Published online Nov 7, 2014. doi: 10.3748/wjg.v20.i41.15087
Revised: February 14, 2014
Accepted: May 28, 2014
Published online: November 7, 2014
Processing time: 381 Days and 3.5 Hours
The liver is a central organ that metabolizes excessive nutrients for storage in the form of glycogen and lipids and supplies energy-producing substrates to the peripheral tissues to maintain their function, even under starved conditions. These processes require a considerable amount of oxygen, which causes a steep oxygen gradient throughout the hepatic lobules. Alcohol consumption and/or excessive food intake can alter the hepatic metabolic balance drastically, which can precipitate fatty liver disease, a major cause of chronic liver diseases worldwide, ranging from simple steatosis, through steatohepatitis and hepatic fibrosis, to liver cirrhosis. Altered hepatic metabolism and tissue remodeling in fatty liver disease further disrupt hepatic oxygen homeostasis, resulting in severe liver hypoxia. As master regulators of adaptive responses to hypoxic stress, hypoxia-inducible factors (HIFs) modulate various cellular and organ functions, including erythropoiesis, angiogenesis, metabolic demand, and cell survival, by activating their target genes during fetal development and also in many disease conditions such as cancer, heart failure, and diabetes. In the past decade, it has become clear that HIFs serve as key factors in the regulation of lipid metabolism and fatty liver formation. This review discusses the molecular mechanisms by which hypoxia and HIFs regulate lipid metabolism in the development and progression of fatty liver disease.
Core tip: Hypoxia occurs in the development and progression of fatty liver disease. Recent reports have shed light on the pathological significance of hypoxia inducible factors (HIFs), master regulators of the hypoxic response, with regard to their regulation of lipid metabolism in context- and isoform-dependent manners. In this review, we summarize recent findings on the various roles of HIF-dependent regulation in fatty liver disease.
- Citation: Suzuki T, Shinjo S, Arai T, Kanai M, Goda N. Hypoxia and fatty liver. World J Gastroenterol 2014; 20(41): 15087-15097
- URL: https://www.wjgnet.com/1007-9327/full/v20/i41/15087.htm
- DOI: https://dx.doi.org/10.3748/wjg.v20.i41.15087
Fatty liver disease (FLD), whether it is alcoholic (AFLD) or non-alcoholic (NAFLD), is a major cause of chronic liver disease worldwide[1,2]. FLD initially begins with simple hepatic steatosis but can irreversibly progress to steatohepatitis, fibrosis, cirrhosis, or hepatocellular carcinoma. Hepatic steatosis was initially thought to be a benign state of FLD, but recent studies have revealed that fat accumulation in the liver clearly predisposes the organ to injury. NAFLD is also highly associated with obesity and diabetes, and insulin resistance is evident in livers that accumulate fat, leading to impaired glucose tolerance[3,4]. Thus, hepatic steatosis is a key step in the development and progression of subsequent pathology. However, pharmacological approaches have not successfully treated hepatic steatosis; therefore, a greater understanding of the mechanisms underlying the disease is required for the development of therapeutics.
In hepatic steatosis, five major pathways determine liver fat volume: (1) the uptake of free fatty acids (FAs) and triglycerides (TG) from the diet; (2) de novo lipogenesis; (3) FA oxidation; (4) the export of TG as very low-density lipoprotein (VLDL) into the bloodstream; and (5) the flux of FAs released from adipose tissue through lipolysis. In the case of AFLD, increased de novo lipogenesis and impaired FA oxidation in the liver are major contributors to lipid accumulation[5]. In contrast, in patients with NAFLD, adipose tissue lipolysis and hepatic de novo lipogenesis accounts for 59% and 26% of fat accumulation in the liver, respectively, with lower amounts derived from the diet (15%), emphasizing the importance of the former two pathways[6]. However, lipid disposal viaβ-oxidation and VLDL formation is only slightly affected[7]. Thus, various pathways related to hepatic lipid metabolism are implicated in the development of hepatic steatosis.
To date, studies in humans and rodents have revealed several major regulators of lipid metabolism. The sterol response element binding protein (SREBP) is a transcription factor that controls de novo lipogenesis[8,9]. SREBP has three isoforms: SREBP-1a, SREBP-1c, and SREBP-2. SREBP-1a and SREBP-1c are splice variants, and the liver predominantly expresses the SREBP-1c isoform together with SREBP-2. SREBP-1 is mainly involved in de novo FA and TG synthesis, whereas SREBP-2 controls cholesterol homeostasis. SREBP-1c promotes FA synthesis by inducing the expression of lipogenic genes such as fatty acid synthase (FAS), acyl-CoA carboxylase (ACC), and stearoyl-CoA desaturase (SCD)-1. These lipogenic genes have been reported to be associated with the development of FLD[5,7,10,11], whereas the importance of SCD-1 in FLD is still controversial[12-15]. Peroxisome proliferator-activated receptors (PPARs) also act as crucial regulators of FA metabolism[16]. The PPAR subfamily consists of PPARα, PPARβ/δ, and PPARγ. PPARs heterodimerize with retinoid X receptor (RXR)α, and bind to peroxisome proliferator hormone response elements (PPREs) of target genes. The PPARα/RXRα complex promotes the expression of genes involved in FA oxidation, such as long-chain acyl CoA dehydrogenase (LCAD), medium-chain acyl CoA dehydrogenase (MCAD), and carnitine palmitoyl-CoA transferase-1 (CPT-1). In AFLD, the expression of these genes is well known to be reduced, which leads to impaired FA oxidation[1,5,10,11]. PPARγ is reportedly upregulated in patients with NAFLD and promotes lipogenesis in the liver[11,17]. However, recent reports found that PPARγ agonists have beneficial effects on NAFLD by improving peripheral insulin sensitivity and may reduce hepatic fat content and fibrotic scarring[18-21]. Furthermore, the carbohydrate response element binding protein (ChREBP) and X-box binding protein (XBP)-1 are also involved in the regulation of hepatic lipid metabolism[4,7,22,23].
Although in vitro and in vivo studies have elucidated the various signaling pathways that regulate lipid metabolism in FLD, little is known regarding upstream stimuli. Historically, in AFLD, hypoxia has been reported in the pericentral zone of hepatic lobules[24-26], and it has also been suggested that an aberrant oxygen gradient can induce hepatic steatosis and subsequent disorders[27]. Recent studies have demonstrated that hypoxia is also observed in NAFLD[28]. In this review, we discuss the role of hypoxia in FLD, focusing on hypoxia-inducible factors (HIFs), which regulate the cellular and tissue adaptive responses to hypoxia, and the association between hypoxia and lipid metabolism.
Aerobic organisms have evolved by using an innovative energy-producing system, mitochondrial oxidative phosphorylation, which requires oxygen as a final electron acceptor. Oxidative phosphorylation generates energy far more efficiently than anaerobic glycolysis, which was a major factor in the evolutionary progression to larger, multicellular organisms with multifunctional cells and organs. Oxygen, in turn, is required for homeostasis and survival of these organisms. However, oxygen deprivation occurs during development and in the cornea and bone marrow where oxygen supply is quite limited. Thus, cells need to activate specific molecular programs to overcome hypoxic challenges in these conditions.
