INTRODUCTION
Hydatid disease results from infestation of a parasite originating from a Mediterranean strain of Echinococcus seen mostly in South America, North Africa, the Middle East and Eastern Europe[1,2]. Hydatid disease is endemic in most parts of the world, especially in the Mediterranean where sheep husbandry is common, and is an important medical health problem in these regions. Furthermore, increasing migration raises the incidence of the disease in areas where it was once rare[3,4]. Approximately 4000 patients per year are diagnosed with hydatid disease in Turkey[5].
The clinical presentation of hydatid disease varies depending on the location, growth rate and size of the cysts, and organ affected. Symptoms typically develop as a result of the compression of adjacent structures or viscera as a result of surrounding inflammation, or from the rupture of the cyst into the bile duct, pleural space, or peritoneal cavity. The liver is most commonly affected, with involvement of the right lobe in 55%-80% of patients. Most patients with hepatic hydatid cysts (HHC) present with an uncomplicated course at diagnosis and treatment planning is not problematic. However, the diagnosis and management of complicated courses of HHC depend on the experience levels of the surgeon, interventional radiologist and therapeutic endoscopist.
Common complications of HHC include rupture into intrahepatic bile ducts or the peritoneal cavity, invasion of other organs, pressure on the biliary tree and other neighboring structures, and infection[6,7]. Of these, intrabiliary rupture (IBR) is the most common and serious complication, occurring in 2%-42% of cases[7-11]. According to Dew[12], who was the first to report this complication, IBR usually occurs in the biliary ducts of the right lobe (55%-60%), less commonly in the left lobe biliary ducts (30%-35%), and rarely in the common bile duct. The clinical presentation of IBR can range from asymptomatic to jaundice, cholecystitis, cholangitis, liver abscess, pancreatitis and septicemia, depending on the size of the cystobiliary communication. However, undiagnosed ruptures can result in biliary leakage, biloma, cavitary infection and obstructive jaundice after HHC surgery[7,13-15].
In the past, IBR complications were diagnosed and treated by surgical methods, accompanied by 50% morbidity and 4.5% mortality rates. More recently, non-invasive or minimally invasive methods have been used to diagnose and treat these complications during both the pre- and the postoperative periods. In clinical practice, it is generally agreed that endoscopic retrograde cholangiopancreatography (ERCP) is indicated for patients with biliary adverse events after surgery, such as persistent biliary fistulae and jaundice, and preoperative frank IBR that is suspected clinically (because of jaundice), biochemically (because of overt cholestasis), or radiologically (as a dilated main biliary ductal system or hydatid elements evident in the bile ducts). However, the use of routine preoperative ERCP and prophylactic endoscopic sphincterotomy (ES) in patients with suspected minor cystobiliary communications remains controversial. Furthermore, the increasing use of magnetic resonance cholangiopancreatography (MRCP) fuels the debate on the routine use of preoperative diagnostic ERCP. The goal of this article is to review the efficacy of ERCP for the diagnosis and treatment of hepatic hydatid disease during the pre- and postoperative periods.
HYDATID CYSTS AND INTRABILIARY RUPTURE
The parasite that causes the hydatid cyst becomes apparent after three weeks and can attain a bulk of 3 cm in the liver parenchyma after three months. Atrophy and fibrosis occur as a result of the pressure of the growing cyst. During this growing period, the intracystic pressure may rise to 80 cm H2O within a live cyst and can cause compression and obstruction of the biliary tree if centrally located. Even if there is no rupture into the bile ducts, cholestasis and partial dilatation of the proximal part of affected ducts can be detected biochemically and radiologically. Continued growth can result in rupture into the bile ducts due to the increased intracystic pressure and fragility of the cyst wall[16-18].
Rupture into the bile ducts is the most common complication of hepatic hydatid disease and its incidence is reported to be as high as 42% in some clinical series[8-11,19,20]. There are two theories describing the pathogenesis of IBR. According to the first theory, progressive necrosis and communication between the cyst and the biliary tree are caused by the compression of the HHC wall. The second theory states that the trapping of small biliary radicals in the pericystic wall causes high intracystic pressure and results in atrophy, followed by the rupture of biliary radicals[21,22].
The communication between the ruptured cyst and bile ducts can be classified as either major or minor/simple based on size. Simple communications are relatively small communications between the cyst wall and small biliary radicals, which are seen in 10%-37% of HHC patients. In these cases, bile ducts are in communication with the cyst, but neither daughter cysts nor germinative membrane is found within the bile ducts. Cystic fluid, scolices or very tiny hydatid elements may enter the bile ducts, but do not cause obstruction due to their size. Simple communications are difficult to demonstrate preoperatively as they do not exhibit indirect symptoms, such as dilatation of bile ducts, seldom cause biliary colic, and patients are generally asymptomatic. Although simple communications can be demonstrated intraoperatively, they are most often recognized by postoperative biliary leakage. In contrast, major, open wide communications between the cyst wall and biliary tree, seen with frank IBR in 3%-17% of HHC patients, can be detected preoperatively. In this type of cystobiliary communication, cystic content empties into the biliary tree causing intermittent or total obstruction[23,24]. Major communications most commonly present as biliary colic, jaundice and cholangitis, which can worsen, resulting in liver abscess, septicemia or anaphylaxis. Although rare, complications such as acute cholecystitis and pancreatitis can also occur due to hydatid remnants within bile ducts[7,12-15].
PREOPERATIVE ERCP
In clinical practice, there are four recommendations concerning preoperative ERCP for diagnosing and treating cystobiliary communications: (1) perform elective surgery to overcome acute conditions such as cholangitis and biliary obstruction; (2) reduce the risk of postoperative persistent biliary fistulae and the duration of hospitalization by preoperative ES in patients with minor communications; (3) remove hydatid elements within the bile ducts and schedule hydatid cyst surgery; and (4) in cases of major rupture, employ a therapeutic approach by emptying hydatid contents within the cyst and bile ducts[10,16,18-20,25,26].
Diagnostic ERCP
The first management approach for hepatic hydatid disease consists of a clinical history, detailed physical examination, routine blood tests (complete blood count, sedimentation, liver biochemistry), serologic examination and imaging methods such as ultrasonography (US) and computed tomography (CT). These evaluations help to determine whether the hydatid disease has a complicated or uncomplicated course[7,16,17,27,28].
Although routine ERCP is not yet accepted for uncomplicated hydatid cysts, some clinical centers advise their use in order to entirely determine bile duct anatomy and describe the communication between the cyst and bile ducts[16,29]. With consideration of cases where cystobiliary communication is common in liver hydatid cyst surgery (e.g., large or multiple cysts, hilar or caudate lobe cysts or cysts accompanied with leukocytosis), the routine use of ERCP in uncomplicated hydatid cystic disease is suggested to prevent or decrease postoperative biliary complications[10,11,20,30,31]. However, postoperative bile leakage has been reported in patients who underwent preoperative ERCP and ES for prevention of fistula formation, not all communications can be detected with diagnostic ERCP, and most of the patients with postoperative bile leakage heal spontaneously without a need for ERCP[3,10,11,20,30,31]. Moreover, in a variable percentage (0%-30%) of patients who underwent ERCP for suspected frank IBR (i.e., presented with jaundice and/or dilated bile ducts), only bile duct compression by the cyst was observed[5,10,18].
There are some limitations associated with ERCP for the diagnosis of hydatid disease. Primarily, minor cystobiliary communications may not be detected with ERCP due to increased intracystic pressure, the minimal communication of cyst and biliary tree, and to daughter cysts temporarily obstructing the cystobiliary opening. Intrahepatic bile ducts may not completely fill with contrast media due to the increased pressure, increasing the false negativity ratio up to 17%-20%. Also, if contrast media is too intense, cysts within the main bile ducts may not be apparent and daughter cysts or old hydatid particles may mimic gallstones. Finally, hydatid elements that are solely in intrahepatic ducts may be overlooked with ERCP. Therefore, surgeons should remain vigilant for preoperative minor cystobiliary communications despite their apparent absence[3,10,16,32-35]. Some of these limitations can be minimized with correct pressure injection from different points or by applying occlusion cholangiography. Moreover, ERCP can reveal cystobiliary fistulae and filling defects due to hydatid elements in bile ducts, as well as indirect signs of minor cystobiliary communication, such as cystic pressure, dilatation and erratic filling of neighboring bile ducts.
Studies have suggested that MRCP is comparable to ERCP as a diagnostic tool because of its high sensitivity in bile duct pathologies, and advise its use in place of ERCP as it is a non-invasive method[10,36,37]. In cases of cystobiliary communication, ERCP can then be used as a therapeutic means. In our clinical practice, we do not perform preoperative diagnostic ERCP if there is no history of cystobiliary pancreatic symptoms, overt cholestasis in blood studies or biliary ductal dilatation distal to the cyst in US and CT images. In cases of minor cholestasis or partial dilatation of intrahepatic bile ducts proximal to the cyst, MRCP in addition to US and CT prove to be more useful than ERCP for diagnosing minor communications in uncomplicated cases.
Although frank IBR can be easily diagnosed with US, CT or MRCP, ERCP is usually the first modality chosen in cases of complicated HHC where frank IBR is suspected by obstructive jaundice, cholangitis or biliary sepsis, abnormal biochemical liver function tests, presence of cholestatic enzymes or hyperbilirubinemia, if hydatid elements are present within the bile ducts, or for any kind of dilatation of main bile ducts[16,19,31,35,38]. Hence, preoperative ERCP is more commonly used therapeutically than diagnostically[3,7,23].
In cases of frank IBR, white, shiny hydatid membranes are mostly seen within the duodenum or protruding from the papilla Vateri during ERCP. On cholangiography, linear wavy filling defects of laminated hydatid membranes can be easily seen within the main bile duct. Daughter cysts can be seen as rounded or oval lucent filled defects. In late phases, membrane particles lose their shiny characteristic and mimic bile stones as they become colorized by bile acids. In patients with uncomplicated HHC, both diagnostic and therapeutic ERCP should be performed if cystic fluid is colorized by bile when using the PAIR (puncture, aspiration, injection and re-aspiration) protocol[7,16].
Therapeutic ERCP
Preoperative therapeutic ERCP has a reported success rate as high as 80%-100%[4,20,39-47], and can be used when cystobiliary communication or hydatid membranes and/or daughter cysts that cause biliary obstruction are demonstrated by cholangiography. ES, extraction by balloon or basket catheter, nasobiliary drainage (NBD) and biliary stenting are some therapeutic methods that can be performed by ERCP. As described above, preoperative ES reduces the risk of postoperative external biliary fistulae, morbidity and the length of hospitalization in patients diagnosed with cystobiliary communication by ERCP. However, in the series reported by Rodriguez et al[39], patients undergoing preoperative ERCP developed uncontrollable fistulae. In another series reported by Galati et al[23] ES was found to reduce postoperative fistulae and cavitary infection due to cystobiliary rupture. Additional advantages of endoscopic treatment for HHC patients are that it allows for elective surgery and significantly decreases morbidity and mortality associated with the procedure. However, further studies are needed to evaluate the prophylactic use of ES for the prevention of biliary fistulae[13,20,47,48].
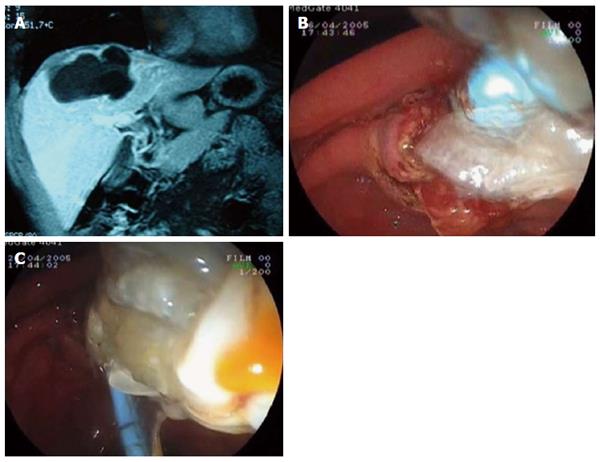
In major ruptures, hydatid membranes or daughter cysts encountered in bile ducts can be emptied out by ES, and a Dormia basket and biliary occlusion balloon can be used to clean out the common and main bile ducts. If hydatid membranes are sufficiently large, they can fill the duodenal lumen and hide the already sphincterotomized papilla orifice. In our ERCP procedure, guidewire placement is routinely used before ES when large amounts of hydatid elements are observed in bile ducts. These techniques can be performed successively many times under C-arm fluoroscopy even when endoscopic manipulation is made difficult by the hydatid materials around the papilla. After emptying hydatid membranes and big daughter vesicles, saline irrigation of the bile duct may be necessary to flush out the hydatid material and small daughter cysts (Figure 1).
Figure 1 Endoscopic retrograde cholangiopancreatography.
A: Magnetic resonance image of a hydatid cyst with major rupture; B: Emptying of hydatid membranes with endoscopic sphincterotomy and a biliary occlusion balloon; C: Extraction of hydatid materials.
As bile ducts cannot be purified from hydatid elements using ES alone, NBD and biliary stenting can be added. NBD may be the first choice of temporary treatment, particularly when life-threatening sepsis from acute cholangitis occurs. Once the obstructed bile ducts are drained, ERCP can be re-performed to completely clean out bile ducts of remaining hydatid elements. If there is an obstruction or stricture in the biliary tree, endoscopic balloon dilatation and biliary stenting may be needed[10,18,19,43,49-51].
There are numerous studies demonstrating that the use of ERCP to clean out bile ducts and cystic materials in cases of frank IRB significantly decreases cyst volume during successive follow-ups and 25% of patients are cured and do not need any further surgical treatment[4,16,35,39,42-46,52,53]. An endoscopic transpapillary treatment for frank IBR in which hydatid elements within the bile ducts were cleaned out using a biliary occlusion balloon and basket catheter, and a saline solution was infused in the cystic cavity by an endoscopically placed NBD catheter was first described in 1987 by al Karawi et al[54]. Five of the 6 patients treated by these authors were cured completely without needing any further surgical treatment[55]. Similar successes were reported in five patients described by Akkiz et al[42] in 1996 and seven patients described by Signh et al[56] in 2006, demonstrating that therapeutic ERCP is both effective and safe for the treatment of ruptured hydatid cysts.
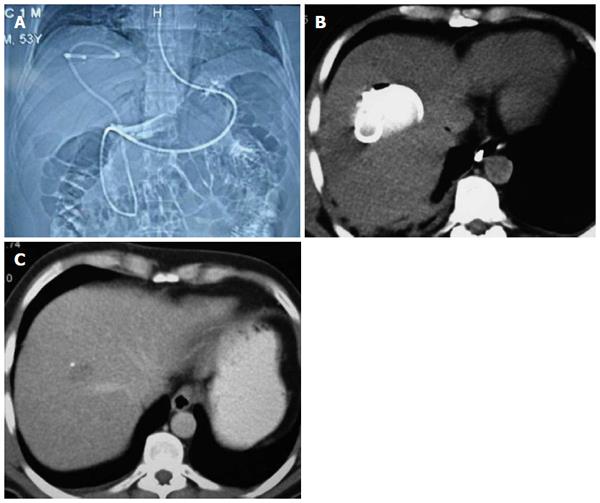
This technique has been performed in our clinic with some modifications, which we refer to as nasocystic-biliary drainage (NCBD). In this procedure, hydatid elements are emptied into the duodenum, and a hydrophilic guidewire is negotiated into the cyst. Hydatid materials are then completely emptied out over the guidewire by balloon and basket catheter, and a redesigned 10F NBD catheter is inserted into the cystic cavity to facilitate full emptying of the cyst contents. The cystic cavity and common bile duct are periodically irrigated using a saline solution through the nasobiliary catheter for seven days to ensure total clearance of the germinal layers and the remaining small daughter cysts. Small additional holes are placed in the choledochal and duodenal portion of the NCBD catheter to avoid the risk of cholangitis due to the continuous drainage of hydatid materials into bile ducts and obstruction. This redesign allows the NCBD catheter to act as a stent between the common bile duct and duodenum, and for periodic irrigation of the cystic cavity. Furthermore, the NCBD decreases the external biliary loss[26]. At present, 12 of the 14 patients that have undergone this procedure have been completely cured (Figure 2). The procedure failed in one patient who underwent a previous major cystobiliary rupture operation (duodenotomy and open sphincteroplasty), and complete extraction of hydatid remnants in the common bile duct could not be achieved due to the lack of dilatation of extrahepatic bile ducts. The procedure could not be completed in another patient who refused treatment on the fourth day[26]. As a result, we suggest that ERCP and NCBD should be the first choice of treatment in patients with major cystobiliary rupture in whom bile ducts are dilated.
Figure 2 Nasocystic-biliary drainage procedure.
A: Topographic images of hydatid cyst with major rupture by nasocystic drainage; B: Drainage catheter within the cystic cavity; C: Computed tomography of the same patient taken at the three-month follow-up.
POSTOPERATIVE ROLE OF ERCP
In the postoperative period, ERCP can be used to clarify the causes of already present or recurrent symptoms or biochemical abnormalities, overcome obstruction or cholangitis due to residual materials within bile ducts, treat postoperative external biliary fistulae, and resolve secondary biliary strictures[5,16,19,52]. Early postoperative complications include persistent biliary fistulae and obstructive jaundice due to hydatid cyst surgery, whereas as sclerosing cholangitis and stenosis of the sphincter of Oddi are considered late postoperative complications[19,50,57].
Biliary stricture is a rare but problematic postoperative complication of hydatid cyst disease. It is reported that 27% of all postoperative biliary strictures are secondary to hydatid cyst surgery[58]. Predisposing factors for caustic sclerosing cholangitis and biliary strictures include scolicidal agents, cystobiliary communications, hydatid cystic elements, secondary biliary infections, distal bile duct obstruction due to gallstones, papillary stenosis and centrally located hydatid cysts[16,58]. A few studies claim that endoscopic or percutaneous methods should be used first for the management of postoperative strictures. Biliary strictures affect mostly long and multiple segments, are located proximally (Bismuth type III and IV), and cholangiographic signs show diffuse involvement as primary sclerosing cholangitis. Although ERCP can be an appropriate method for the treatment of complications due to stricture, such as jaundice, cholangitis and sepsis, the repairs are costly and lengthy and can result in cholangitis and liver abscess formation. However, therapeutic ERCP should be preferred for biliary strictures distally located and involving single short segments, whereas percutaneous transhepatic cholangiography should be used for proximally located, multiple and long segment biliary strictures[16,35,39,58].
The formation of biliary fistulae is the most common postoperative complication, with a postoperative incidence as high a 50%-63%[53,59]. Although biliary leakage risk is higher in the early postoperative period, it begins to decrease from the tenth day after surgery. Persistent biliary fistulae are defined by a high amount of biliary drainage lasting more than ten days after surgery, and ERCP use is advised in these cases[5,15,60]. It is difficult to determine whether postoperative biliary fistulae will subside spontaneously or will turn into a persistent form. However, the presence of a large amount of leaking bile in the early postoperative period, or drainage that does not decrease day by day suggests that the fistula will not spontaneously subside. In such cases, there is no need to wait ten days, and ERCP can be performed early in the postoperative period, as any delay will cause the chronicity of fistulae and infective complications. In contrast, small or decreasing amounts of drainage may be followed conservatively[61,62].
When biliary fistulae are observed, it is important to evaluate the clinical effectiveness of biliary drainage. Effective biliary drainage is displayed by controlled fistulae, which show no signs of localized or generalized peritonitis, and no pathophysiologic signs of cholestasis. In uncontrolled biliary fistulae, there is generally insufficient biliary drainage and this condition results in intra-abdominal bile collection. The condition requires urgent hemodynamic stabilization of the patient, and drainage of the collection percutaneously, laparoscopically or surgically. Undrained bile will become infected, resulting in subphrenic, suphepatic abscess formation or generalized peritonitis. In some cases, ineffective biliary draining can lead to life-threatening cholangitis, intrahepatic abscesses and septicemia, which require urgent manipulations[51].
To treat biliary fistulae, ERCP with endoscopic treatments, such as ES, NBD and biliary stenting, are used to bypass the sphincter of Oddi, reduce duodenobiliary pressure differences, and to divert the bile flow into the duodenum. As pressure within the biliary tree decreases, bile will flow into the duodenum by way of the papilla Vateri instead of flowing into the cystic cavity. Although the advantages of these techniques are debated, the success rate has been reported as high as 90% accompanied by a low mortality rate[16]. In the patients who underwent ES in the study by Rodriguez et al[39] postoperative fistulae were closed successfully in approximately 25 d. In the study by Tekant et al[63] postoperative persistent biliary fistulae subsided spontaneously in seven days in all but one patient who underwent ES. The same study suggested that late ES may delay fistula closing, and thus ERCP should be performed at an early period. Reports in the literature show that postoperative ES fistulae close within 3-43 d, yet hospitalization typically lasts only 3-9 d for ERCP procedures[7,43,49,52,64].
In a study by Bilsel et al[50] ES was considered to be the appropriate treatment modality as stent usage was not cost-effective and required a second ERCP for removal. However, the same study reported that ES should be combined with biliary stenting for chronic and high-output fistulae in major cystobiliary communications, which was also indicated in a study by Adas et al[65]. Akcakaya et al[66] suggest that ES should be the first method selected, but in specific cases where there is a defect in emptying of hydatid materials, or a stricture or gallstone is present in the common bile duct, biliary stenting should be used directly. However, Cicek et al[5] recommend using NBD, rather than ES, with biliary stenting, as NBD is more effective in decreasing intrabiliary pressure and permits follow-up by cholangiography. Moreover, preoperative NBD aids in the intraoperative localization of cystobiliary communications[67-69]. Despite these apparent advantages, ES may be needed in some cases where hydatid membranes are within the bile ducts, and because of the low tolerability of lengthy hospitalization for NBD.
In our experience, ES is the primary choice as it is a safe and effective treatment for biliary fistulae resulting from hydatid cyst surgery. In addition, early ERCP is limited to patients with fistula output that is high or does not decrease within the first week. Because small hydatid particles cannot be visualized clearly with cholangiography, all of our patients undergo ES, bile ducts are controlled routinely with balloons and basket catheters, and hydatid elements are cleaned out if present. If biliary strictures accompany biliary fistulae, ES is supplemented with biliary stenting. Finally, NCBD is performed following ES in cases of biliary sepsis, particularly when the bile ducts are filled with hydatid elements.
CONCLUSION
Preoperative ERCP is the primary diagnostic and therapeutic method for frank IBR. Following a diagnosis of frank IBR, cystic material can be emptied by NBD, without a need for further surgical intervention. However, preoperative ERCP is not indicated for cases involving large or multiple cysts, hilar or caudate lobe cysts, or if there is minimal dilatation of intrahepatic bile ducts proximal to the cyst. Moreover, ERCP can fail to detect simple communications and may promote the formation of postoperative biliary fistulae. Even in the most experienced hands, serious complications can result from ERCP, including pancreatitis, bleeding, infection or perforation. Therefore, suspected minor communications should be investigated by non-invasive methods such as MRCP, US and CT.
Therapeutic ERCP is indicated for early (persistent biliary fistula and obstructive jaundice) and late (sclerosing cholangitis and sphincter of Oddi stenosis) postoperative biliary adverse events, of which biliary fistulae are the most common. Although some fistulae spontaneously resolve, high-output or persistent fistulae require ERCP. If biliary fistulae are accompanied by strictures or do not close after ES, biliary stenting should be added. However, biliary stenting and NBD should be confined to rare cases.
ACKNOWLEDGMENTS
Authors would like to thank to Dr Pelin Basim for wrote the English language of this review article.
P- Reviewer: Moralioglu S S- Editor: Qi Y L- Editor: A E- Editor: Liu XM