Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15125
Revised: May 23, 2014
Accepted: June 21, 2014
Published online: November 7, 2014
Processing time: 231 Days and 13.7 Hours
Surgery for rectal cancer in complex and entails many challenges. While the laparoscopic approach in general and specific to colon cancer has been long proven to have short term benefits and to be oncologically safe, it is still a debatable topic for rectal cancer. The attempt to benefit rectal cancer patients with the known advantages of the laparoscopic approach while not compromising their oncologic outcome has led to the conduction of many studies during the past decade. Herein we describe our technique for laparoscopic proctectomy and assess the current literature dealing with short term outcomes, immediate oncologic measures (such as lymph node yield and specimen quality) and long term oncologic outcomes of laparoscopic rectal cancer surgery. We also briefly evaluate the evolving issues of robotic assisted rectal cancer surgery and the current innovations and trends in the minimally invasive approach to rectal cancer surgery.
Core tip: Surgery for rectal cancer entails many challenges and several debates exist regarding the appropriate way to deal with this disease. One of these debates is the choice of surgical approach and whether laparoscopy is appropriate. This article reviews the current knowledge about the use of the laparoscopic approach for rectal cancer. Herein we describe our technique for laparoscopic proctectomy and assess the current literature dealing with the outcomes and the oncologic safety of laparoscopic rectal cancer surgery. We also briefly evaluate the evolving issues of robotic assisted rectal cancer surgery and the current innovations in this field.
- Citation: Shussman N, Wexner SD. Current status of laparoscopy for the treatment of rectal cancer. World J Gastroenterol 2014; 20(41): 15125-15134
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15125.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15125
The laparoscopic approach to intra-abdominal surgical procedures was introduced over two decades ago. At first it was used as a diagnostic measure and later on to perform small scale surgery, with the classic example of cholecystectomy. Laparoscopic surgery is associated with well-known benefits as a result of the reduced tissue trauma. These advantages include but are not limited to reduced postoperative pain, a reduced incidence of postoperative respiratory complications and wound related complications (wound infections and incisional hernias), early ambulation and discharge and improved cosmetic outcomes as compared to laparotomy[1].
Laparoscopic colectomies were introduced in the early 1990s[2-3]. They were performed at first for benign indications due to doubts regarding the oncologic outcomes and the fear of port-site metastases. Subsequently, the application of laparoscopic colon resections for colon cancer was evaluated and found to be similar to open colon resections, while it still had the short term benefits of laparoscopy[4-9]. The COST study group has randomized 872 patients in 48 institutions to undergo open versus laparoscopic assisted colectomy for colon cancer[4]. These patients were followed for long term (90% for five years or more)[5]. This study has shown a disease free five year survival (69.2% for laparoscopy, 68.4% for open surgery), an overall five year survival (76.4% for laparoscopy, 74.6% for open surgery) and a recurrence pattern that did not differ for the two approaches. Guillou et al[6] have randomized 794 patients in 27 institutions to undergo open versus laparoscopic assisted surgery for either colon or rectal cancer. They did not follow the patients for long term but they have shown similar short term outcomes for the colon cancer patients. Later on, the long term outcomes were published and showed non inferiority of the laparoscopic approach[7]. They have found no differences between laparoscopically assisted and open surgery in terms of overall survival, disease-free survival, and local and distant recurrence. Wound/port-site recurrence in the laparoscopic arm was 2.4%.
It was also found that patients who were operated laparoscopically had an earlier return of bowel function than did patients who underwent laparotomy, with most studies showing approximately a one day reduction in the duration of ileus[1,4,6,8,9].
Surgery for rectal cancer entails many challenges and several debates exist regarding the appropriate way to deal with this disease. Some of these many debates refer to systemic decision making regarding the treatment of a patient diagnosed with rectal cancer, like the best means to achieve preoperative staging, the use of neo-adjuvant chemoradiation and the appropriate use of local surgical modalities (trans-anal excision, trans-anal endoscopic microsurgery etc.) for early localized lesions. Other debates relate purely to the technical aspects of the radical surgery for rectal cancer like the appropriate terms for sphincter preservation, the best functional reconstruction of the rectal reservoir following a restorative proctectomy and the choice of surgical approach. These technical debates evolved due to several technical challenges posed by rectal and pelvic surgery: The deep and narrow cavity composed of the pelvic bones in which the surgeon needs to operate; the possible proximity of tumor to the circumferential resection margin (CRM); the possible proximity of the distal resection margin (DRM) to the sphincter complex; the absence of the rectal reservoir hence the need to reconstruct it after resection and more.
This article is aimed at reviewing the current knowledge about the use of the laparoscopic approach for rectal cancer. Herein we describe our technique for laparoscopic proctectomy and review the current literature dealing with both short and long term outcomes and the oncologic safety of laparoscopic rectal cancer surgery. We also briefly discuss the evolving issues of robotic assisted rectal cancer surgery and the current innovations and trends in the minimally invasive approach to rectal cancer.
As in any other surgical operation, there are many various possible techniques to perform a laparoscopic assisted proctectomy. Herein we present the way we perform this operation, with our personal preferences and tips.