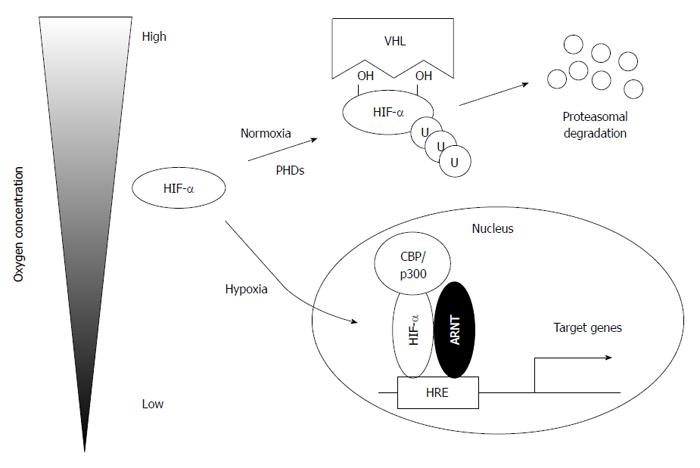
Hypoxia inducible factors (HIFs) play central roles in the cellular and tissue adaptation to hypoxia. HIF was first discovered as a transcriptional regulator of erythropoietin, which is a hematopoietic regulator protein expressed predominantly in the kidney and, to a lesser extent, in the liver[29]. HIFs consist of an oxygen-sensitive α subunit (HIFα) and a constitutively expressed β-subunit [aryl hydrocarbon receptor nuclear translocator (ARNT), HIF-1β][30]. Three isoforms of HIFα subunits (HIF-1α, HIF-2α, and HIF-3α) have been identified. Both HIF-1 and HIF-2 exert overlapping roles in the regulation of erythropoiesis, angiogenesis, cell proliferation, and apoptosis, and each HIF regulates a unique subset of target genes (Table 1)[31-34]. HIF-1 upregulates almost all glycolytic enzymes, including hexokinase (HK) 1/2, phosphofructokinase, and pyruvate kinase (PK), especially in solid tumors and stem cells[33]. HIF-2 is an important inducer of anti-oxidant genes, including superoxide dismutase (SOD)-2[35]. HIF-3α lacks a transactivation domain and therefore is considered to be transcriptionally inactive. However, a splice variant of HIF-3α, named inhibitory of PAS domain protein, interacts directly with HIF-1α and prevents its binding to DNA, which inactivates its ability to induce downstream targets[36].
| Hypoxia inducible gene | Function | HIF-1 target gene | HIF-2 target gene |
| Oxygen supply | |||
| Erythropoietin | Erythropoiesis | + | + |
| VEGF | Angiogenesis | + | + |
| iNOS | Vasodilation | + | - |
| PAI-1 | Blood flow | + | + |
| ANGPTL2 | Vascular remodeling | - | + |
| Cellular metabolism | |||
| HK1/2 | Glycolysis | + | - |
| PGK1 | Glycolysis | + | - |
| LDHA | Glycolysis | + | - |
| GLUT-1 | Glucose transporter | + | + |
| ADRP/ADFP | Lipid metabolism | + | + |
| DEC1/2 | Lipid metabolism | + | - |
| ANGPTL3 | Lipid metabolism | + | + |
| Cell cycle | |||
| TGFα | Growth factor | - | + |
| Cyclin D1 | Cell cycle progression | - | + |
| BNIP3 | Apoptosis | + | - |
| Oxidative stress | |||
| HO-1 | Anti-oxidant | + | - |
| SOD1/2 | Anti-oxidant | + | |
| Catalase | Anti-oxidant | + | |
| GPX1 | Anti-oxidant | + | |
The stability of HIFα subunits is strictly regulated by oxygen concentration (Figure 1)[31]. In the presence of oxygen, HIFα subunits are hydroxylated at specific proline residues by prolyl hydroxylase domain proteins (PHD1, PHD2, and PHD3) in an oxygen-dependent manner[37,38]. HIFα subunits are subsequently recognized and targeted for degradation by the von Hippel-Lindau protein, which has E3 ubiquitin ligase activity[37,39]. However, under hypoxic conditions, HIFα subunits escape from proteasomal degradation and translocate to the nucleus to heterodimerize with ARNT to exert their transcriptional activity with its cofactor CREB-binding protein (CBP)/p300. In addition, factor-inhibiting HIF (FIH) also hydroxylates the asparagine residue of HIFα, which prevents the interaction between HIFα and CBP/p300, leading to suppressed transcriptional activity[40].
Hypoxia is associated with the progression of various diseases, such as cardiovascular disease, cancer, and inflammatory diseases[41-43], and is also present in rodent models of liver disease[44]. In fatty liver disease, the “hypermetabolic state” may be a driving force for lobular hypoxia. Chronic ethanol consumption promotes the expression of cytochrome P450 2E1 (CYP2E1) in the pericentral zone of hepatic lobules, which metabolizes ethanol and acetaldehyde using oxygen as a co-substrate[45,46]. In addition, hepatocytes reoxidize excessive reducing equivalents of NADH produced by oxidation of ethanol via alcohol dehydrogenase and aldehyde dehydrogenase in mitochondria[47-49]. Indeed, oxygen consumption has been reported to increase in the liver of alcohol-fed rats, leading to a reduction in the oxygen content of hepatic venous blood[50]. In NAFLD, the excessive intake of foods containing lipids may promote β-oxidation of FA, which requires a considerable amount of oxygen[51,52]. Constriction of hepatic sinusoids and/or prevention of substrate exchange due to swelling of hepatocytes and accumulation of a fibrotic scar may trigger the development of hypoxia in NAFLD[53]. In addition, reactive oxygen species (ROS) are produced in the pathogenesis of liver disease, which leads to the stabilization of HIFα subunits by inhibition of PHDs. Indeed, HIF expression is increased in liver diseases, including AFLD and NAFLD[54-60]. These observations imply that HIFs are involved in the development of fatty liver and the pathological sequelae.
Hypoxia is tightly associated with lipid homeostasis, as both in vitro and in vivo studies reveal that ischemic and hypoxic stress increases cellular lipid deposition[61-64]. Hypoxia also upregulates genes involved in lipogenesis, lipid uptake, and lipid droplet formation[65-67]. In addition, several studies have implicated the role of HIFs in lipid homeostasis. In von hippel-lindau (VHL)-deficient renal cell carcinomas that highly express both HIF-1α and HIF-2α, neutral lipids accumulate, which makes the cells appear clear[68]. Moreover, heterozygous deletion of VHL or liver-specific inactivation of VHL results in hepatocellular steatosis[69,70].
