared as previously described[15]. Briefly, rat livers were rapidly excised and placed in medium containing 250 mmol/L sucrose, 10 mmol/L Tris-HCl, and 1 mmol/L EGTA, pH 7.3, at 4 °C. The tissue was scissor-minced and homogenized in ice using a Teflon Potter homogenizer. The homogenate was centrifuged at 600 g for 10 min. The supernatant was centrifuged for 10 min at 10000 g to obtain the mitochondrial pellet. A mitochondrial suspension containing 30-40 mg/mL of mitochondrial protein was prepared, and stored on ice before the assay of mitochondrial respiration.
The mitochondrial oxygen consumption was polarographically[16] measured using a Gilson 5/6H Oxygraph (Gilson Medical Eletronics, Inc., Middleton, WI) in a closed reaction vessel fitted with a Clark oxygen electrode (Yellow Springs Instruments Co., Yellow Springs, OH) at 28 °C. The incubation medium consisted of 120 mmol/L KCl, 2 mmol/L sodium phosphate, 10 μmol/L rotenone, and 1 mmol/L EGTA [Ethylene glycol-bis(2-aminoethylether)-N,N,N’,N’-tetraacetic acid], and was buffered at pH 7.3 with 5 mmol/L Tris-HCl. Mitochondria were energized with potassium succinate as substrate at a final concentration of 10 mmol/L. After a brief equilibration period, state 3 (activated state, S3) respiration was induced by the addition of 280 nmol adenosine diphosphate (ADP). The added ADP was phosphorylated to adenosine triphosphate (ATP) and the state 4 (basal state, S4) respiration was then measured. The oxygen consumption ratio in the presence of ADP to that in absence (respiratory control rate, RCR) and the ADP/O ratio were calculated as indices of mitochondrial oxidation and phosphorylation activities[17].
RCR = Oxygen consumption in the S3/oxygen consumption in the S4.
ADP/O = Moles of ATP formed from ADP per atom of oxygen consumed.
S3 and S4 were measured and reported as nmol oxygen per milligram mitochondrial protein per minute. Mitochondria protein content was determined by the method of Lowry et al[18].
MDA formation was used to indicate the occurrence of lipid peroxidation in the tissues and was estimated as thiobarbituric acid-reactive substances (TBARS). Liver tissues (100 mg/mL) were homogenized in 1.15% KCl buffer, and centrifuged at 14000 g for 20 min. An aliquot of the supernatant was then added to a reaction mixture consisting of 1.5 mL 0.8% thiobarbituric acid, 200 μL 8.1% (v/v) sodium dodecyl sulfate, 1.5 mL 20% acetic acid (pH 3.5), and 600 μL distilled water. The mixture was then heated at 90 °C for 45 min. After cooling to room temperature, the samples were cleared by centrifugation (10000 g for 10 min), and the absorbance was measured at 532 nm using malondialdehyde bis (dimethyl acetyl) as external standard. The content of lipid peroxides was expressed as nmol MDA per mg of protein[19].
Serum levels of nitrite-nitrate were determining using a commercial assay kit (R and D Systems Inc, MN, USA) according to the manufacturer’s guidelines.
Serum levels of TNF-α, IL-6, IL-10, and TGF-β1 were determined by ELISA using commercial kits (Invitrogen, CA, USA).
Liver samples were fixed in 10% buffered formalin for standard HE staining. Histological evaluation of the liver sections was performed by the same pathologist in a blinded manner. The severity of histological injury was analyzed according to the scoring system proposed by Quireze et al[20].
Increases in lung microvascular permeability were quantified by the Evans blue dye (EBD) extravasation technique as described previously[18]. EBD 20 mg/kg body weight was injected via dorsal penial vein 15 min before euthanasia. After collecting blood sampling, lungs were perfused with 30-50 mL of NaCl 0.9% at 10 mL/min, using a syringe pump (model 975) from Harvard Apparatus, and weighed. One small fragment was dried at 60 °C for calculation of total dry weight. To extract the dye, the lung was incubated with formamide, 4 mL/mg of tissue, for 24 h at room temperature. The concentration of EBD extracted into formamide was quantified spectrophotometrically at 620 nm using the Ultra Microplate Reader ELX 808 from Bio-Tek Instruments (Winooski, VT). The results are expressed as microgram of EBD per gram of dry weight tissue. Expression of results as a function of dry weight avoids underevaluation due to edema formation[21].