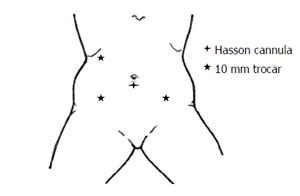
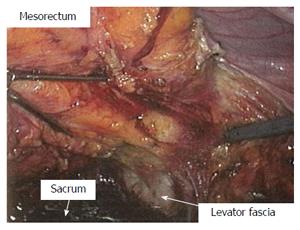
After induction of general endotracheal anesthesia, the patient is positioned in the supine modified lithotomy position. Insertion of bilateral ureteric catheters via cystoscopy is an option to enhance ureteral visualization and identification at the time of surgery, and is done at this stage if at all. The abdomen and pelvis, and perineum are prepped and draped in a sterile manner. Using the Hasson technique, a 10 mm cannula is placed through a vertical infraumbilical incision, and 15 mmHg pneumoperitoneum is achieved. A 30-degree laparoscope is introduced and evaluation of the peritoneal cavity takes place, 2-3 additional 10 mm ports are then placed under direct vision, 1 in the right lower quadrant, 1 in the right upper quadrant, and a possible additional 1 at the left lower quadrant (or through the colostomy site in the case of an abdominoperineal resection) (Figure 1). With positioning the patient at right side down and alternating head up and head down, the entire left colon is mobilized along the line of Toldt up to around the splenic flexure. The transverse colon is freed from the gastrocolic ligament and full mobilization of the splenic flexure and of the left mesocolon takes place. The left ureter is identified throughout this process and is reflected out of harm’s way. The inferior mesenteric artery is then divided at its origin from the aorta with a bipolar sealing device, followed by division of the inferior mesenteric vein just lateral to the duodenum. The base of the left mesocolon is being divided from just distal to the mid colic vessels to the sacral promontory, while assuring continuity of the marginal arcade. The pre-sacral space is then entered and a total mesorectal excision (TME) is undertaken. The dissection continues to the level of the levator muscles. The laparoscopic approach enables superb view at this stage of the operation (Figure 2). The remainder of the procedure is dependent according to whether the tumor is far enough from the sphincter complex and hence a reconstructive procedure is feasible, or not.
Under direct manual and visual guidance from both abdominal and perineal fields, a 60 mm articulating endoscopic linear cutting stapler is placed across the distal rectum, optimally 2 cm cephalad to the dentate line (if possible with achieving clear DRM). The stapler is fired and the rectum is removed from the pelvis. Copious irrigation and verification of meticulous hemostasis in the pelvis takes place, after which the infra-umbilical incision is lengthened to 3-4 cm. Through this incision a wound protector is placed and the entire left colon is withdrawn. The mesentery is divided with a bipolar sealing device from the inferior mesenteric artery high ligation to the sigmoid-descending junction and the bowel is divided with a GIA stapler. The specimen is removed from the field. Our common practice is to reconstruct the rectal reservoir with a colonic J pouch whenever possible. The pouch is fashioned at this stage at a length of 8 cm with a linear stapler. A purse-string stitch is placed at the apical enterotomy into which the anvil of a circular stapling device is secured and the colon is returned into the abdominal cavity. The abdomen is re-insufflated and under direct manual and visual guidance from both abdominal and perineal fields, the circular stapling device is carefully trans-anally introduced until the cartridge rests flushed with the cross staple line. If the patient is female, great care is taken at this step to ensure that the vagina is free of the rectum. The stapling device’s trocar is made to protrude and the anvil is connected to it with care taken to maintain appropriate orientation of the colon and its mesentery. The stapler is then closed, carefully excluding extraneous structures and especially the vagina and fired. The stapler is then removed and the presence of circumferentially intact donuts is verified. The pelvis is filled with saline and the descending colon gently occluded. Flexible sigmoidoscopy is performed to assure a patent, intact, hemostatic and airtight anastomosis. The pelvis is irrigated once more, hemostasis is verified and a drain placed through a stab wound in the left lower quadrant. We routinely create a diverting loop ileostomy following a restorative proctectomy, 40-60 cm cephalad to the ileocecal valve. The loop of ileum is delivered through a stoma site created in a standard fashion through the right rectus muscle in a tension-free manner; a rod is placed under the mesenteric margin and sutured in place. The fascial and skin incisions are then closed and the stoma’s afferent limb is matured everted and the efferent limb sutured flush to the skin.
Needs to be done, following the total mesorectal excision to the level of the levator muscles the surgery continues with the next steps:
The left colon mesentery is divided laparoscopically with the bipolar sealing device from the inferior mesenteric artery high ligation to the sigmoid-descending junction and the bowel is divided with a 60 mm endoscopic linear cutting stapler.
From the perineal aspect, a wide cylindrical abdominoperineal excision is undertaken. After the perineal proctectomy is completed the specimen is removed from the perineal incision, which is then irrigated, hemostasis verified and the incision closed in layers including closure of the skin. The abdomen is re-insufflated, the pelvic hemostasis is verified and a drain placed through a stab wound. The left-sided colostomy site is developed as a stoma around the port and the descending colon gently delivered through the stoma site such that it rests above skin in a tension-free manner. The abdomen is desufflated, fascial and skin incisions are closed and the stoma matured everted.
Different nuances in the technique of laparoscopic proctetomy have been described by different authors. One example is a single stapled technique for low anterior resection, in which an intracorporeal purse-string suture is placed on the distal rectum and the specimen is extracted trans-anally[10].