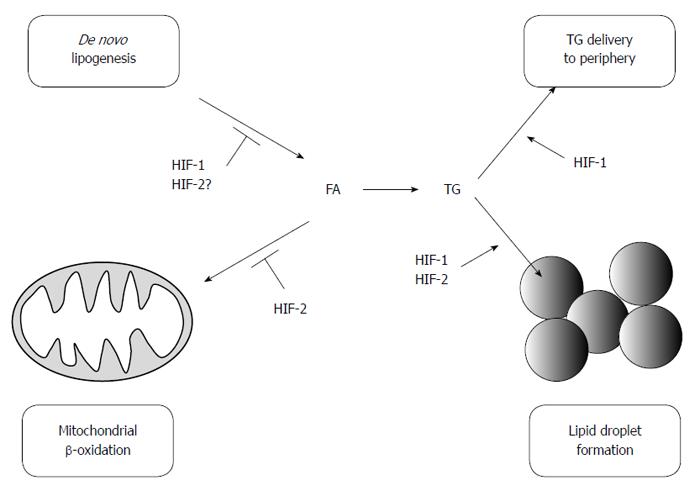
These observations have encouraged researchers to investigate whether HIFs play a role in lipid metabolism in the liver. Rankin et al[71] demonstrated that HIF-2 functions as an important regulator of hepatic lipid metabolism, as it impairs FA β-oxidation and increases lipid storage capacity. In VHL-deficient mice, deletion of HIF-2α results in reduced lipid accumulation, whereas this effect is not observed in HIF-1α-deleted mice. In VHL-null animals, HIF-2 reduces the expression of genes involved in β-oxidation (Acsl1, Cpt1, Crot) and promotes lipid droplet formation by inducing the expression of a lipid droplet associated protein (Adfp). A subsequent investigation using Adeno-Cre mediated liver-specific disruption of VHL suggested that downregulation of β-oxidation in VHL-deficient mice may be attributable to impaired mitochondrial respiration, which is due in part to a decrease in the Fe-S cluster assembly protein levels, IscU1/2[72]. In addition, tamoxifen-induced temporal disruption of VHL in the liver results in increased hepatic lipid accumulation, which is reversed by the simultaneous disruption of HIF-2α but not HIF-1α[73]. Interestingly, the mechanisms involved in lipid accumulation appear to differ depending on the time after tamoxifen treatment. The expression of genes involved in FA synthesis (Srebp-1c and Fasn) increases 3 d post-treatment, but it is significantly suppressed by 14 d. Genes involved in β-oxidation (Cpt1a, Cpt2, Acox1, and Ppara) are also reduced 14 d post-treatment. Moreover, angiopoietin-like 3 (Angptl3), an endogenous lipoprotein lipase inhibitor, is induced after disruption of VHL and is strongly upregulated by HIF-2. The importance of HIF-2 in lipid metabolism is further confirmed in Phd2/3-double knockout mice[74]. Although the majority of studies indicate that HIF-2 promotes fat accumulation, some reports indicate the contrary. Germ-line deletion of HIF-2α paradoxically results in severe steatosis due to impaired β-oxidation through ROS-mediated mitochondrial dysfunction[35]. In addition, activation of HIF-2 in hepatocytes only has a minimal effect on hepatic lipid accumulation[75]. Simultaneous activation of HIF-1 and HIF-2 results in severe steatosis, suggesting that the regulation of lipid metabolism via HIFs may be more complex than originally thought.
On the other hand, recent studies have also demonstrated the physiological and pathological effect of HIF-1 levels in the fatty liver. Using a liver-specific HIF-1α-deleted mouse model, we found that HIF-1α prevents excessive lipid accumulation by suppressing the SREBP-1c-dependent lipogenic pathway in the alcoholic fatty liver[59]. Chronic ethanol administration results in hypoxia in the pericentral zone of hepatic lobules and HIF-1α is correspondingly expressed in this region. HIF-1 enhances the expression of a circadian helix-loop-helix (HLH) transcription factor, differentiated embryo-chondrocyte 1 (DEC1), which reduces the expression of SREBP-1c and its downstream lipogenic genes. These findings are in a good agreement with previous reports that HIF-1 induces transcriptional activation of DEC1, which in turn represses SREBP-1c-mediated gene expression by competing with other HLH transcription factors to bind the E-box of the SREBP promoter and/or by directly interacting with the SREBP-1c protein[76,77]. In addition, the administration of DMOG, a pharmacological inhibitor of PHDs, reduces ethanol-induced lipid accumulation in the liver in a HIF-1-dependent manner. It has been consistently shown that the ablation of ARNT promotes lipogenic gene expression (SCD-1, FAS) in the liver, which suggests that HIF-1 prevents lipid synthesis[78]. Protective effects of HIF-1 activation against fatty liver disease are further supported by a recent report suggesting that HIF-1 promotes mitochondrial β-oxidation and prevents lipid peroxidation by regulating mitochondrial biogenesis in the liver of high-fat diet (HFD)-fed animals[58]. Collectively, these results indicate that HIF-1 serves as a protective factor against the development of fatty liver and that pharmacological prolyl hydroxylase inhibitors may lead to the development of therapies for the disease. In contrast, Nath et al[60] found that HIF-1 is critical for the development of hepatic steatosis. The authors demonstrated that a combined treatment of ethanol and an inflammatory stimulus (LPS) aggravates hepatic steatosis through induction of HIF-1 via monocyte chemoattractant protein1 (MCP1). It is not clear why opposing results are obtained from these two studies. One possible explanation is that the presence of inflammation may rewire the HIF-1 pathway, which leads to a different gene expression profile compared to that observed in simple steatosis. In support of this hypothesis, a combination of hypoxia and proinflammatory stimuli strongly enhances the expression of HIF-1 due to a positive feedback loop mechanism between HIF-1 and nuclear factor κB (NFκB), which may mimic the overexpression studies of HIF-1α[79-82]. In addition, it is implicated that inflammation promotes lipid accumulation by the tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) pathways[83-86].
In addition to the development of fatty liver, HIFs also play a critical role in the pathological sequelae, especially in hepatic fibrosis. Consistent observations suggest that angiogenesis is tightly associated with fibrogenesis in experimental models and human diseases. In fact, vascular endothelial growth factor (VEGF) is now a potential target for the treatment of fibrosis[87-89]. Hypoxic areas are present in the fibrotic liver and co-localize with VEGF expression in endothelial cells, hepatocytes, and hepatic stellate cells (HSCs)[90-92]. In addition, a clinical study revealed that in the human cirrhotic liver, heme oxygenase-1, a well-known target of HIF-1, and HIF-2α are detected in the hepatocytes of regenerative nodules and cells of fibrotic septa[92]. In agreement with these reports, Moon et al[93] demonstrated that bile duct ligation in mice induces hypoxia and HIF-1α activation. Deletion of HIF-1α attenuates fibrogenesis due to the reduced expression of profibrogenic genes, such as platelet-derived growth factor (PDGF)-A, PDGF-B, and plasminogen activator inhibitor-1. Considering the lack of transcriptional activation of PDGFs in the hepatocytes of the fibrotic liver, non-parenchymal cells, presumably macrophages and/or stellate cells, are major contributors to hepatic fibrosis[94-96]. Consistent with this hypothesis, ARNT in myeloid cells contributes to the development of hepatic fibrogenesis via induction of PDGF-B expression[97]. In addition, another report found that overexpression of HIF-2 but not HIF-1 in hepatocytes promotes inflammation and fibrosis in the liver[73]. Although, these in vivo studies have provided evidence that HIFs are important regulators of hepatic fibrosis, the role of each HIF isoform in each cell type is not fully understood, particularly in the context of NAFLD. It is worth noting that macrophages have opposing roles during the progression of and recovery from hepatic fibrosis[98]. It is hypothesized that alternatively activated macrophages (AAMs) promote fibrosis by inducing profibrogenic factors, while classically activated macrophages (CAMs) mediate recovery responses by secreting matrix metalloproteinases and/or removing fibrillar collagen[99]. In addition, CAMs and AAMs predominantly express HIF-1α and HIF-2α, respectively[100]. These observations suggest that HIF-1 and HIF-2 in macrophages may have different roles during fibrosis development.
Overall, the regulation of lipid metabolism by HIFs is complex (Figure 2), and genetic and environmental factors may affect its role in the pathogenesis of fatty liver. The involvement of DECs and ChREBP in the HIF-1 pathway suggests that lifestyle and diet may affect HIF-1-mediated lipid metabolism via lipogenic transcription factors, such as SREBP-1c and PPARγ[76,77,101]. In addition, the role of different HIF isoforms in various cell types during the progression of NAFLD needs to be elucidated. Further studies are required to identify the functional interactions between HIFs and other factors that produce mixed phenotypes observed in FLD.
Obstructive sleep apnea (OSA) is another risk factor for the development of NAFLD. OSA is an obstruction of the upper airway and is associated with intermittent hypoxia (IH), temporal deprivation of oxygen from the blood[102]. Studies of human OSA have revealed an association between OSA and NAFLD. Independent of the presence of obesity, IH induces insulin resistance, liver injury, and fibrosis, and the effects are more severe in obese patients[103-107]. While the presence of hepatic steatosis and inflammation in lean patients with OSA was not conclusively determined in these studies, other clinical studies have demonstrated that a combination of OSA and obesity promotes excessive fat accumulation and progression of NAFLD to non-alcoholic steatohepatitis (NASH)[108,109]. Recently, an important meta-analysis of 11 clinical studies with 404 controls and 668 OSA patients was conducted, clarifying that OSA is associated with liver injury, fatty liver and fibrosis but not inflammation[110]. To investigate the role of OSA in the development of disease, Fletcher et al[111] first established a rodent model of IH, in which N2 or air is repeatedly infused into a chamber, mimicking the abnormal fluctuation of oxyhemoglobin saturation observed in OSA patients. This model has been used to elucidate the mechanism(s) by which IH affects lipid metabolism in the fatty liver. Short-term exposure to IH in lean mice induces hepatic steatosis and dyslipidemia through SREBP-1c and its downstream target SCD-1[112], whereas long-term exposure abolishes this effect[113]. In contrast, long-term IH exposure increases lipid accumulation in the liver of leptin-deficient mice (ob/ob), which upregulates lipogenic genes such as SREBP-1, SCD-1, and glycerol 3-phosphate acyltransferase[114]. Furthermore, a combination of high fat diet-induced obesity and long-term IH exacerbates hepatic steatosis and inflammation[115]. Overall, these observations suggest that long-term IH better mimics human OSA in NAFLD.
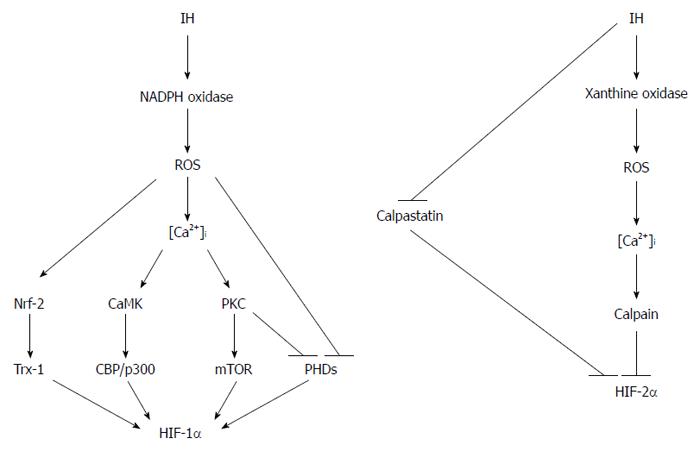
IH and chronic hypoxia control the stability of HIF proteins via different mechanisms (Figure 3). In the case of HIF-1α, IH induces ROS production by nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, which then increases Ca2+ concentration in the cytoplasm[116]. This increase in Ca2+ activates protein kinase C, which in turn inactivates PHDs and stimulates translation of HIF-1α by activating mammalian target of rapamycin. Ca2+ also activates calmodulin kinase, which enhances transcriptional activity of HIF-1 by phosphorylating CBP/p300[117]. In addition, ROS inhibit the activity of PHDs, independent of Ca2+ concentration, which leads to reduced prolyl hydroxylation of HIF-1α[116]. Moreover, ROS also induce the expression of thioredoxin 1 via nuclear factor erythroid 2-related factor 2, which further augments HIF-1 signaling[118]. In contrast, Ca2+ signaling activates calpain protease, which in turn degrades HIF-2α[119]. IH also reduces the expression of calpastatin, which serves as an inhibitor of calpain. Moreover, a recent study found that xanthine oxidase, but not NADPH oxidase, is required for ROS-mediated HIF-2α degradation[120]. These results clearly suggest that IH promotes the expression of HIF-1α, while destabilizing HIF-2α.
HIFs also serve as central regulators in the development of NAFLD in OSA. Li et al[121] demonstrated that the heterozygous deletion of HIF-1α in mice reduces serum and hepatic TG contents. HIF-1 induces the expression of SREBP cleavage activation protein in hepatocytes, which escorts SREBP from the ER to the Golgi apparatus and triggers SREBP cleavage. The subsequent accumulation of active SREBP fragments in the nucleus results in SCD-1 up-regulation. Thus, IH causes hepatic steatosis and hyperlipidemia presumably through the induction of HIF-1α in the liver. However, neuronal or inter-organ effects must be considered to interpret the mechanisms involved in NAFLD development of OSA. In fact, HIF-1 in the carotid body regulates systemic responses to IH by activating the sympathetic nerve system (SNS), which in turn stimulates adipose tissue lipolysis[122], suggesting that SNS affects hepatic lipid content. In addition, HIF-1 prevents the secretion of adiponectin from adipose tissue, which suppresses de novo lipogenesis in the liver[123-125]. Therefore, the extent to which HIF-1 in the liver contributes to serum and hepatic lipid contents during IH remains to be determined.
Hypoxia is associated with the development of fatty liver, and it is now clear that hypoxia-inducible transcription factors regulate lipid metabolism in hepatocytes. OSA is an independent risk factor for developing NAFLD. Obesity and OSA synergistically accelerate tissue hypoxia, which further leads to the development of severe hepatic steatosis and inflammation. In addition to the imbalance between oxygen supply and demand, other factors such as inflammation and nutrients as well as inter-organ metabolic and signaling crosstalk must be considered to interpret the hypoxic response during fatty liver development. In addition, each HIF target gene has a unique optimal dose of HIFs for their transcription, and the context-dependent regulation of this process is a major contributor to disease outcome. Therefore, the manipulation of HIF activity may be a desirable therapeutic target for the treatment of fatty liver.
P- Reviewer: Gorrell MD, Liu H, Nagarajan P, Pirola CJ, Sacco R, Yan M S- Editor: Ma YJ L- Editor: A E- Editor: Ma S
| 1. | Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572-1585. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1244] [Cited by in RCA: 1493] [Article Influence: 106.6] [Reference Citation Analysis (0)] |
| 2. | Cohen JC, Horton JD, Hobbs HH. Human fatty liver disease: old questions and new insights. Science. 2011;332:1519-1523. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1720] [Cited by in RCA: 1657] [Article Influence: 118.4] [Reference Citation Analysis (2)] |
| 3. | Savage DB, Semple RK. Recent insights into fatty liver, metabolic dyslipidaemia and their links to insulin resistance. Curr Opin Lipidol. 2010;21:329-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 73] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 4. | Tilg H, Moschen AR. Insulin resistance, inflammation, and non-alcoholic fatty liver disease. Trends Endocrinol Metab. 2008;19:371-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 335] [Cited by in RCA: 368] [Article Influence: 21.6] [Reference Citation Analysis (0)] |
| 5. | Crabb DW, Liangpunsakul S. Alcohol and lipid metabolism. J Gastroenterol Hepatol. 2006;21 Suppl 3:S56-S60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 78] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 6. | Donnelly KL, Smith CI, Schwarzenberg SJ, Jessurun J, Boldt MD, Parks EJ. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343-1351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2112] [Cited by in RCA: 2591] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 7. | Postic C, Girard J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J Clin Invest. 2008;118:829-838. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 838] [Cited by in RCA: 940] [Article Influence: 55.3] [Reference Citation Analysis (0)] |
| 8. | Goldstein JL, DeBose-Boyd RA, Brown MS. Protein sensors for membrane sterols. Cell. 2006;124:35-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1162] [Cited by in RCA: 1292] [Article Influence: 68.0] [Reference Citation Analysis (0)] |
| 9. | Raghow R, Yellaturu C, Deng X, Park EA, Elam MB. SREBPs: the crossroads of physiological and pathological lipid homeostasis. Trends Endocrinol Metab. 2008;19:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 251] [Cited by in RCA: 249] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 10. | Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33:191-205. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 244] [Cited by in RCA: 245] [Article Influence: 15.3] [Reference Citation Analysis (1)] |
| 11. | Reddy JK, Rao MS. Lipid metabolism and liver inflammation. II. Fatty liver disease and fatty acid oxidation. Am J Physiol Gastrointest Liver Physiol. 2006;290:G852-G858. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 556] [Cited by in RCA: 586] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 12. | Fernández Gianotti T, Burgueño A, Gonzales Mansilla N, Pirola CJ, Sookoian S. Fatty liver is associated with transcriptional downregulation of stearoyl-CoA desaturase and impaired protein dimerization. PLoS One. 2013;8:e76912. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 13. | Dobrzyn A, Ntambi JM. Stearoyl-CoA desaturase as a new drug target for obesity treatment. Obes Rev. 2005;6:169-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 136] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 14. | Kurikawa N, Takagi T, Wakimoto S, Uto Y, Terashima H, Kono K, Ogata T, Ohsumi J. A novel inhibitor of stearoyl-CoA desaturase-1 attenuates hepatic lipid accumulation, liver injury and inflammation in model of nonalcoholic steatohepatitis. Biol Pharm Bull. 2013;36:259-267. [PubMed] |
| 15. | Silbernagel G, Kovarova M, Cegan A, Machann J, Schick F, Lehmann R, Häring HU, Stefan N, Schleicher E, Fritsche A. High hepatic SCD1 activity is associated with low liver fat content in healthy subjects under a lipogenic diet. J Clin Endocrinol Metab. 2012;97:E2288-E2292. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 16. | Evans RM, Barish GD, Wang YX. PPARs and the complex journey to obesity. Nat Med. 2004;10:355-361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1138] [Cited by in RCA: 1207] [Article Influence: 57.5] [Reference Citation Analysis (0)] |
| 17. | Matsusue K, Haluzik M, Lambert G, Yim SH, Gavrilova O, Ward JM, Brewer B, Reitman ML, Gonzalez FJ. Liver-specific disruption of PPARgamma in leptin-deficient mice improves fatty liver but aggravates diabetic phenotypes. J Clin Invest. 2003;111:737-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 245] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 18. | Zheng H, Li S, Ma L, Cheng L, Deng C, Chen Z, Xie C, Xiang M, Jiang W, Chen L. A novel agonist of PPAR-γ based on barbituric acid alleviates the development of non-alcoholic fatty liver disease by regulating adipocytokine expression and preventing insulin resistance. Eur J Pharmacol. 2011;659:244-251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Ratziu V, Giral P, Jacqueminet S, Charlotte F, Hartemann-Heurtier A, Serfaty L, Podevin P, Lacorte JM, Bernhardt C, Bruckert E. Rosiglitazone for nonalcoholic steatohepatitis: one-year results of the randomized placebo-controlled Fatty Liver Improvement with Rosiglitazone Therapy (FLIRT) Trial. Gastroenterology. 2008;135:100-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 479] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 20. | Nakagami H, Kiomy Osako M, Nakagami F, Shimosato T, Minobe N, Moritani T, Shimamura M, Miyake T, Shimizu H, Takeya Y. Prevention and regression of non-alcoholic steatohepatitis (NASH) in a rat model by metabosartan, telmisartan. Int J Mol Med. 2010;26:477-481. [PubMed] |
| 21. | Hirata T, Tomita K, Kawai T, Yokoyama H, Shimada A, Kikuchi M, Hirose H, Ebinuma H, Irie J, Ojiro K. Effect of Telmisartan or Losartan for Treatment of Nonalcoholic Fatty Liver Disease: Fatty Liver Protection Trial by Telmisartan or Losartan Study (FANTASY). Int J Endocrinol. 2013;2013:587140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 68] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 22. | Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492-1496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 777] [Cited by in RCA: 787] [Article Influence: 46.3] [Reference Citation Analysis (0)] |
| 23. | Ma L, Robinson LN, Towle HC. ChREBP*Mlx is the principal mediator of glucose-induced gene expression in the liver. J Biol Chem. 2006;281:28721-28730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 259] [Cited by in RCA: 286] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 24. | Videla L, Bernstein J, Israel Y. Metabolic alterations produced in the liver by chronic ethanol administration. Increased oxidative capacity. Biochem J. 1973;134:507-514. [PubMed] |
| 25. | Arteel GE, Iimuro Y, Yin M, Raleigh JA, Thurman RG. Chronic enteral ethanol treatment causes hypoxia in rat liver tissue in vivo. Hepatology. 1997;25:920-926. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 120] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 26. | French SW. The role of hypoxia in the pathogenesis of alcoholic liver disease. Hepatol Res. 2004;29:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 47] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 27. | Jungermann K, Kietzmann T. Oxygen: modulator of metabolic zonation and disease of the liver. Hepatology. 2000;31:255-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 312] [Cited by in RCA: 303] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 28. | Mantena SK, Vaughn DP, Andringa KK, Eccleston HB, King AL, Abrams GA, Doeller JE, Kraus DW, Darley-Usmar VM, Bailey SM. High fat diet induces dysregulation of hepatic oxygen gradients and mitochondrial function in vivo. Biochem J. 2009;417:183-193. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 225] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 29. | Wang GL, Jiang BH, Rue EA, Semenza GL. Hypoxia-inducible factor 1 is a basic-helix-loop-helix-PAS heterodimer regulated by cellular O2 tension. Proc Natl Acad Sci USA. 1995;92:5510-5514. [PubMed] |
| 30. | Semenza GL. Regulation of oxygen homeostasis by hypoxia-inducible factor 1. Physiology (Bethesda). 2009;24:97-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 524] [Cited by in RCA: 617] [Article Influence: 38.6] [Reference Citation Analysis (0)] |
| 31. | Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40:294-309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1923] [Cited by in RCA: 1812] [Article Influence: 120.8] [Reference Citation Analysis (0)] |
| 32. | Keith B, Johnson RS, Simon MC. HIF1α and HIF2α: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12:9-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1159] [Cited by in RCA: 1389] [Article Influence: 99.2] [Reference Citation Analysis (0)] |
| 33. | Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108-113. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 650] [Cited by in RCA: 632] [Article Influence: 35.1] [Reference Citation Analysis (0)] |
| 34. | Wenger RH, Stiehl DP, Camenisch G. Integration of oxygen signaling at the consensus HRE. Sci STKE. 2005;2005:re12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 460] [Cited by in RCA: 664] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 35. | Scortegagna M, Ding K, Oktay Y, Gaur A, Thurmond F, Yan LJ, Marck BT, Matsumoto AM, Shelton JM, Richardson JA. Multiple organ pathology, metabolic abnormalities and impaired homeostasis of reactive oxygen species in Epas1-/- mice. Nat Genet. 2003;35:331-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 395] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 36. | Makino Y, Kanopka A, Wilson WJ, Tanaka H, Poellinger L. Inhibitory PAS domain protein (IPAS) is a hypoxia-inducible splicing variant of the hypoxia-inducible factor-3alpha locus. J Biol Chem. 2002;277:32405-32408. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 262] [Cited by in RCA: 256] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 37. | Kaelin WG, Ratcliffe PJ. Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol Cell. 2008;30:393-402. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2073] [Cited by in RCA: 2451] [Article Influence: 144.2] [Reference Citation Analysis (0)] |
| 38. | Aragonés J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metab. 2009;9:11-22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 215] [Cited by in RCA: 216] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 39. | Safran M, Kaelin WG. HIF hydroxylation and the mammalian oxygen-sensing pathway. J Clin Invest. 2003;111:779-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 98] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 40. | Lando D, Gorman JJ, Whitelaw ML, Peet DJ. Oxygen-dependent regulation of hypoxia-inducible factors by prolyl and asparaginyl hydroxylation. Eur J Biochem. 2003;270:781-790. [PubMed] |
| 41. | Simon MC, Liu L, Barnhart BC, Young RM. Hypoxia-induced signaling in the cardiovascular system. Annu Rev Physiol. 2008;70:51-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 58] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 42. | Wilson WR, Hay MP. Targeting hypoxia in cancer therapy. Nat Rev Cancer. 2011;11:393-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2218] [Cited by in RCA: 2376] [Article Influence: 169.7] [Reference Citation Analysis (0)] |
| 43. | Eltzschig HK, Carmeliet P. Hypoxia and inflammation. N Engl J Med. 2011;364:656-665. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1400] [Cited by in RCA: 1559] [Article Influence: 111.4] [Reference Citation Analysis (0)] |
| 44. | Nath B, Szabo G. Hypoxia and hypoxia inducible factors: diverse roles in liver diseases. Hepatology. 2012;55:622-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 231] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 45. | Lieber CS. Cytochrome P-4502E1: its physiological and pathological role. Physiol Rev. 1997;77:517-544. [PubMed] |
| 46. | Gorsky LD, Koop DR, Coon MJ. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. J Biol Chem. 1984;259:6812-6817. [PubMed] |
| 47. | Thurman RG, Ji S, Matsumura T, Lemasters JJ. Is hypoxia involved in the mechanism of alcohol-induced liver injury? Fundam Appl Toxicol. 1984;4:125-133. [PubMed] |
| 48. | French SW, Benson NC, Sun PS. Centrilobular liver necrosis induced by hypoxia in chronic ethanol-fed rats. Hepatology. 1984;4:912-917. [PubMed] |
| 49. | Sato N, Kamada T, Kawano S, Hayashi N, Kishida Y, Meren H, Yoshihara H, Abe H. Effect of acute and chronic ethanol consumption on hepatic tissue oxygen tension in rats. Pharmacol Biochem Behav. 1983;18 Suppl 1:443-447. [PubMed] |
| 50. | Tsukamoto H, Xi XP. Incomplete compensation of enhanced hepatic oxygen consumption in rats with alcoholic centrilobular liver necrosis. Hepatology. 1989;9:302-306. [PubMed] |
| 51. | Henly DC, Berry MN. Effect of palmitate concentration on the relative contributions of the beta-oxidation pathway and citric acid cycle to total O2 consumption of isolated rat hepatocytes. Biochim Biophys Acta. 1993;1175:269-276. [PubMed] |
| 52. | Nasrin N, Wu X, Fortier E, Feng Y, Bare’ OC, Chen S, Ren X, Wu Z, Streeper RS, Bordone L. SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem. 2010;285:31995-32002. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 240] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 53. | Kondo K, Sugioka T, Tsukada K, Aizawa M, Takizawa M, Shimizu K, Morimoto M, Suematsu M, Goda N. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, improves hepatic microcirculatory patency and oxygen availability in a high-fat-diet-induced fatty liver in mice. Adv Exp Med Biol. 2010;662:77-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 54. | Kaelin WG. ROS: really involved in oxygen sensing. Cell Metab. 2005;1:357-358. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 55. | Klimova T, Chandel NS. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death Differ. 2008;15:660-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 285] [Cited by in RCA: 317] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 56. | Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. 2013;58:395-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 311] [Cited by in RCA: 385] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 57. | Wang X, Wu D, Yang L, Gan L, Cederbaum AI. Cytochrome P450 2E1 potentiates ethanol induction of hypoxia and HIF-1α in vivo. Free Radic Biol Med. 2013;63:175-186. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 58. | Carabelli J, Burgueño AL, Rosselli MS, Gianotti TF, Lago NR, Pirola CJ, Sookoian S. High fat diet-induced liver steatosis promotes an increase in liver mitochondrial biogenesis in response to hypoxia. J Cell Mol Med. 2011;15:1329-1338. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 75] [Cited by in RCA: 93] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 59. | Nishiyama Y, Goda N, Kanai M, Niwa D, Osanai K, Yamamoto Y, Senoo-Matsuda N, Johnson RS, Miura S, Kabe Y. HIF-1α induction suppresses excessive lipid accumulation in alcoholic fatty liver in mice. J Hepatol. 2012;56:441-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 60. | Nath B, Levin I, Csak T, Petrasek J, Mueller C, Kodys K, Catalano D, Mandrekar P, Szabo G. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology. 2011;53:1526-1537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 164] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 61. | Gordon GB, Barcza MA, Bush ME. Lipid accumulation of hypoxic tissue culture cells. Am J Pathol. 1977;88:663-678. [PubMed] |
| 62. | Whitmer JT, Idell-Wenger JA, Rovetto MJ, Neely JR. Control of fatty acid metabolism in ischemic and hypoxic hearts. J Biol Chem. 1978;253:4305-4309. [PubMed] |
| 63. | Boström P, Magnusson B, Svensson PA, Wiklund O, Borén J, Carlsson LM, Ståhlman M, Olofsson SO, Hultén LM. Hypoxia converts human macrophages into triglyceride-loaded foam cells. Arterioscler Thromb Vasc Biol. 2006;26:1871-1876. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 137] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 64. | Piguet AC, Stroka D, Zimmermann A, Dufour JF. Hypoxia aggravates non-alcoholic steatohepatitis in mice lacking hepatocellular PTEN. Clin Sci (Lond). 2010;118:401-410. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 65. | Saarikoski ST, Rivera SP, Hankinson O. Mitogen-inducible gene 6 (MIG-6), adipophilin and tuftelin are inducible by hypoxia. FEBS Lett. 2002;530:186-190. [PubMed] |
| 66. | Parathath S, Mick SL, Feig JE, Joaquin V, Grauer L, Habiel DM, Gassmann M, Gardner LB, Fisher EA. Hypoxia is present in murine atherosclerotic plaques and has multiple adverse effects on macrophage lipid metabolism. Circ Res. 2011;109:1141-1152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 131] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 67. | Furuta E, Pai SK, Zhan R, Bandyopadhyay S, Watabe M, Mo YY, Hirota S, Hosobe S, Tsukada T, Miura K. Fatty acid synthase gene is up-regulated by hypoxia via activation of Akt and sterol regulatory element binding protein-1. Cancer Res. 2008;68:1003-1011. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 316] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 68. | Pinthus JH, Whelan KF, Gallino D, Lu JP, Rothschild N. Metabolic features of clear-cell renal cell carcinoma: mechanisms and clinical implications. Can Urol Assoc J. 2011;5:274-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 69. | Haase VH, Glickman JN, Socolovsky M, Jaenisch R. Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci USA. 2001;98:1583-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 345] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 70. | Ma W, Tessarollo L, Hong SB, Baba M, Southon E, Back TC, Spence S, Lobe CG, Sharma N, Maher GW. Hepatic vascular tumors, angiectasis in multiple organs, and impaired spermatogenesis in mice with conditional inactivation of the VHL gene. Cancer Res. 2003;63:5320-5328. [PubMed] |
| 71. | Rankin EB, Rha J, Selak MA, Unger TL, Keith B, Liu Q, Haase VH. Hypoxia-inducible factor 2 regulates hepatic lipid metabolism. Mol Cell Biol. 2009;29:4527-4538. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 271] [Cited by in RCA: 266] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 72. | Kucejova B, Sunny NE, Nguyen AD, Hallac R, Fu X, Peña-Llopis S, Mason RP, Deberardinis RJ, Xie XJ, Debose-Boyd R. Uncoupling hypoxia signaling from oxygen sensing in the liver results in hypoketotic hypoglycemic death. Oncogene. 2011;30:2147-2160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 73. | Qu A, Taylor M, Xue X, Matsubara T, Metzger D, Chambon P, Gonzalez FJ, Shah YM. Hypoxia-inducible transcription factor 2α promotes steatohepatitis through augmenting lipid accumulation, inflammation, and fibrosis. Hepatology. 2011;54:472-483. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 153] [Cited by in RCA: 156] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 74. | Minamishima YA, Moslehi J, Padera RF, Bronson RT, Liao R, Kaelin WG. A feedback loop involving the Phd3 prolyl hydroxylase tunes the mammalian hypoxic response in vivo. Mol Cell Biol. 2009;29:5729-5741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 128] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 75. | Kim WY, Safran M, Buckley MR, Ebert BL, Glickman J, Bosenberg M, Regan M, Kaelin WG. Failure to prolyl hydroxylate hypoxia-inducible factor alpha phenocopies VHL inactivation in vivo. EMBO J. 2006;25:4650-4662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 206] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 76. | Yun Z, Maecker HL, Johnson RS, Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331-341. [PubMed] |
| 77. | Choi SM, Cho HJ, Cho H, Kim KH, Kim JB, Park H. Stra13/DEC1 and DEC2 inhibit sterol regulatory element binding protein-1c in a hypoxia-inducible factor-dependent mechanism. Nucleic Acids Res. 2008;36:6372-6385. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 78. | Wang XL, Suzuki R, Lee K, Tran T, Gunton JE, Saha AK, Patti ME, Goldfine A, Ruderman NB, Gonzalez FJ. Ablation of ARNT/HIF1beta in liver alters gluconeogenesis, lipogenic gene expression, and serum ketones. Cell Metab. 2009;9:428-439. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 71] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 79. | Bonello S, Zähringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, Kietzmann T, Görlach A. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol. 2007;27:755-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 523] [Article Influence: 29.1] [Reference Citation Analysis (0)] |
| 80. | Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807-811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1279] [Cited by in RCA: 1214] [Article Influence: 71.4] [Reference Citation Analysis (0)] |
| 81. | Kuhlicke J, Frick JS, Morote-Garcia JC, Rosenberger P, Eltzschig HK. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS One. 2007;2:e1364. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 82. | Kim SY, Choi YJ, Joung SM, Lee BH, Jung YS, Lee JY. Hypoxic stress up-regulates the expression of Toll-like receptor 4 in macrophages via hypoxia-inducible factor. Immunology. 2010;129:516-524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 141] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 83. | Huang W, Metlakunta A, Dedousis N, Zhang P, Sipula I, Dube JJ, Scott DK, O’Doherty RM. Depletion of liver Kupffer cells prevents the development of diet-induced hepatic steatosis and insulin resistance. Diabetes. 2010;59:347-357. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 365] [Cited by in RCA: 420] [Article Influence: 28.0] [Reference Citation Analysis (0)] |
| 84. | Miura K, Kodama Y, Inokuchi S, Schnabl B, Aoyama T, Ohnishi H, Olefsky JM, Brenner DA, Seki E. Toll-like receptor 9 promotes steatohepatitis by induction of interleukin-1beta in mice. Gastroenterology. 2010;139:323-324.e7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 527] [Cited by in RCA: 622] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 85. | Stienstra R, Saudale F, Duval C, Keshtkar S, Groener JE, van Rooijen N, Staels B, Kersten S, Müller M. Kupffer cells promote hepatic steatosis via interleukin-1beta-dependent suppression of peroxisome proliferator-activated receptor alpha activity. Hepatology. 2010;51:511-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 370] [Article Influence: 24.7] [Reference Citation Analysis (0)] |
| 86. | Kersten S. Regulation of nutrient metabolism and inflammation. Results Probl Cell Differ. 2010;52:13-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 87. | Rosmorduc O, Housset C. Hypoxia: a link between fibrogenesis, angiogenesis, and carcinogenesis in liver disease. Semin Liver Dis. 2010;30:258-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 168] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 88. | Cannito S, Paternostro C, Busletta C, Bocca C, Colombatto S, Miglietta A, Novo E, Parola M. Hypoxia, hypoxia-inducible factors and fibrogenesis in chronic liver diseases. Histol Histopathol. 2014;29:33-44. [PubMed] |
| 89. | Paternostro C, David E, Novo E, Parola M. Hypoxia, angiogenesis and liver fibrogenesis in the progression of chronic liver diseases. World J Gastroenterol. 2010;16:281-288. [PubMed] |
| 90. | Corpechot C, Barbu V, Wendum D, Kinnman N, Rey C, Poupon R, Housset C, Rosmorduc O. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 352] [Cited by in RCA: 369] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 91. | Novo E, Cannito S, Zamara E, Valfrè di Bonzo L, Caligiuri A, Cravanzola C, Compagnone A, Colombatto S, Marra F, Pinzani M. Proangiogenic cytokines as hypoxia-dependent factors stimulating migration of human hepatic stellate cells. Am J Pathol. 2007;170:1942-1953. [PubMed] |
| 92. | Novo E, Povero D, Busletta C, Paternostro C, di Bonzo LV, Cannito S, Compagnone A, Bandino A, Marra F, Colombatto S. The biphasic nature of hypoxia-induced directional migration of activated human hepatic stellate cells. J Pathol. 2012;226:588-597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 67] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 93. | Moon JO, Welch TP, Gonzalez FJ, Copple BL. Reduced liver fibrosis in hypoxia-inducible factor-1alpha-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2009;296:G582-G592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 181] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 94. | Copple BL, Bustamante JJ, Welch TP, Kim ND, Moon JO. Hypoxia-inducible factor-dependent production of profibrotic mediators by hypoxic hepatocytes. Liver Int. 2009;29:1010-1021. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 69] [Cited by in RCA: 75] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 95. | Kuwabara K, Ogawa S, Matsumoto M, Koga S, Clauss M, Pinsky DJ, Lyn P, Leavy J, Witte L, Joseph-Silverstein J. Hypoxia-mediated induction of acidic/basic fibroblast growth factor and platelet-derived growth factor in mononuclear phagocytes stimulates growth of hypoxic endothelial cells. Proc Natl Acad Sci USA. 1995;92:4606-4610. [PubMed] |
| 96. | Copple BL, Bai S, Burgoon LD, Moon JO. Hypoxia-inducible factor-1α regulates the expression of genes in hypoxic hepatic stellate cells important for collagen deposition and angiogenesis. Liver Int. 2011;31:230-244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 97. | Copple BL, Kaska S, Wentling C. Hypoxia-inducible factor activation in myeloid cells contributes to the development of liver fibrosis in cholestatic mice. J Pharmacol Exp Ther. 2012;341:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 53] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 98. | Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 729] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 99. | Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245-257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1127] [Cited by in RCA: 1074] [Article Influence: 71.6] [Reference Citation Analysis (0)] |
| 100. | Takeda N, O’Dea EL, Doedens A, Kim JW, Weidemann A, Stockmann C, Asagiri M, Simon MC, Hoffmann A, Johnson RS. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 501] [Article Influence: 33.4] [Reference Citation Analysis (0)] |
| 101. | Noordeen NA, Khera TK, Sun G, Longbottom ER, Pullen TJ, da Silva Xavier G, Rutter GA, Leclerc I. Carbohydrate-responsive element-binding protein (ChREBP) is a negative regulator of ARNT/HIF-1beta gene expression in pancreatic islet beta-cells. Diabetes. 2010;59:153-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 54] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 102. | Gastaut H, Tassinari CA, Duron B. Polygraphic study of the episodic diurnal and nocturnal (hypnic and respiratory) manifestations of the Pickwick syndrome. Brain Res. 1966;1:167-186. [PubMed] |
| 103. | Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165:670-676. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 822] [Cited by in RCA: 803] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 104. | Tanné F, Gagnadoux F, Chazouillères O, Fleury B, Wendum D, Lasnier E, Lebeau B, Poupon R, Serfaty L. Chronic liver injury during obstructive sleep apnea. Hepatology. 2005;41:1290-1296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 183] [Article Influence: 9.2] [Reference Citation Analysis (0)] |
| 105. | Kallwitz ER, Herdegen J, Madura J, Jakate S, Cotler SJ. Liver enzymes and histology in obese patients with obstructive sleep apnea. J Clin Gastroenterol. 2007;41:918-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 106. | Jouët P, Sabaté JM, Maillard D, Msika S, Mechler C, Ledoux S, Harnois F, Coffin B. Relationship between obstructive sleep apnea and liver abnormalities in morbidly obese patients: a prospective study. Obes Surg. 2007;17:478-485. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 68] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 107. | Polotsky VY, Patil SP, Savransky V, Laffan A, Fonti S, Frame LA, Steele KE, Schweizter MA, Clark JM, Torbenson MS. Obstructive sleep apnea, insulin resistance, and steatohepatitis in severe obesity. Am J Respir Crit Care Med. 2009;179:228-234. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 162] [Cited by in RCA: 153] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 108. | Kheirandish-Gozal L, Sans Capdevila O, Kheirandish E, Gozal D. Elevated serum aminotransferase levels in children at risk for obstructive sleep apnea. Chest. 2008;133:92-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 109. | Aron-Wisnewsky J, Minville C, Tordjman J, Lévy P, Bouillot JL, Basdevant A, Bedossa P, Clément K, Pépin JL. Chronic intermittent hypoxia is a major trigger for non-alcoholic fatty liver disease in morbid obese. J Hepatol. 2012;56:225-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 168] [Cited by in RCA: 188] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 110. | Sookoian S, Pirola CJ. Obstructive sleep apnea is associated with fatty liver and abnormal liver enzymes: a meta-analysis. Obes Surg. 2013;23:1815-1825. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 111. | Fletcher EC, Lesske J, Qian W, Miller CC, Unger T. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19:555-561. [PubMed] |
| 112. | Li J, Thorne LN, Punjabi NM, Sun CK, Schwartz AR, Smith PL, Marino RL, Rodriguez A, Hubbard WC, O’Donnell CP. Intermittent hypoxia induces hyperlipidemia in lean mice. Circ Res. 2005;97:698-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 237] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 113. | Savransky V, Nanayakkara A, Vivero A, Li J, Bevans S, Smith PL, Torbenson MS, Polotsky VY. Chronic intermittent hypoxia predisposes to liver injury. Hepatology. 2007;45:1007-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 192] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 114. | Li J, Grigoryev DN, Ye SQ, Thorne L, Schwartz AR, Smith PL, O’Donnell CP, Polotsky VY. Chronic intermittent hypoxia upregulates genes of lipid biosynthesis in obese mice. J Appl Physiol (1985). 2005;99:1643-1648. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 150] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 115. | Drager LF, Li J, Reinke C, Bevans-Fonti S, Jun JC, Polotsky VY. Intermittent hypoxia exacerbates metabolic effects of diet-induced obesity. Obesity (Silver Spring). 2011;19:2167-2174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 116. | Yuan G, Nanduri J, Khan S, Semenza GL, Prabhakar NR. Induction of HIF-1alpha expression by intermittent hypoxia: involvement of NADPH oxidase, Ca2+ signaling, prolyl hydroxylases, and mTOR. J Cell Physiol. 2008;217:674-685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 264] [Cited by in RCA: 252] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 117. | Yuan G, Nanduri J, Bhasker CR, Semenza GL, Prabhakar NR. Ca2+/calmodulin kinase-dependent activation of hypoxia inducible factor 1 transcriptional activity in cells subjected to intermittent hypoxia. J Biol Chem. 2005;280:4321-4328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 187] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 118. | Malec V, Gottschald OR, Li S, Rose F, Seeger W, Hänze J. HIF-1 alpha signaling is augmented during intermittent hypoxia by induction of the Nrf2 pathway in NOX1-expressing adenocarcinoma A549 cells. Free Radic Biol Med. 2010;48:1626-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 119] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 119. | Nanduri J, Wang N, Yuan G, Khan SA, Souvannakitti D, Peng YJ, Kumar GK, Garcia JA, Prabhakar NR. Intermittent hypoxia degrades HIF-2alpha via calpains resulting in oxidative stress: implications for recurrent apnea-induced morbidities. Proc Natl Acad Sci USA. 2009;106:1199-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 120. | Nanduri J, Vaddi DR, Khan SA, Wang N, Makerenko V, Prabhakar NR. Xanthine oxidase mediates hypoxia-inducible factor-2α degradation by intermittent hypoxia. PLoS One. 2013;8:e75838. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 60] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 121. | Li J, Bosch-Marce M, Nanayakkara A, Savransky V, Fried SK, Semenza GL, Polotsky VY. Altered metabolic responses to intermittent hypoxia in mice with partial deficiency of hypoxia-inducible factor-1alpha. Physiol Genomics. 2006;25:450-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 125] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 122. | Peng YJ, Yuan G, Ramakrishnan D, Sharma SD, Bosch-Marce M, Kumar GK, Semenza GL, Prabhakar NR. Heterozygous HIF-1alpha deficiency impairs carotid body-mediated systemic responses and reactive oxygen species generation in mice exposed to intermittent hypoxia. J Physiol. 2006;577:705-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 290] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 123. | Jiang C, Kim JH, Li F, Qu A, Gavrilova O, Shah YM, Gonzalez FJ. Hypoxia-inducible factor 1α regulates a SOCS3-STAT3-adiponectin signal transduction pathway in adipocytes. J Biol Chem. 2013;288:3844-3857. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 59] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 124. | Jiang C, Qu A, Matsubara T, Chanturiya T, Jou W, Gavrilova O, Shah YM, Gonzalez FJ. Disruption of hypoxia-inducible factor 1 in adipocytes improves insulin sensitivity and decreases adiposity in high-fat diet-fed mice. Diabetes. 2011;60:2484-2495. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 219] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 125. | Sun K, Halberg N, Khan M, Magalang UJ, Scherer PE. Selective inhibition of hypoxia-inducible factor 1α ameliorates adipose tissue dysfunction. Mol Cell Biol. 2013;33:904-917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 192] [Article Influence: 14.8] [Reference Citation Analysis (0)] |