Lung MPO activity was used as an indicator of the neutrophil content in lung parenchyma. Samples of 300 mg wet lung tissue were homogenized with a polytron homogenizer (Polytron PT-2100 homogenizer, Kinematica AG, Luzern, Switzerland) for 60 s in 1 mL of sodium phosphate buffer, pH 6.2, containing 0.5 g/dL hexadecyltrimethyl ammonium bromide and 5 mmol/L of ethylenediaminetetraacetic acid. Homogenized samples were then sonicated at 40 Hz for 60 s, and centrifuged at 3000 g for 30 min at 4 °C. MPO activity in the supernatant was assayed by measuring the change in absorption at 460 nm (A460) resulting from the metabolism of hydrogen peroxide in the presence of O-dianisidine[22,23]. MPO content was expressed as units of MPO activity per gram tissue.
Serum levels of creatinine were determined at 4 and 24 h after reperfusion by a modified Jaffe method.
Results were presented as mean values ± SEM. Continuous variables were compared using analysis of variance. Results from the histological analysis were compared using the Kruskal-Wallis test. The level of P < 0.05 was considered as statistically significant. The GraphPad 6 Prism Software (GraphPad Software, San Diego, CA) was used for statistical analysis.
At 4 and 24 h after reperfusion serum AST and ALT activities were significantly elevated in groups with I/R compared to the control group; however the diazoxide group presented elevation of AST, with ALT serum levels significantly lower than the saline group (Figure 1A and B).
Four hours after reperfusion, there was a decrease in the oxygen consumption rate by liver mitochondria in state 3 (S3), in the RCR, and in the ADP/O ratio in animals from the saline group when compared to the diazoxide and control groups (Figure 2A-C). No differences were found in state 4 (S4) between the saline and diazoxide groups.
MDA content in the liver was used as measure of lipid peroxidation in the organ and it was not affected by diazoxide administration (Table 1).
| Control | Saline | Diazoxide | |
| Liver MDA (nmol/mg prot) | 1.49 ± 0.20a | 3.24 ± 0.26 | 2.60 ± 0.19 |
| Lung EBD (µg/g dry weight) | 70.13 ± 10.19a | 200.02 ± 54.44 | 155.70 ± 43.44 |
| Lung MPO Activity/g tissue | 0.032 ± 0.007a | 0.062 ± 0.008 | 0.065 ± 0.012 |
| Serum creatinine1 | 0.3 ± 0.1a | 0.8 ± 0.1 | 0.9 ± 0.1 |
| Serum Creatinine2 | 0.3 ± 0.1a | 1.0 ± 0.1 | 0.8 ± 0.2 |
Four hours after reperfusion it was observed that there was a significant reduction of nitrite-nitrate serum levels in the animals treated with diazoxide when compared to animals in the saline group (Figure 3).
At 4 h after liver reperfusion there was a significant increase in serum levels of TNF-α, IL-6, and IL-10 in the saline group when compared with the diazoxide and control groups (Figure 4A-C).
Transforming growth factor (TGF-β1) was also reduced 24 h after liver reperfusion in diazoxide treated animals and in the control group when compared to the saline group (Figure 4D).
The severity of histological injury analyzed according to the scoring system proposed by Quireze et al[20] was similar in both the diazoxide and saline groups.
Lung microvascular permeability evaluated through Evans blue dye (EBD) extravasation, was increased 4 h after reperfusion compared to the control group; however it was not affected by diazoxide treatment. Similar results were observed with the evaluation of lung neutrophil infiltration by lung MPO activity determination (Table 1).
Serum levels of creatinine were used as evaluation of kidney injury after I/R liver and these were not affected by diazoxide administration (Table 1).
Transient periods of nonlethal ischemia and reperfusion that confer protection against organ I/R injury have been named ischemic preconditioning. This ischemic preconditioning effect has been extensively studied as a method useful to reduce organ damage, including liver I/R injury[24-27]. In fact this is the only strategy used in clinical practice to reduce liver ischemic/reperfusion injury[28].
There are several pieces of experimental evidence that the main mechanism of organ protection in the ischemic preconditioning effect is the opening of the mitoKATP channel[29-32]. The main physiologic action of the mitoKATP channel is the regulation of the mitochondrial volume and therefore it regulates electron transport which is important in mitochondrial bioenergetic functions[33,34].
MitoKATP opening is followed by mitochondrial K+ uptake inducing matrix alkalization that causes complex I reactive oxygen species production and activation of protein kinase C-e and therefore inhibition of membrane permeability transition (MPT) pore opening[35]. Inhibition of MPT pore opening keeps mitochondrial integrity during I/R injury since the MPT pore opening leads to dissipation of proton motive force resulting in ATP depletion and cell energetic failure.