As mentioned above, many studies over the past two decades have shown better immediate postoperative outcomes of laparoscopy than of laparotomy. The benefits of laparoscopy have been proven for other abdominal surgeries including for colon resections[1,4,6,8,9]. Nevertheless, the question of whether there is benefit in laparoscopy specifically for rectal cancer surgery is not that straight forward. That is due to the increased technical complexity of these operations, that raises the question how much of the expected postoperative morbidity would be due to the abdominal wall incision per say, and how much would be secondary to stages in the operation that are being done regardless of the surgical approach, for example the pelvic dissection.
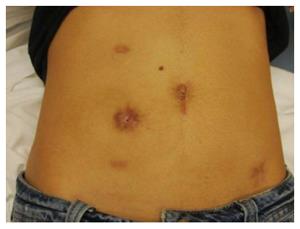
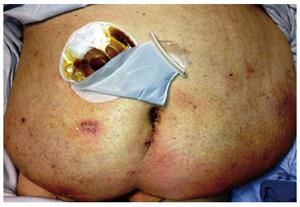
The short term outcomes after performing laparoscopic proctectomy using the technique described above at the authors’ institution have previously been published[11]. The results showed a mean operative time of 245 min, a mean hospital stay of 7 d and a significantly lower rate of 30 d postoperative morbidity compared to open surgery, as well as a high patient satisfaction from the cosmetic outcome (Figures 3 and 4).
van der Pas et al[12] have prospectively randomized 1103 patients with rectal cancer to undergo either a laparoscopic or open surgery, of which 1044 were eligible for analysis. Patients in the laparoscopic group suffered less blood loss than did those in the open group (200 mL vs 400 mL, P < 0.0001). In the laparoscopic group, bowel function returned earlier than in the open group (2 d vs 3 d, P < 0.0001) and the hospital stay was shorter (8 d vs 9 d, P = 0.036). Laparoscopic procedures took longer (240 min vs 188 min, P < 0.0001). The 28 d morbidity and mortality did not differ in between the two groups.
Boutros et al[11] in a study from our institution retrospectively reviewed and compared 234 patients who underwent either an open or a laparoscopic TME for rectal cancer over a period of 57 mo. This study has shown that while laparoscopy was associated with longer operative time (245 min vs 213 min, P = 0.002), it was also associated with less blood loss (284 mL vs 388 mL, P = 0.01), shorter hospital stay (7 d vs 8 d, P = 0.05) and lower rates of 30 d postoperative general morbidity (25% vs 43%, P = 0.04) and specifically surgical site infections (9% vs 20%, P = 0.04).
Lee et al[13] have conducted a retrospective study of 160 patients who underwent either laparoscopic or open surgery for stage I rectal cancer. Overall morbidity and mortality did not differ in between the groups. Operating time was longer (221 min vs 184 min, P = 0.008) for the laparoscopic group, but blood loss (150 mL vs 200 mL, P = 0.03), time to first bowel movement (2.44 d vs 3.54 d, P < 0.001), rate of superficial surgical-site infection (0% vs 7.5%, P = 0.03) and postoperative hospital stay (8 d vs 11 d, P < 0.001) were all improved in the laparoscopic group compared to the open group.
Arezzo et al[14] conducted a meta-analysis of prospective trials comparing open and laparoscopic rectal resection for cancer. They included 23 studies, 8 of which were randomized, representing 4539 patients. They observed a mortality incidence of 1.0% in the laparoscopic group compared to 2.4% in the open group (P = 0.048) and an overall morbidity rate of 31.8% in the laparoscopic group compared to 35.4% in the open group (P < 0.001).
A specific aspect of the short term outcomes of rectal cancer surgery is sexual and urinary dysfunction, which is an established risk after pelvic surgery, due to the proximity of the autonomic nerves innervating the urogenital system to the surgical field. McGlone et al[15] have analyzed the impact of the surgical approach (open vs laparoscopic) used in patients undergoing rectal cancer resection on functional urogenital outcomes. They used questionnaires which were sent to surviving patients to assess their postoperative sexual and urinary functions. They compared 78 patients who undergone laparoscopic rectal resection (49 men and 29 women) to 65 who had an open resection (41 men and 24 women). They have found that both groups were associated with deterioration in urinary and sexual function. While there was no difference in the deterioration of urinary function in between the groups in either gender, the deterioration in sexual function in the laparoscopic group was not as bad with significantly higher incidence of successful penetration in men and significantly better outcomes in all aspects of sexual activity in women.
The above mentioned are the most recent publications dealing with the short term outcomes of laparoscopic rectal cancer resection, but are definitely not the only ones. Other studies as well have evaluated the short term outcomes of laparoscopy versus open surgery for rectal cancer and have shown superiority of the laparoscopic approach[16-24].
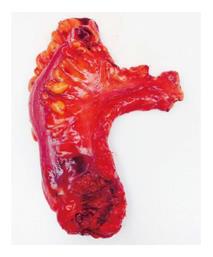
During the past 10 years, many studies have been published that evaluate the quality of the pathological specimen achieved in laparoscopic rectal cancer surgery, and compare it to the quality of the specimen achieved in open rectal cancer surgery. The quality of the specimen can be measured by several factors. First of all, the completeness of tumor resection with no involved specimen margin with the tumor is essential. It has become a standard for pathologists to describe the distance of the tumor from both the DRM and CRM as a factor to evaluate the completeness of resection. Distances of the tumor from the DRM of at least 1 cm and from the CRM of at least 1 mm have been shown to provide acceptable oncologic outcome and are considered nowadays a standard of care[25]. Another way to evaluate the completeness of resection according to the anatomic CRM (which is the mesorectal fascia in TME) is a descriptive measure of whether the TME is complete or not (Figure 5)[26-27]. In addition, the completeness of resection could be evaluated indirectly by the number of lymph nodes in the specimen. Lymph node status is considered to be the strongest pathologic predictor of patient outcome[28]. The standard of care is for at least 12 lymph nodes to be present at the specimen. This is due to the to the National Cancer Institute’s recommendation that a minimum of 12 lymph nodes negative for disease must be examined to confirm that the disease does not involve the nodes[29].