Diazoxide is a strong mitoKATP opener and therefore could protect organs from I/R injury. In fact its protective effect has been demonstrated in cerebral, renal and heart ischemia[29,36-38].
In the present study we observed a reduction in serum AST and ALT levels in the diazoxide group, reflecting the reduction in liver damage from I/R injury. Reduction of inflammatory mediators (TNF-α, IL-6, IL-10) observed in the present study is also related to a reduction of liver damage; however, the severity of histological injury analyzed according to the scoring system proposed by Quireze et al[20] was similar in both the diazoxide and the saline group. Despite reduction of inflammatory mediators, no attenuation of lung and kidney injury was observed in diazoxide treated animals. The preservation of mitochondrial function observed in this study by an increase in the oxygen consumption rate by liver mitochondria in state 3, in RCR, and in ADP/O ratio in animals in the diazoxide group when compared to the saline group, suggests that diazoxide may act by sustaining the mitochondrial energetics through mitoKATP opening, MPT pore opening inhibition and reduction of ATP depletion.
The reversible redox conversion of nitrite and nitric oxide (NO) allows us to evaluate the production of NO in pathologic conditions by determination of serum nitrate-nitrite levels. In the present study we observed a significant reduction in nitrate-nitrite serum levels in the group of animals treated with diazoxide compared to the saline group.
Reduction in TGF-β1 in diazoxide group also indicates a reduction in liver damage since TGF-β1 has been recognized as a key mediator in tissue fibrosis by stimulating matrix-producing fibrogenic cells and promoting extracellular matrix deposition following tissue injury.
MitoKATP opening inducing partial mitochondrial membrane depolarization reduces the driving force for Ca2+ influx during ischemia. In fact, it has been reported that diazoxide reduces Ca2+ influx during cardiac reperfusion[38]. Since intracellular Ca2+ overload may cause mitochondrial damage, the beneficial effect of diazoxide in liver I/R injury may also be related to reduction in hepatocyte intracellular Ca2+. The suppression of hepatocyte calcium overload by diazoxide recently demonstrated[12] is therefore an additional effect of diazoxide protecting liver mitochondria from I/R injury. The study of other activators of these mitoKATP channels may provide new therapeutic strategies for treatment of liver I/R injury.
In conclusion diazoxide administration by mitoKATP opening maintains liver mitochondrial function, increases liver tolerance to I/R injury, and reduces systemic inflammatory response. Since diazoxide has also a hypotensive effect its administration may also reduce bleeding in liver surgery during hepatic parenchyma transection. These effects require further evaluation before use in a clinical setting.
The mitochondrial ATP-sensitive potassium channel (mitoKATP) channel has been related to preconditioning protection in several organs including the liver. Diazoxide is a selective mitoKATP opener which has a protective effect on liver ischemia/reperfusion (I/R) injury; however the mechanism of this protection is not well understood.
The authors demonstrated in this study that diazoxide protects the liver from I/R injury by reduction of mitochondrial dysfunction and also by significant reduction of inflammatory cytokines.
Although the protective effects of diazoxide on liver I/R injury had been previously shown, we demonstrated that these effects include mitochondrial function preservation and also a significant reduction in inflammatory cytokines. The authors concluded that diazoxide reduces mitochondrial dysfunction and reduces inflammatory cytokines protecting the liver but has no effect on distant organ damage.
Diazoxide as a hypotensive drug can be used during liver resection reducing bleeding and also protecting liver from I/R injury.
This study evaluated the effects of mitochondrial K+ channel opener diazoxide on liver I/R injury using a rat in vivo warm I/R model. It shows that diazoxide decreased AST and ALT release after hepatic warm I/R but did not protect against liver histological changes and lung and kidney injury. It provides some interesting data on the protective effects of diazoxide after hepatic warm I/R in rats.
P- Reviewer: Zhong Z S- Editor: Ding Y L- Editor: O’Neill M E- Editor: Wang CH
| 1. | Kicińska A, Szewczyk A. Protective effects of the potassium channel opener-diazoxide against injury in neonatal rat ventricular myocytes. Gen Physiol Biophys. 2003;22:383-395. [PubMed] |
| 2. | Noma A. ATP-regulated K+ channels in cardiac muscle. Nature. 1983;305:147-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1685] [Cited by in RCA: 1644] [Article Influence: 39.1] [Reference Citation Analysis (0)] |
| 3. | Bajgar R, Seetharaman S, Kowaltowski AJ, Garlid KD, Paucek P. Identification and properties of a novel intracellular (mitochondrial) ATP-sensitive potassium channel in brain. J Biol Chem. 2001;276:33369-33374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 218] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 4. | Debska G, Kicinska A, Skalska J, Szewczyk A, May R, Elger CE, Kunz WS. Opening of potassium channels modulates mitochondrial function in rat skeletal muscle. Biochim Biophys Acta. 2002;1556:97-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 77] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 5. | Inoue I, Nagase H, Kishi K, Higuti T. ATP-sensitive K+ channel in the mitochondrial inner membrane. Nature. 1991;352:244-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 553] [Cited by in RCA: 536] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 6. | Wang L, Kinnear C, Hammel JM, Zhu W, Hua Z, Mi W, Caldarone CA. Preservation of mitochondrial structure and function after cardioplegic arrest in the neonate using a selective mitochondrial KATP channel opener. Ann Thorac Surg. 2006;81:1817-1823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 7. | Liang BT, Gross GJ. Direct preconditioning of cardiac myocytes via opioid receptors and KATP channels. Circ Res. 1999;84:1396-1400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 108] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Wakahara N, Katoh H, Yaguchi Y, Uehara A, Satoh H, Terada H, Fujise Y, Hayashi H. Difference in the cardioprotective mechanisms between ischemic preconditioning and pharmacological preconditioning by diazoxide in rat hearts. Circ J. 2004;68:156-162. [PubMed] |
| 9. | Oldenburg O, Yang XM, Krieg T, Garlid KD, Cohen MV, Grover GJ, Downey JM. P1075 opens mitochondrial K(ATP) channels and generates reactive oxygen species resulting in cardioprotection of rabbit hearts. J Mol Cell Cardiol. 2003;35:1035-1042. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Lenzsér G, Kis B, Bari F, Busija DW. Diazoxide preconditioning attenuates global cerebral ischemia-induced blood-brain barrier permeability. Brain Res. 2005;1051:72-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 11. | Roseborough G, Gao D, Chen L, Trush MA, Zhou S, Williams GM, Wei C. The mitochondrial K-ATP channel opener, diazoxide, prevents ischemia-reperfusion injury in the rabbit spinal cord. Am J Pathol. 2006;168:1443-1451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 12. | Nakagawa Y, Yoshioka M, Abe Y, Uchinami H, Ohba T, Ono K, Yamamoto Y. Enhancement of liver regeneration by adenosine triphosphate-sensitive K+ channel opener (diazoxide) after partial hepatectomy. Transplantation. 2012;93:1094-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Yoshizumi T, Yanaga K, Soejima Y, Maeda T, Uchiyama H, Sugimachi K. Amelioration of liver injury by ischaemic preconditioning. Br J Surg. 1998;85:1636-1640. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 86] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 14. | Figueira ER, Bacchella T, Coelho AM, Sampietre SN, Molan NA, Leitão RM, Machado MC. Timing-dependent protection of hypertonic saline solution administration in experimental liver ischemia/reperfusion injury. Surgery. 2010;147:415-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 15. | Coelho AM, Machado MC, Sampietre SN, Leite KR, Oliveira VL, Pinotti HW. Hepatic damage during acute pancreatitis in the rat. Braz J Med Biol Res. 1997;30:947-953. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Estabrook RW. Mitochondrial respiratory control and the polarographic measurement of ADP/O ratios. Estabrook and ME Pullman: Methods in enzymology. New York: Academic Press 1967; 41-47. |
| 17. | CHANCE B, WILLIAMS GR. A simple and rapid assay of oxidative phosphorylation. Nature. 1955;175:1120-1121. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 299] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 18. | Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265-275. [PubMed] |