Sara et al[30] have published in 2010 a retrospective case matched study of 200 patients undergoing either laparoscopic or open surgery for rectal cancer. They have found non significant differences in CRM positivity (4% laparoscopic vs 9% open) and mean number of lymph nodes harvested (13.76 laparoscopic vs 12.74 open).
More recently, Boutros et al[11] have shown no differences in between laparoscopic and open TME groups in CRM, proportion of DRM < l cm and completeness of TME, while the laparoscopic group had significantly more lymph nodes (26 vs 21, P = 0.02) in the specimen than did the open group. van der Pas et al[12] have shown no difference in specimen quality between open and laparoscopic resection of rectal cancer groups. In their study both macroscopic completeness of the resection did not differ in between the groups. Microscopically as well, the results were similar with a CRM of less than 2 mm in 10% of patients in each group (P = 0.85) and a median distance from tumor to DRM of 3 cm in both groups (P = 0.676).
Lujan et al[31] in a recently published prospective non randomized multicenter study of 4405 patients, also reported superior results of the laparoscopic approach in both macroscopically and microscopically results.
Penninckx et al[32] reviewed retrospectively 2660 patients in 82 hospitals who underwent either an open or a laparosopic TME for a mid to low rectal cancer over a period of 6 years. They have shown equivalent rates of incomplete TME, CRM positivity and lymph node yield between the two groups. They have also shown a lower morbidity and a shorter length of stay in the laparoscopic group.
These and many other studies suggest that there is no difference between the laparoscopic and open approaches concerning the specimen’s DRM, CRM positivity or number of harvested lymph nodes. These data hence suggest that laparoscopic rectal resection is not only technically feasible but also seems to be oncologically safe.
As important as the short term outcomes and the quality of the specimen might be, one must always consider the long term oncologic outcome as a factor of utmost importance whenever dealing with cancer surgery. The same concerns of long term outcome, especially wound or port site recurrence that originally existed for the safety of laparoscopy in colon cancer still exist regarding rectal cancer.
Morino et al[33] in 2005 have conducted an analysis of 191 consecutive patients who underwent either a laparoscopic (n = 98) or open (n = 93) surgery for rectal cancer. They have shown equivalent 5 year overall survival (OS) and disease free survival (DFS) (80.0% and 65.4% in the laparoscopic group vs 68.9% and 58.9% in the open group, not significant) but a significantly lower local recurrence rate in the laparoscopic group (3.2% vs 12.6%, P < 0.05) than in the open group.
Green et al[34] have recently reported the long term outcome of the patients that were originally included in the classic trial. The classic trial was a randomized study for patients with colorectal cancer to undergo either a laparoscopic or an open surgery[6]. In a subgroup analysis of the patients with rectal cancer, no difference was found in median OS and DFS in between the open and laparoscopic groups. A higher rate of distant recurrence was found in rectal cancer patient than in colon cancer patients, but no difference was found in between the open and laparoscopic surgery for rectal cancer.
Lee et al[13] have recently shown no difference in 5 year OS (98.6% vs 97.1%, P = 0.41) or DFS (98.2% vs 96.4%, P = 0.30) between open and laparoscopic resection of stage I rectal cancer in a retrospective study on 160 patients.
Another study from Finland compared the 5 year DFS as well as 5 year local recurrence rate in matched groups of rectal 191 cancer patients, and found that there was no difference in these parameters between laparoscopic or open resection[35]. Laurent et al[36] from France have recently retrospectively compared laparoscopy vs open surgery for low rectal cancer that requires intersphincteric resection. They included 110 patients who had laparoscopic surgery and 65 who had open surgery. There found no difference in 5 year local recurrence (5% vs 2%, P = 0.349) and 5 year DFS (70% vs 71%, P = 0.862). Interestingly unlike many other studies this one has found short term morbidity to be similar in both groups. Another study which specifically looked at intermediate term (mean 34 mo follow up) results of intersphincteric resections for low rectal cancers concluded safety and adequacy of the laparoscopic approach[19]. The local recurrence rates were similar in the 2 groups (laparoscopy 2.6% vs open 7.7%, P = 0.18) and 3 year DFS for all stages was 82.1% in the laparoscopic group and 77.0% in the open group (P = 0.52).
Other retrospective studies have shown laparoscopy to have equal intermediate and long term oncologic outcomes to open surgery in the treatment of rectal cancer[21-22,37-39].
To date, no long term oncologic results of a randomized study that compares laparoscopic vs open resection for advanced stage rectal cancer have been published. Some data from prospective randomized trials for low stage cancer or as subgroups of colorectal cancer do exist, as mentioned above, but specific wide scale data are yet to be published. These would include the long term results of the COLOR II trial[12]. Another study that is still active is the ACOSOG z6051[40]. This is a multicenter randomized phase III trial comparing laparoscopic to open resection of stage IIA, IIIA, or IIIB rectal cancer. Within the next few years, data will be available from both these studies that will shed light on the true long term oncologic outcome of laparoscopic rectal cancer resection.
Robotic assisted surgery has several presumed advantages: The robotic platform has the ability to downscale the surgeon’s movements, to overcome the physiologic tremor and to supply with a 3D visualization. On the other hand, there is loss of tactile feedback and it requires a learning curve, hence the question rises whether there is any advantage for the patient in using it. Due to the technical complexity of the surgical treatment of rectal cancer, an assumption exists that with robotic assistance improved technical success, and with it, improved outcome, should be anticipated. The question persists whether robotic assisted laparoscopic proctectomy indeed bares any advantages over laparoscopic proctectomy in any of the above mentioned aspects of improved short term recovery, improved specimen quality or improved oncologic outcome. The question of long term oncologic outcomes remains to be answered, since the experience with robotic assisted surgery has not reached long term yet. Never the less, up to date, many studies have been published comparing the short term outcome of robotic assisted vs laparoscopic surgery for rectal cancer.
A recently published systematic review of the literature on the topics of robotic assisted surgery and laparoscopic surgery for rectal cancer concluded that robotic assisted surgery was associated with increased cost and operating time, but lower conversion rates regardless of the surgeon’s experience[41]. The authors also mentioned a non-significant marginally better outcome in anastomotic leak rates, CRM positivity and perseveration of autonomic function.
Kang et al[24] conducted a case matched retrospective study, comparing robotic assisted, laparoscopic and open surgery for rectal cancer with 165 patients in each arm. They have found better outcome of the robotic group in the time to resume solid diet and in the length of stay compared to the laparoscopic group, with both these groups having improved outcome compared to open surgery. The robotic group also had improved postoperative pain scores, less voiding problems and less CRM involvement. No significant difference in 2 year DFS was observed among the 3 groups.
Yang et al[42] in a recently published meta-analysis of studies comparing robotic assisted surgery to conventional laparoscopic surgery in colorectal diseases, included 7 studies dealing with rectal cancer only and performed a separate analysis for these studies. They have concluded that robotic assisted surgery was associated with less blood loss (P < 0.001) and a lower conversion rate (P < 0.001) than conventional laparoscopy.
Kim et al[43] have prospectively compared the urogenital function of 39 patients who underwent laparoscopic TME to 30 patients who underwent robotic assisted TME. They used uroflowmetric studies and questionnaires preoperatively and then at 1, 3, 6 and 12 mo postoperatively to prospectively evaluate the urogenital function. They have found that the urogenital function of all the patients deteriorated after surgery, as expected. The function of the patients in the robotic assisted group recovered faster than the patients in the laparoscopic group, but eventually both groups achieved the same improvement.
In contrast to the above mentioned studies supporting the robotic assistance, and concluding that the robotic assisted approach does have some potential benefits, several studies in the few recent years have shown no benefit in the robotic assisted approach.
Baek et al[44] have published a case matched study of 82 patients undergoing either a robotic assisted or laparoscopic TME. No statistically significant differences were found in DRM, CRM, lymph node harvest or postoperative course or complication rate.
Kwak et al[45] have also conducted a case matched study with 59 patients in each group of robotic assisted and laparoscopic rectal cancer resection. They found no differences between the groups by means of DRM, CRM, number of lymph nodes harvested or postoperative morbidity or mortality. Operating time was longer in the robotic group than in the laparoscopic group (270 min vs 228 min, P < 0.0001).
Park et al[23] included 263 consecutive patients who underwent curative surgery for rectal cancer in a retrospective study. The patients were classified into an open surgery group (n= 88), a laparoscopic surgery group (n = 123), and a robotic assisted group (n = 52). Mean operating time was significantly longer for the laparoscopic group than for the robotic and open groups (158 min of laparoscopic, 232 min of robotic and 233 min of open, P < 0.001). Patients from both the laparoscopic and robotic groups recovered significantly faster than did those from the open group (P < 0.05) but there was no difference between the laparoscopic group and robotic group. The specimen quality (DRM, CRM and lymph nodes) and the postoperative morbidity did not differ among the three groups.
Park et al[46] retrospectively compared 40 patients with distal rectal cancer who underwent a robotic assisted intersphincteric resection to 40 patients who underwent laparoscopic intersphincteric resection. The mean operative time was significantly longer in the robotic group than in the laparoscopic group and no difference was observed in the postoperative morbidity and pathological outcomes between the groups.
Baek et al[47] have focused on analyzing the costs of robotic assisted surgery compared to conventional laparoscopy. They retrospectively analyzed 154 robotic assisted surgeries and compared them to 150 laparoscopic surgeries for rectal cancer. They have found that while postoperative course and complications were similar, for the robotic assisted cases operative time was significantly longer (285 min vs 219 min, P = 0.018) and costs were significantly higher ($14647 vs $9978, P = 0.001).
To conclude, the data to date regarding the use of robotic assisted surgery for rectal cancer show feasibility of this approach, but disagreement exists regarding the true benefits of its use. Until prospective randomized studies evaluating both short term recovery and long term oncologic outcome will take place, there will be no definite answer to this disagreement. Never the less, all the studies which looked at costs agreed upon the increased cost of the use of the robot. Hence, the use of the robotic assisted approach should be considered in a cost-benefit perspective.
Given the growing body of evidence regarding the safety and feasibility of laparoscopy for rectal cancer, other minimally invasive approaches to the radical resection of rectal cancer have recently been described.
Single port laparoscopy is an approach that has been shown to be feasible for other laparoscopic procedures and in some centers has become routinely used for simpler procedures than rectal cancer resection. Recently single-port laparoscopic surgery has been described in case reports and short series as a feasible approach to TME as well, with good short term outcomes and acceptable quality specimen in selected patients with rectal cancer[48,49]. Never the less, there are no reports of large series or of long term outcomes for this approach yet.
The growing evidence of the feasibility and safety of the laparoscopic approach to rectal cancer on one hand, with the growing experience of local excision of early rectal tumors via transanal endoscopic microsurgery (TEM) on the other hand, have led to the development of a hybrid technique. In 2010 Sylla et al[50] have described a porcine survival study evaluating TEM rectosigmoid resection with or without trans-gastric natural orifice transluminal endoscopic surgery (NOTES) assistance. They had no mortalities and concluded that NOTES for rectosigmoid resection using TEM is feasible and associated with low morbidity in a porcine survival model. The same group has published their experience in a series of 32 human cadavers with or without laparoscopic assistance[51]. They achieved a 100% rate of intact TME using this approach, but had 9 events of bowel perforation. Soon after, reports of the transanal approach for the performance of TME in human patients have emerged[52]. Recently, de Lacy et al[53] have published their experience with 20 selected patients with rectal cancer who underwent minilaparoscopy-assisted NOTES TME. They had no conversions and achieved complete TME in all cases. Pathologic analysis revealed negative distal and circumferential margins in all specimens and an average of 15.9 retrieved lymph nodes.
In summary, during the last few years, reports have emerged on newly designed minimally invasive approaches to the radical resection of rectal cancer. Some reports have shown feasibility of these approaches but no data exist regarding its wide application in non-selected patients. Even though recent studies have shown them to meets the oncologic requirements for high-quality rectal cancer surgery, no data exist regarding the long term oncologic safety of these approaches. Even if single port laparoscopy and NOTES will prove to be feasible and safe in both perspectives of short term outcomes and long term oncologic safety, the question remains what benefit they offer to patients over a standard laparoscopic approach. Therefore, it is the authors’ opinion that these approaches should be used in the context of clinical trials until there will be evidence for their safety, feasibility and at least non-inferiority related to the standard laparoscopic approach.
Surgery for rectal cancer in complex and entails many challenges. While the laparoscopic approach in general and specifically to colon cancer has been proven long ago to have short term benefits and to be oncologically safe, it is still a debatable topic when dealing with rectal cancer. The attempt to benefit rectal cancer patients with the known advantages of the laparoscopic approach while not compromising their oncologic outcome has led to the conduction of many studies during the past decade.
Many studies have evaluated the short term outcomes of laparoscopy vs open surgery for rectal cancer and have shown superiority of the laparoscopic approach. Many of the same and other studies suggest that there is no difference between the laparoscopic and open approaches concerning the specimen’s quality. These studies as well as many retrospective studies that evaluated intermediate and long term oncologic outcomes suggest that laparoscopic rectal resection is oncologically safe. Never the less, to date, no long term oncologic results of randomized study that compares laparoscopic vs open resection for advanced stage rectal cancer have been published. Some studies are active and when they will be concluded and published they are expected to shed light on the true long term oncologic outcome of laparoscopic rectal cancer resection.
Regarding the evolving issues of robotic assisted surgery and innovative minimally invasive approaches for rectal cancer surgery, they are yet to prove to have any advantages over the standard laparoscopic approach.
P- Reviewer: Arezzo A, M'Koma AE S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
| 1. | Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005;CD003145. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3573] [Cited by in RCA: 2754] [Article Influence: 1377.0] [Reference Citation Analysis (0)] |
| 2. | Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc. 1991;1:144-150. [PubMed] |
| 3. | Schlinkert RT. Laparoscopic-assisted right hemicolectomy. Dis Colon Rectum. 1991;34:1030-1031. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 133] [Cited by in RCA: 130] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 4. | The Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050-2059. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2606] [Cited by in RCA: 2518] [Article Influence: 119.9] [Reference Citation Analysis (0)] |
| 5. | Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW, Hellinger M, Flanagan R, Peters W, Nelson H. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655-662; discussion 662-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 789] [Cited by in RCA: 801] [Article Influence: 44.5] [Reference Citation Analysis (0)] |
| 6. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2298] [Article Influence: 114.9] [Reference Citation Analysis (0)] |
| 7. | Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 830] [Cited by in RCA: 737] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 8. | Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, Pique JM. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 407] [Cited by in RCA: 436] [Article Influence: 25.6] [Reference Citation Analysis (0)] |
| 9. | Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477-484. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1691] [Cited by in RCA: 1680] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 10. | Kim HJ, Choi GS, Park JS, Park SY. Comparison of intracorporeal single-stapled and double-stapled anastomosis in laparoscopic low anterior resection for rectal cancer: a case-control study. Int J Colorectal Dis. 2013;28:149-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 11. | Boutros M, Hippalgaonkar N, Silva E, Allende D, Wexner SD, Berho M. Laparoscopic resection of rectal cancer results in higher lymph node yield and better short-term outcomes than open surgery: a large single-center comparative study. Dis Colon Rectum. 2013;56:679-688. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1030] [Cited by in RCA: 1213] [Article Influence: 101.1] [Reference Citation Analysis (0)] |
| 13. | Lee SD, Park SC, Park JW, Kim DY, Choi HS, Oh JH. Laparoscopic versus open surgery for stage I rectal cancer: long-term oncologic outcomes. World J Surg. 2013;37:646-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Arezzo A, Passera R, Scozzari G, Verra M, Morino M. Laparoscopy for rectal cancer reduces short-term mortality and morbidity: results of a systematic review and meta-analysis. Surg Endosc. 2013;27:1485-1502. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 94] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 15. | McGlone ER, Khan O, Flashman K, Khan J, Parvaiz A. Urogenital function following laparoscopic and open rectal cancer resection: a comparative study. Surg Endosc. 2012;26:2559-2565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 34] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Trastulli S, Cirocchi R, Listorti C, Cavaliere D, Avenia N, Gullà N, Giustozzi G, Sciannameo F, Noya G, Boselli C. Laparoscopic vs open resection for rectal cancer: a meta-analysis of randomized clinical trials. Colorectal Dis. 2012;14:e277-e296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 123] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 17. | Biondo S, Kreisler E, Fraccalvieri D, Basany EE, Codina-Cazador A, Ortiz H. Risk factors for surgical site infection after elective resection for rectal cancer. A multivariate analysis on 2131 patients. Colorectal Dis. 2012;14:e95-e102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Seshadri RA, Srinivasan A, Tapkire R, Swaminathan R. Laparoscopic versus open surgery for rectal cancer after neoadjuvant chemoradiation: a matched case-control study of short-term outcomes. Surg Endosc. 2012;26:154-161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Park JS, Choi GS, Jun SH, Hasegawa S, Sakai Y. Laparoscopic versus open intersphincteric resection and coloanal anastomosis for low rectal cancer: intermediate-term oncologic outcomes. Ann Surg. 2011;254:941-946. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 20. | Liang X, Hou S, Liu H, Li Y, Jiang B, Bai W, Li G, Wang W, Feng Y, Guo J. Effectiveness and safety of laparoscopic resection versus open surgery in patients with rectal cancer: a randomized, controlled trial from China. J Laparoendosc Adv Surg Tech A. 2011;21:381-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Baik SH, Gincherman M, Mutch MG, Birnbaum EH, Fleshman JW. Laparoscopic vs open resection for patients with rectal cancer: comparison of perioperative outcomes and long-term survival. Dis Colon Rectum. 2011;54:6-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 22. | da Luz Moreira A, Mor I, Geisler DP, Remzi FH, Kiran RP. Laparoscopic resection for rectal cancer: a case-matched study. Surg Endosc. 2011;25:278-283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 23. | Park JS, Choi GS, Lim KH, Jang YS, Jun SH. S052: a comparison of robot-assisted, laparoscopic, and open surgery in the treatment of rectal cancer. Surg Endosc. 2011;25:240-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 147] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 24. | Kang J, Yoon KJ, Min BS, Hur H, Baik SH, Kim NK, Lee KY. The impact of robotic surgery for mid and low rectal cancer: a case-matched analysis of a 3-arm comparison--open, laparoscopic, and robotic surgery. Ann Surg. 2013;257:95-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 25. | Balch GC, De Meo A, Guillem JG. Modern management of rectal cancer: a 2006 update. World J Gastroenterol. 2006;12:3186-3195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 83] [Cited by in RCA: 82] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479-1482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1867] [Cited by in RCA: 1914] [Article Influence: 49.1] [Reference Citation Analysis (0)] |
| 27. | Nagtegaal ID, van de Velde CJ, van der Worp E, Kapiteijn E, Quirke P, van Krieken JH. Macroscopic evaluation of rectal cancer resection specimen: clinical significance of the pathologist in quality control. J Clin Oncol. 2002;20:1729-1734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 660] [Cited by in RCA: 680] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 28. | Baxter NN, Virnig DJ, Rothenberger DA, Morris AM, Jessurun J, Virnig BA. Lymph node evaluation in colorectal cancer patients: a population-based study. J Natl Cancer Inst. 2005;97:219-225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 384] [Article Influence: 19.2] [Reference Citation Analysis (0)] |
| 29. | Nelson H, Petrelli N, Carlin A, Couture J, Fleshman J, Guillem J, Miedema B, Ota D, Sargent D. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst. 2001;93:583-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 928] [Cited by in RCA: 947] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 30. | Sara S, Poncet G, Voirin D, Laverriere MH, Anglade D, Faucheron JL. Can adequate lymphadenectomy be obtained by laparoscopic resection in rectal cancer? Results of a case-control study in 200 patients. J Gastrointest Surg. 2010;14:1244-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Lujan J, Valero G, Biondo S, Espin E, Parrilla P, Ortiz H. Laparoscopic versus open surgery for rectal cancer: results of a prospective multicentre analysis of 4,970 patients. Surg Endosc. 2013;27:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 66] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 32. | Penninckx F, Kartheuser A, Van de Stadt J, Pattyn P, Mansvelt B, Bertrand C, Van Eycken E, Jegou D, Fieuws S. Outcome following laparoscopic and open total mesorectal excision for rectal cancer. Br J Surg. 2013;100:1368-1375. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 61] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 33. | Morino M, Allaix ME, Giraudo G, Corno F, Garrone C. Laparoscopic versus open surgery for extraperitoneal rectal cancer: a prospective comparative study. Surg Endosc. 2005;19:1460-1467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 34. | Green BL, Marshall HC, Collinson F, Quirke P, Guillou P, Jayne DG, Brown JM. Long-term follow-up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg. 2013;100:75-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 483] [Cited by in RCA: 497] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 35. | Kellokumpu IH, Kairaluoma MI, Nuorva KP, Kautiainen HJ, Jantunen IT. Short- and long-term outcome following laparoscopic versus open resection for carcinoma of the rectum in the multimodal setting. Dis Colon Rectum. 2012;55:854-863. [PubMed] |
| 36. | Laurent C, Paumet T, Leblanc F, Denost Q, Rullier E. Intersphincteric resection for low rectal cancer: laparoscopic vs open surgery approach. Colorectal Dis. 2012;14:35-41; discussion 42-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 37. | Li S, Chi P, Lin H, Lu X, Huang Y. Long-term outcomes of laparoscopic surgery versus open resection for middle and lower rectal cancer: an NTCLES study. Surg Endosc. 2011;25:3175-3182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 38. | Lam HD, Stefano M, Tran-Ba T, Tinton N, Cambier E, Navez B. Laparoscopic versus open techniques in rectal cancer surgery: a retrospective analysis of 121 sphincter-saving procedures in a single institution. Surg Endosc. 2011;25:454-462. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 39. | Zheng MH, Feng B, Hu CY, Lu AG, Wang ML, Li JW, Hu WG, Zang L, Mao ZH, Dong TT. Long-term outcome of laparoscopic total mesorectal excision for middle and low rectal cancer. Minim Invasive Ther Allied Technol. 2010;19:329-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 40. | Available from: http://www.cancer.gov/clinicaltrials/search/view?cdrid=601816&version=HealthProfessional. |
| 41. | Scarpinata R, Aly EH. Does robotic rectal cancer surgery offer improved early postoperative outcomes? Dis Colon Rectum. 2013;56:253-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 111] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 42. | Yang Y, Wang F, Zhang P, Shi C, Zou Y, Qin H, Ma Y. Robot-assisted versus conventional laparoscopic surgery for colorectal disease, focusing on rectal cancer: a meta-analysis. Ann Surg Oncol. 2012;19:3727-3736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 144] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 43. | Kim JY, Kim NK, Lee KY, Hur H, Min BS, Kim JH. A comparative study of voiding and sexual function after total mesorectal excision with autonomic nerve preservation for rectal cancer: laparoscopic versus robotic surgery. Ann Surg Oncol. 2012;19:2485-2493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 264] [Article Influence: 20.3] [Reference Citation Analysis (0)] |
| 44. | Baek JH, Pastor C, Pigazzi A. Robotic and laparoscopic total mesorectal excision for rectal cancer: a case-matched study. Surg Endosc. 2011;25:521-525. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 45. | Kwak JM, Kim SH, Kim J, Son DN, Baek SJ, Cho JS. Robotic vs laparoscopic resection of rectal cancer: short-term outcomes of a case-control study. Dis Colon Rectum. 2011;54:151-156. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 46. | Park SY, Choi GS, Park JS, Kim HJ, Ryuk JP. Short-term clinical outcome of robot-assisted intersphincteric resection for low rectal cancer: a retrospective comparison with conventional laparoscopy. Surg Endosc. 2013;27:48-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 116] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 47. | Baek SJ, Kim SH, Cho JS, Shin JW, Kim J. Robotic versus conventional laparoscopic surgery for rectal cancer: a cost analysis from a single institute in Korea. World J Surg. 2012;36:2722-2729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 121] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 48. | Sourrouille I, Dumont F, Goéré D, Honoré C, Elias D. Resection of rectal cancer via an abdominal single-port access: short-term results and comparison with standard laparoscopy. Dis Colon Rectum. 2013;56:1203-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 49. | Bulut O, Aslak KK, Rosenstock S. Technique and short-term outcomes of single-port surgery for rectal cancer: a feasibility study of 25 patients. Scand J Surg. 2014;103:26-33. [PubMed] |
| 50. | Sylla P, Sohn DK, Cizginer S, Konuk Y, Turner BG, Gee DW, Willingham FF, Hsu M, Mino-Kenudson M, Brugge WR. Survival study of natural orifice translumenal endoscopic surgery for rectosigmoid resection using transanal endoscopic microsurgery with or without transgastric endoscopic assistance in a swine model. Surg Endosc. 2010;24:2022-2030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 51. | Telem DA, Han KS, Kim MC, Ajari I, Sohn DK, Woods K, Kapur V, Sbeih MA, Perretta S, Rattner DW. Transanal rectosigmoid resection via natural orifice translumenal endoscopic surgery (NOTES) with total mesorectal excision in a large human cadaver series. Surg Endosc. 2013;27:74-80. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 52. | Sylla P, Rattner DW, Delgado S, Lacy AM. NOTES transanal rectal cancer resection using transanal endoscopic microsurgery and laparoscopic assistance. Surg Endosc. 2010;24:1205-1210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 507] [Cited by in RCA: 534] [Article Influence: 35.6] [Reference Citation Analysis (0)] |
| 53. | de Lacy AM, Rattner DW, Adelsdorfer C, Tasende MM, Fernández M, Delgado S, Sylla P, Martínez-Palli G. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: “down-to-up” total mesorectal excision (TME)--short-term outcomes in the first 20 cases. Surg Endosc. 2013;27:3165-3172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 195] [Article Influence: 16.3] [Reference Citation Analysis (0)] |