| 19. | Soriano FG, Liaudet L, Szabó E, Virág L, Mabley JG, Pacher P, Szabó C. Resistance to acute septic peritonitis in poly(ADP-ribose) polymerase-1-deficient mice. Shock. 2002;17:286-292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 129] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 20. | Quireze C, Montero EF, Leitão RM, Juliano Y, Fagundes DJ, Poli-de-Figueiredo LF. Ischemic preconditioning prevents apoptotic cell death and necrosis in early and intermediate phases of liver ischemia-reperfusion injury in rats. J Invest Surg. 2006;19:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Jancar S, De Giaccobi G, Mariano M, Mencia-Huerta JM, Sirois P, Braquet P. Immune complex induced pancreatitis: effect of BN 52021, a selective antagonist of platelet-activating factor. Prostaglandins. 1988;35:757-770. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 40] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Goldblum SE, Wu KM, Jay M. Lung myeloperoxidase as a measure of pulmonary leukostasis in rabbits. J Appl Physiol (1985). 1985;59:1978-1985. [PubMed] |
| 23. | Warren JS, Yabroff KR, Mandel DM, Johnson KJ, Ward PA. Role of O2- in neutrophil recruitment into sites of dermal and pulmonary vasculitis. Free Radic Biol Med. 1990;8:163-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 24. | Kume M, Yamamoto Y, Saad S, Gomi T, Kimoto S, Shimabukuro T, Yagi T, Nakagami M, Takada Y, Morimoto T. Ischemic preconditioning of the liver in rats: implications of heat shock protein induction to increase tolerance of ischemia-reperfusion injury. J Lab Clin Med. 1996;128:251-258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 149] [Cited by in RCA: 147] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 25. | Yellon DM, Baxter GF, Garcia-Dorado D, Heusch G, Sumeray MS. Ischaemic preconditioning: present position and future directions. Cardiovasc Res. 1998;37:21-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 164] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 26. | Peralta C, Prats N, Xaus C, Gelpí E, Roselló-Catafau J. Protective effect of liver ischemic preconditioning on liver and lung injury induced by hepatic ischemia-reperfusion in the rat. Hepatology. 1999;30:1481-1489. [PubMed] |
| 27. | Zhang WX, Yin W, Zhang L, Wang LH, Bao L, Tuo HF, Zhou LF, Wang CC. Preconditioning and postconditioning reduce hepatic ischemia-reperfusion injury in rats. Hepatobiliary Pancreat Dis Int. 2009;8:586-590. [PubMed] |
| 28. | Petrowsky H, McCormack L, Trujillo M, Selzner M, Jochum W, Clavien PA. A prospective, randomized, controlled trial comparing intermittent portal triad clamping versus ischemic preconditioning with continuous clamping for major liver resection. Ann Surg. 2006;244:921-928; discussion 928-930. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 164] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 29. | Rahgozar M, Willgoss DA, Gobé GC, Endre ZH. ATP-dependent K+ channels in renal ischemia reperfusion injury. Ren Fail. 2003;25:885-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 30. | Facundo HT, Carreira RS, de Paula JG, Santos CC, Ferranti R, Laurindo FR, Kowaltowski AJ. Ischemic preconditioning requires increases in reactive oxygen release independent of mitochondrial K+ channel activity. Free Radic Biol Med. 2006;40:469-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 54] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 31. | Facundo HT, de Paula JG, Kowaltowski AJ. Mitochondrial ATP-sensitive K+ channels are redox-sensitive pathways that control reactive oxygen species production. Free Radic Biol Med. 2007;42:1039-1048. [PubMed] |
| 32. | Nishida H, Sato T, Fukasawa M, Miyazaki M, Nakaya H. Oxytocin potentiates the opening of mitochondrial ATPsensitive K channels and reduces infarct size in rabbit hearts. J Pharmacol Sci. 2007;103 Suppl I:102P. [RCA] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 33. | Nishida H, Matsumoto A, Tomono N, Hanakai T, Harada S, Nakaya H. Biochemistry and physiology of mitochondrial ion channels involved in cardioprotection. FEBS Lett. 2010;584:2161-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 34. | Nishida H, Sato T, Ogura T, Nakaya H. New aspects for the treatment of cardiac diseases based on the diversity of functional controls on cardiac muscles: mitochondrial ion channels and cardioprotection. J Pharmacol Sci. 2009;109:341-347. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 35. | Garlid KD, Costa AD, Quinlan CL, Pierre SV, Dos Santos P. Cardioprotective signaling to mitochondria. J Mol Cell Cardiol. 2009;46:858-866. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 151] [Cited by in RCA: 150] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 36. | Domoki F, Bari F, Nagy K, Busija DW, Siklós L. Diazoxide prevents mitochondrial swelling and Ca2+ accumulation in CA1 pyramidal cells after cerebral ischemia in newborn pigs. Brain Res. 2004;1019:97-104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 37. | Iwai T, Tanonaka K, Koshimizu M, Takeo S. Preservation of mitochondrial function by diazoxide during sustained ischaemia in the rat heart. Br J Pharmacol. 2000;129:1219-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 49] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 38. | González G, Zaldívar D, Carrillo E, Hernández A, García M, Sánchez J. Pharmacological preconditioning by diazoxide downregulates cardiac L-type Ca(2+) channels. Br J Pharmacol. 2010;161:1172-1185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |